Bioactives Overproduction through Operational Strategies in the Ichthyotoxic Microalga Heterosigma akashiwo Culture
Abstract
:1. Introduction
2. Results and Discussion
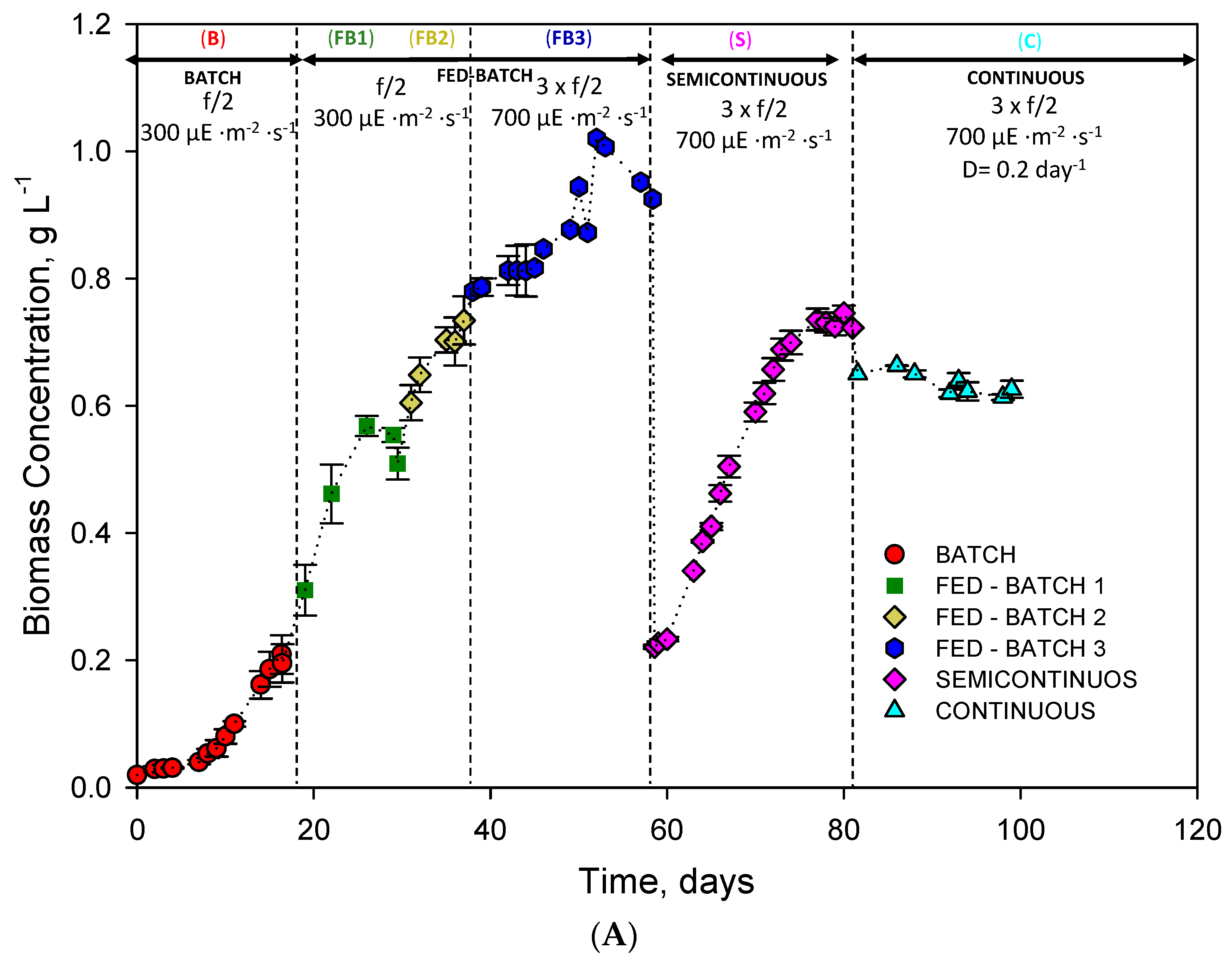
2.1. Operational Conditions in the LED-Based Bubble Column PBR
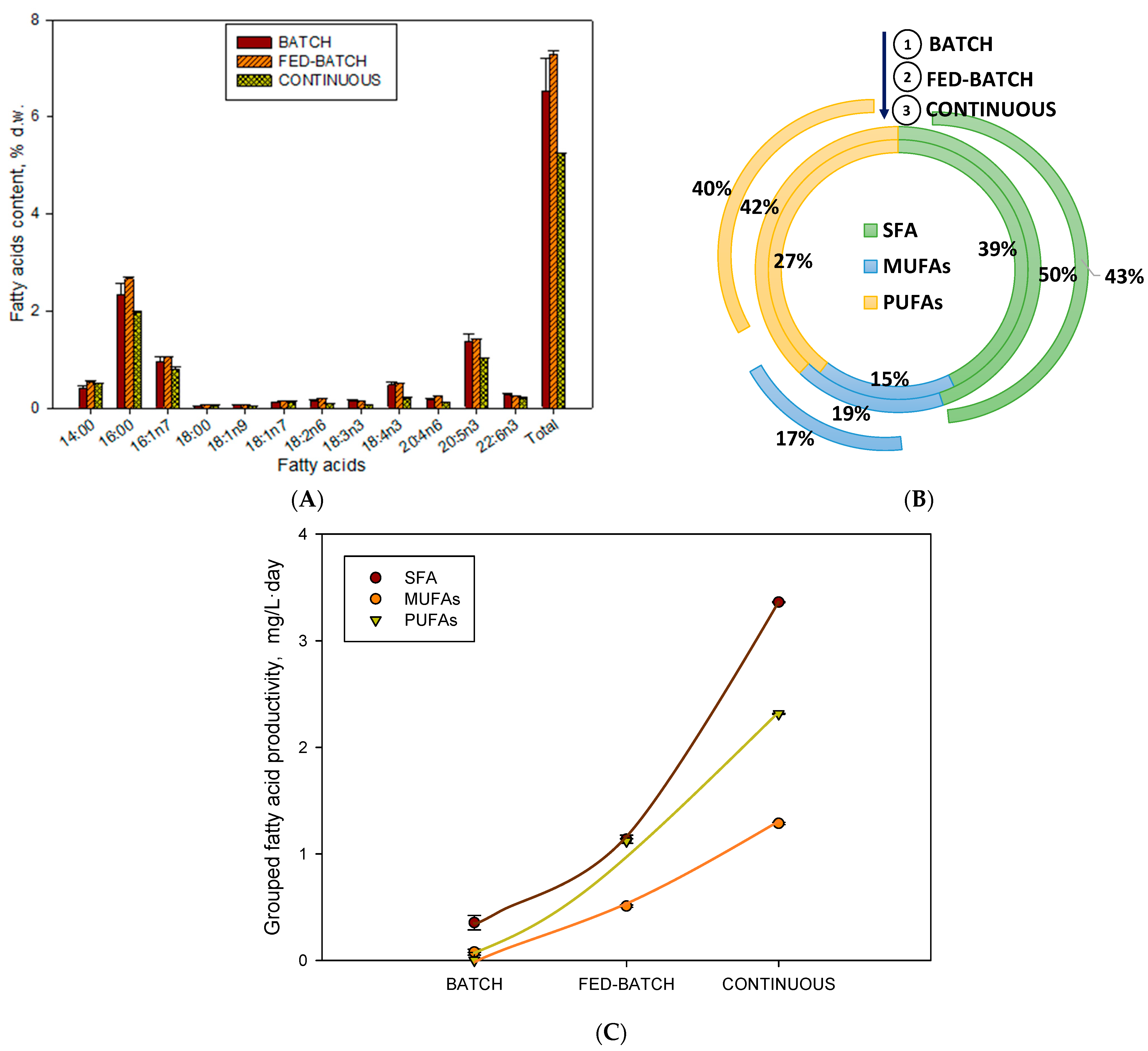
2.2. Influence of Operational Conditions on PUFA Profile and Content
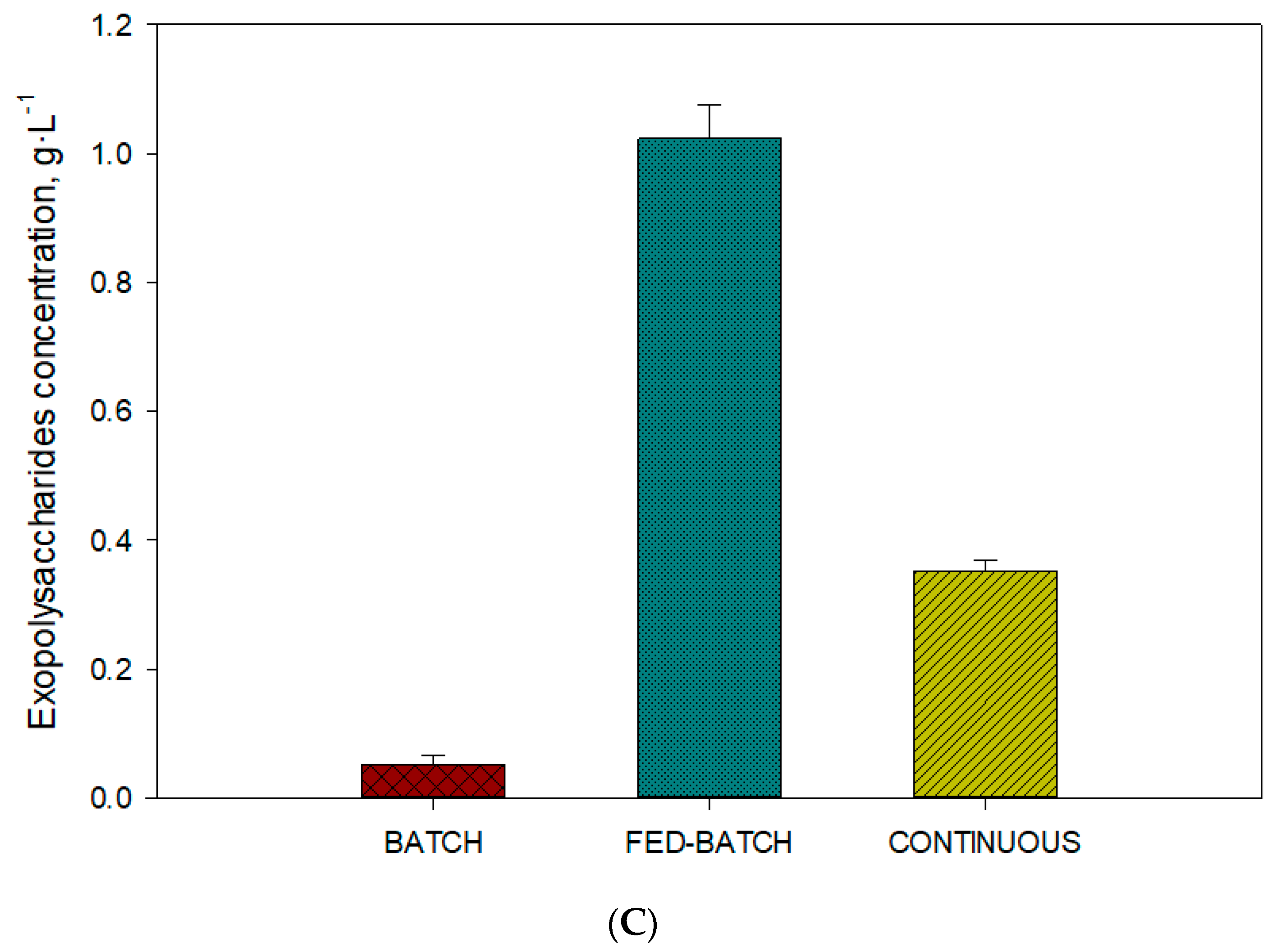
2.3. Influence of Operational Conditions on Carotenoid and Exopolysaccharides Content
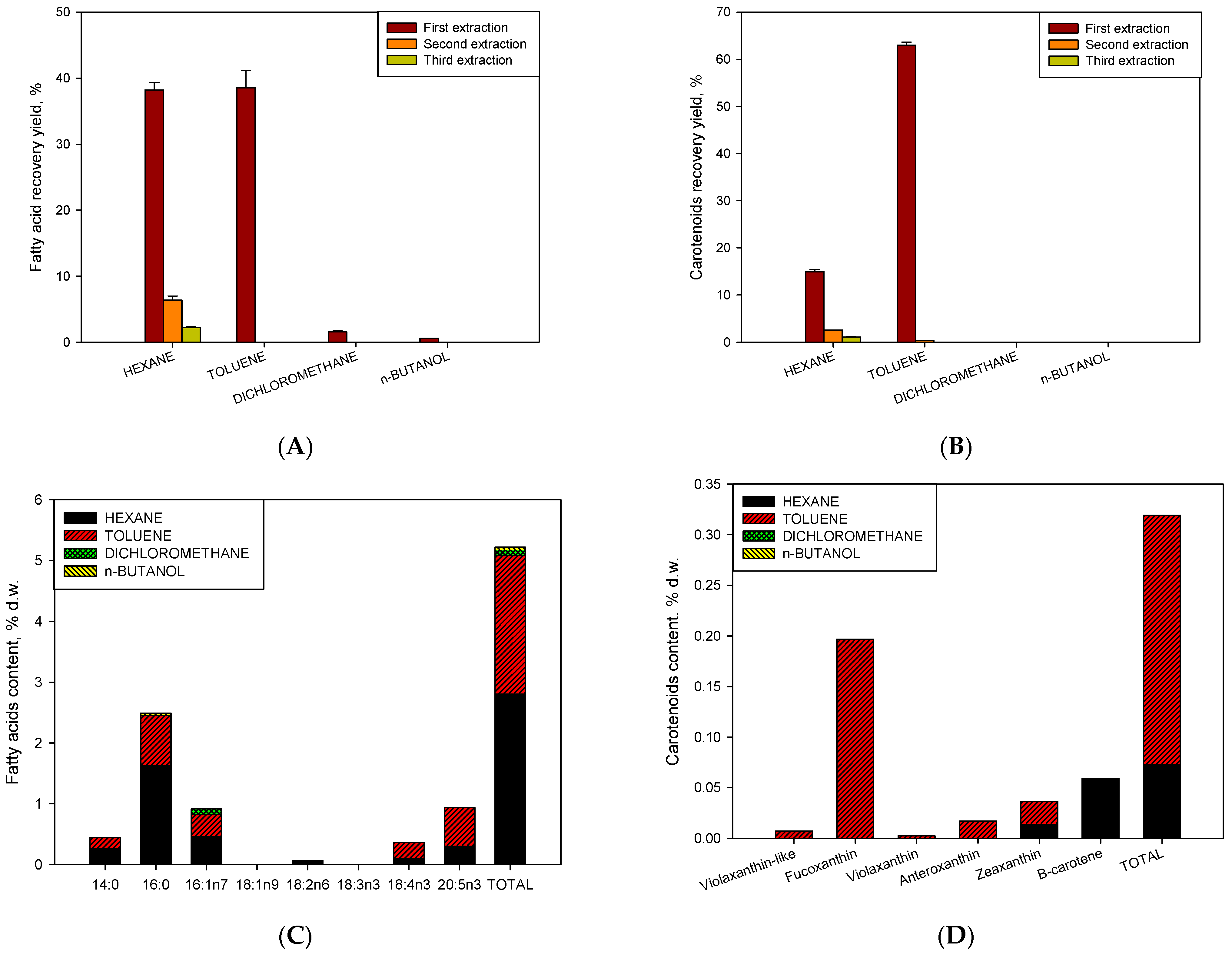
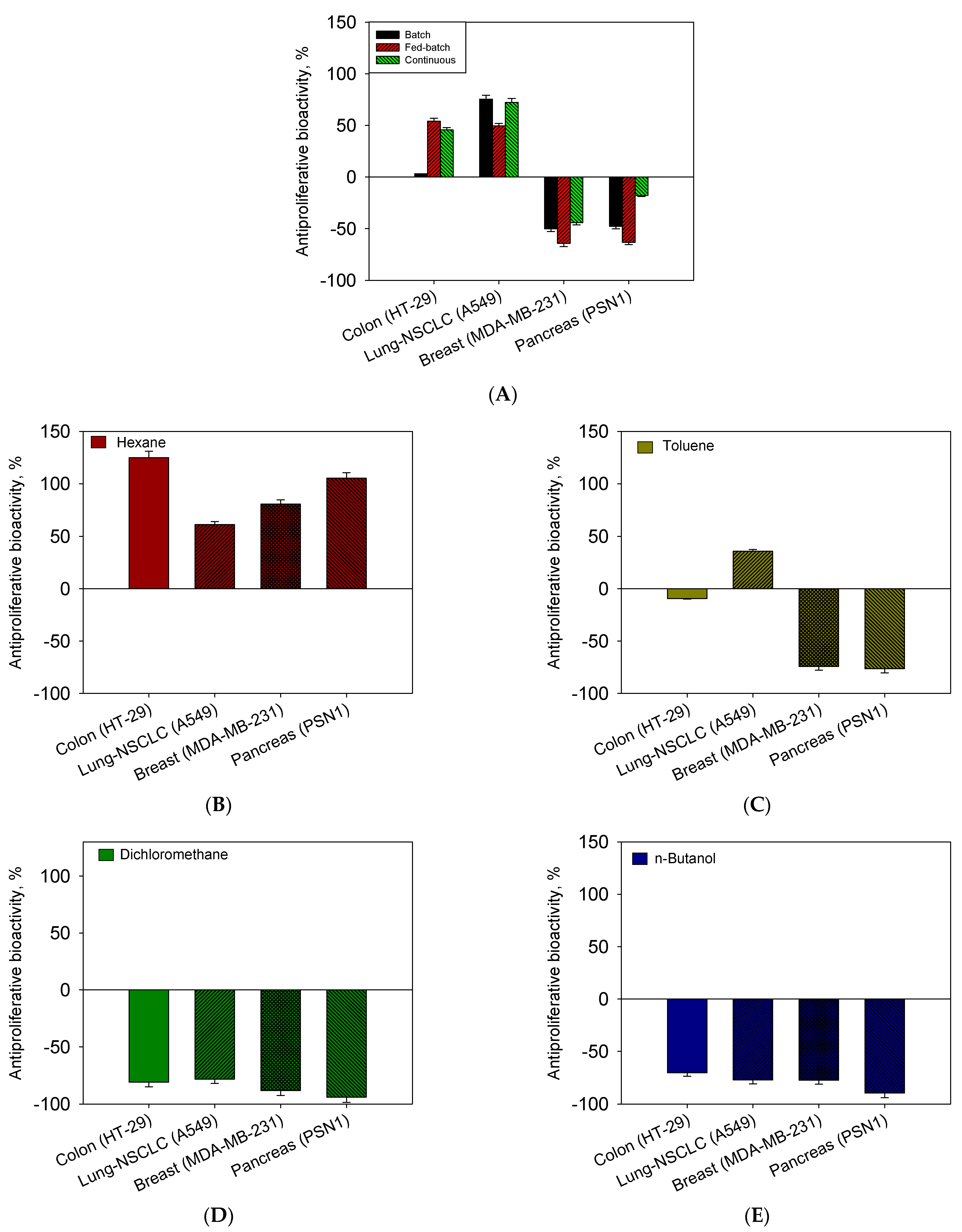
2.4. Influence of Operational Conditions on Growth and Sequential Extraction Liquid–Liquid on Bioactivity Assays as Antiproliferative against Human Tumour Cells
3. Conclusions
4. Materials and Methods
4.1. The Microalgae, Inoculum, and Its Maintenance
4.2. The LED-Based Bubble Column PBR
4.3. Kinetic Parameters
4.4. Analytical Procedures
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Archibald, J.M.; Simpson, A.G.B. Handbook of the Protists. In Handbook of the Protists; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Keppler, C.J.; Lewitus, A.J.; Ringwood, A.H.; Hoguet, J.; Staton, T. Sublethal cellular effects of short-term raphidophyte and brevetoxin exposures on the eastern oyster Crassostrea virginica. Mar. Ecol. Prog. Ser. 2006, 312, 141–147. [Google Scholar] [CrossRef]
- Khan, S.; Arakawa, O.; Onoue, Y. Neurotoxins in a toxic red tide of Heterosigma akashiwo (Raphidophyceae) in Kagoshima Bay, Japan. Aquac. Res. 1997, 28, 9–14. [Google Scholar] [CrossRef]
