Multicolor Visual Detection of Deoxynivalenol in Grain Based on Magnetic Immunoassay and Enzymatic Etching of Plasmonic Gold Nanobipyramids
Abstract
1. Introduction
2. Results and Discussion