- Gallardo Rodríguez, J.J.; Sánchez Mirón, A.; García Camacho, F.; Cerón García, M.C.; Belarbi, E.H.; Molina Grima, E. Culture of dinoflagellates in a fed-batch and continuous stirred-tank photobioreactors: Growth, oxidative stress and toxin production. Process Biochem. 2010, 45, 660–666. [Google Scholar] [CrossRef]
- Camacho, F.G.; Rodríguez, J.G.; Mirón, A.S.; García, M.C.C.; Belarbi, E.H.; Chisti, Y.; Grima, E.M. Biotechnological significance of toxic marine dinoflagellates. Biotechnol. Adv. 2007, 25, 176–194. [Google Scholar] [CrossRef]
- Shimizu, Y. Microalgal metabolites. Curr. Opin. Microbiol. 2003, 6, 236–243. [Google Scholar] [CrossRef]
- Marshall, J.A.; Ross, T.; Pyecroft, S.; Hallegraeff, G. Superoxide production by marine microalgae: II. Towards understanding ecological consequences and possible functions. Mar. Biol. 2005, 147, 541–549. [Google Scholar] [CrossRef]
- Oda, T.; Nakamura, A.; Okamoto, T.; Ishimatsu, A.; Muramatsu, T. Lectin-induced enhancement of superoxide union production by red tide phytoplankton. Mar. Biol. 1998, 131, 383–390. [Google Scholar] [CrossRef]
- Twiner, M.J.; Trick, C.G. Possible physiological mechanisms for production of hydrogen peroxide by the ichthyotoxic flagellate Heterosigma akashiwo. J. Plankton Res. 2000, 22, 1961–1975. [Google Scholar] [CrossRef]
- De Boer, M.K.; Tyl, M.R.; Vrieling, E.G.; Van Rijssel, M. Effects of salinity and nutrient conditions on growth and haemolytic activity of Fibrocapsa japonica (Raphidophyceae). Aquat. Microb. Ecol. 2004, 37, 171–181. [Google Scholar] [CrossRef]
- Fu, M.; Koulman, A.; Van Rijssel, M.; Lützen, A.; De Boer, M.K.; Tyl, M.R.; Liebezeit, G. Chemical characterisation of three haemolytic compounds from the microalgal species Fibrocapsa japonica (Raphidophyceae). Toxicon 2004, 43, 355–363. [Google Scholar] [CrossRef]
- Ling, C.; Trick, C.G. Expression and standardized measurement of hemolytic activity in Heterosigma akashiwo. Harmful Algae 2010, 9, 522–529. [Google Scholar] [CrossRef]
- Black, E.A. Differentiation of Morphology and Toxicity in Harmful Algal Blooms Caused by the Raphidophyte Alga Heterosigma akashiwo. Ph.D. Thesis, University of Victoria, Victoria, BC, Canada, 2000; 207p. [Google Scholar]
- Powers, L.; Creed, I.F.; Trick, C.G. Sinking of Heterosigma akashiwo results in increased toxicity of this harmful algalbloom species. Harmful Algae 2012, 13, 95–104. [Google Scholar] [CrossRef]
- Fuentes-Grünewald, C.; Garcés, E.; Alacid, E.; Rossi, S.; Camp, J. Biomass and Lipid Production of Dinoflagellates and Raphidophytes in Indoor and Outdoor Photobioreactors. Mar. Biotechnol. 2013, 15, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Grünewald, C.; Garcés, E.; Alacid, E.; Sampedro, N.; Rossi, S.; Camp, J. Improvement of lipid production in the marine strains Alexandrium minutum and Heterosigma akashiwo by utilizing abiotic parameters. J. Ind. Microbiol. Biotechnol. 2012, 39, 207–216. [Google Scholar] [CrossRef]
- Fuentes-Grünewald, C.; Garcés, E.; Rossi, S.; Camp, J. Use of the dinoflagellate Karlodinium veneficum as a sustainable source of biodiesel production. J. Ind. Microbiol. Biotechnol. 2009, 36, 1215–1224. [Google Scholar] [CrossRef]
- Martínez, R.; Orive, E.; Laza-Martínez, A.; Seoane, S. Growth response of six strains of Heterosigma akashiwo to varying temperature, salinity and irradiance conditions. J. Plankton Res. 2010, 32, 529–538. [Google Scholar] [CrossRef]
- Kok, J.W.K.; Yeo, D.C.J.; Leong, S.C.Y. Growth and physiological responses of a tropical toxic marine microgalga Heterosigma akashiwo (Heterokontophyta: Raphidophyceae) from Singapore waters to varying nitrogen sources and light conditions. Ocean Sci. J. 2015, 50, 491–508. [Google Scholar] [CrossRef]
- Wang, C.; Lin, X.; Li, L.; Lin, S. Differential growth responses of marine phytoplankton to herbicide glyphosate. PLoS ONE 2016, 11, e0151633. [Google Scholar] [CrossRef]
- Stewart, J.J.; Bianco, C.M.; Miller, K.R.; Coyne, K.J. The marine microalga, Heterosigma akashiwo, converts industrial waste gases into valuable biomass. Front. Energy Res. 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Nichols, P.D.; Volkman, J.K.; Hallegraeff, G.M.; Blackburn, S.I. Sterols and fatty acids of the red tide flagellates Heterosigma akashiwo and Chattonella antiqua (Raphidophyceae). Phytochemistry 1987, 26, 2537–2541. [Google Scholar] [CrossRef]
- Fiksdahl, A.; Withers, N.; Liaaen-Jensen, S. Carotenoids of Heterosigma akashiwo: A chemosystematic contribution. Biochem. Syst. Ecol. 1984, 12, 355–356. [Google Scholar] [CrossRef]
- Wang, R.; Tang, X. Allelopathic effects of macroalga Corallina pilulifera on the red-tide forming alga Heterosigma akashiwo under laboratory conditions. Chin. J. Oceanol. Limnol. 2016, 34, 314–321. [Google Scholar] [CrossRef]
- Gallardo-Rodríguez, J.J.; Astuya-Villalón, A.; Avello, V.; Llanos-Rivera, A.; Krock, B.; Agurto-Muñoz, C.; Sánchez-Mirón, A.; García-Camacho, F. Production of extracts with anaesthetic activity from the culture of Heterosigma akashiwo in pilot-scale photobioreactors. Algal Res. 2020, 45, 101760. [Google Scholar] [CrossRef]
- Bianco, C.M.; Stewart, J.J.; Miller, K.R.; Fitzgerald, C.; Coyne, K.J. Light intensity impacts the production of biofuel intermediates in Heterosigma akashiwo growing on simulated flue gas containing carbon dioxide and nitric oxide. Bioresour. Technol. 2016, 219, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Saéz, L.M.; Abreu, A.C.; Camacho-Rodríguez, J.; González-López, C.V.; Cerón-García, M.C.; Fernández, I. NMR Metabolomics as an Effective Tool to Unravel the Effect of Light Intensity and Temperature on the Composition of the Marine Microalgae Isochrysis galbana. J. Agric. Food Chem. 2019, 67, 3879–3889. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Rodríguez, J.; Cerón-García, M.C.; Fernández-Sevilla, J.M.; Molina-Grima, E. The influence of culture conditions on biomass and high value product generation by Nannochloropsis gaditana in aquaculture. Algal Res. 2015, 11, 63–73. [Google Scholar] [CrossRef]
- Okumura, Y.; Yamasaki, M.; Suzuki, T. Pigment profile and violaxanthin cycle of Heterosigma akashiwo (Raphidophyceae). J. Shellfish Res. 2001, 20, 1263–1268. [Google Scholar]
- Subramanian, S.; Barry, A.N.; Pieris, S.; Sayre, R.T. Comparative energetics and kinetics of autotrophic lipid and starch metabolism in chlorophytic microalgae: Implications for biomass and biofuel production. Biotechnol. Biofuels 2013, 6, 150. [Google Scholar] [CrossRef]
- González-Cardoso, M.A.; Cerón-García, M.C.; Navarro-López, E.; Molina-Miras, A.; Sánchez-Mirón, A.; Contreras-Gómez, A.; García-Camacho, F. Alternatives to classic solvents for the isolation of bioactive compounds from Chrysochromulina rotalis. Bioresour. Technol. 2023, 379, 129057. [Google Scholar] [CrossRef]
- Takahashi, K.; Hosokawa, M.; Kasajima, H.; Hatanaka, K.; Kudo, K.; Shimoyama, N.; Miyashita, K. Anticancer effects of fucoxanthin and fucoxanthinol on colorectal cancer cell lines and colorectal cancer tissues. Oncol. Lett. 2015, 10, 1463–1467. [Google Scholar] [CrossRef]
- Méresse, S.; Fodil, M.; Fleury, F.; Chénais, B. Fucoxanthin, a marine-derived carotenoid from brown seaweeds and microalgae: A promising bioactive compound for cancer therapy. Int. J. Mol. Sci. 2020, 21, 9273. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, M.; Le Ferrec, E.; Mayer, C.; Mimouni, V.; Lagadic-Gossmann, D.; Schoefs, B.; Ulmann, L. Microalgal carotenoids and phytosterols regulate biochemical mechanisms involved in human health and disease prevention. Biochimie 2019, 167, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Pajot, A.; Huynh, G.H.; Picot, L.; Marchal, L.; Nicolau, E. Fucoxanthin from Algae to Human, an Extraordinary BioreSource: Insights and Advances in up and Downstream Processes. Mar. Drugs 2022, 20, 222. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Chihara, M. Morphology, Ultrastructure and Taxonomy of the Raphidophycean Alga Heterosigma akashiwo. Bot. Mag. Tokyo 1987, 100, 151–163. [Google Scholar] [CrossRef]
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms. I. cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.A. Algal Culturing Techniques; Elservier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Zhu, X.; Jańczewski, D.; Lee, S.S.C.; Teo, S.L.M.; Vancso, G.J. Cross-linked polyelectrolyte multilayers for marine antifouling applications. ACS Appl. Mater. Interfaces 2013, 5, 5961–5968. [Google Scholar] [CrossRef]
- López-Rosales, L.; García-Camacho, F.; Sánchez-Mirón, A.; Contreras-Gómez, A.; Molina-Grima, E. An optimisation approach for culturing shear-sensitive dinoflagellate microalgae in bench-scale bubble column photobioreactors. Bioresour. Technol. 2015, 197, 375–382. [Google Scholar] [CrossRef]
- American Public Health Association. APHA Method 4500-H pH Value: Standard Methods for the Examination of Water and Wastewater. Stand. Methods Exam. Water Wastewater 2017, 552, 4500. Available online: https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/scientific/technical-documents/white-papers/apha-biochemical-oxygen-demand-white-paper.pdf (accessed on 25 February 2021).
- Cerón-García, M.C.; González-López, C.V.; Camacho-Rodríguez, J.; López-Rosales, L.; García-Camacho, F.; Molina-Grima, E. Maximizing carotenoid extraction from microalgae used as food additives and determined by liquid chromatography (HPLC). Food Chem. 2018, 257, 316–324. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, J.; Belarbi, E.H.; Sánchez, J.L.G.; Alonso, D.L. Rapid simultaneous lipid extraction and transesterification for fatty acid analyses. Biotechnol. Tech. 1998, 12, 689–691. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, R.; Jiang, P.; Liu, Z. Production of an exopolysaccharide bioflocculant by Sorangium cellulosum. Lett. Appl. Microbiol. 2002, 34, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bishop, P.L.; Kinkle, B.K. Comparison of extraction methods for quantifying extracellular polymers in biofilms. Water Sci. Technol. 1999, 39, 211–218. [Google Scholar] [CrossRef]
- Bala Subramanian, S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular polymeric substances (EPS) producing bacterial strains of municipal wastewater sludge: Isolation, molecular identification, EPS characterization and performance for sludge settling and dewatering. Water Res. 2010, 44, 2253–2266. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 19th ed.; APHA: New York, NY, USA, 1995. [Google Scholar]
- Abreu, A.C.; Molina-Miras, A.; Aguilera-Saéz, L.M.; López-Rosales, L.; Cerón-Garcia, M.D.C.; Sánchez-Mirón, A.; Olmo-Garcia, L.; Carrasco-Pancorbo, A.; Garcia-Camacho, F.; Molina-Grima, E.; et al. Production of Amphidinols and Other Bioproducts of Interest by the Marine Microalga Amphidinium carterae Unraveled by Nuclear Magnetic Resonance Metabolomics Approach Coupled to Multivariate Data Analysis. J. Agric. Food Chem. 2019, 67, 9667–9682. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macías-de la Rosa, A.; González-Cardoso, M.Á.; Cerón-García, M.d.C.; López-Rosales, L.; Gallardo-Rodríguez, J.J.; Seoane, S.; Sánchez-Mirón, A.; García-Camacho, F. Bioactives Overproduction through Operational Strategies in the Ichthyotoxic Microalga Heterosigma akashiwo Culture. Toxins 2023, 15, 349. https://doi.org/10.3390/toxins15050349
Macías-de la Rosa A, González-Cardoso MÁ, Cerón-García MdC, López-Rosales L, Gallardo-Rodríguez JJ, Seoane S, Sánchez-Mirón A, García-Camacho F. Bioactives Overproduction through Operational Strategies in the Ichthyotoxic Microalga Heterosigma akashiwo Culture. Toxins. 2023; 15(5):349. https://doi.org/10.3390/toxins15050349
Chicago/Turabian StyleMacías-de la Rosa, Adrián, Miguel Ángel González-Cardoso, María del Carmen Cerón-García, Lorenzo López-Rosales, Juan José Gallardo-Rodríguez, Sergio Seoane, Asterio Sánchez-Mirón, and Francisco García-Camacho. 2023. "Bioactives Overproduction through Operational Strategies in the Ichthyotoxic Microalga Heterosigma akashiwo Culture" Toxins 15, no. 5: 349. https://doi.org/10.3390/toxins15050349
APA StyleMacías-de la Rosa, A., González-Cardoso, M. Á., Cerón-García, M. d. C., López-Rosales, L., Gallardo-Rodríguez, J. J., Seoane, S., Sánchez-Mirón, A., & García-Camacho, F. (2023). Bioactives Overproduction through Operational Strategies in the Ichthyotoxic Microalga Heterosigma akashiwo Culture. Toxins, 15(5), 349. https://doi.org/10.3390/toxins15050349





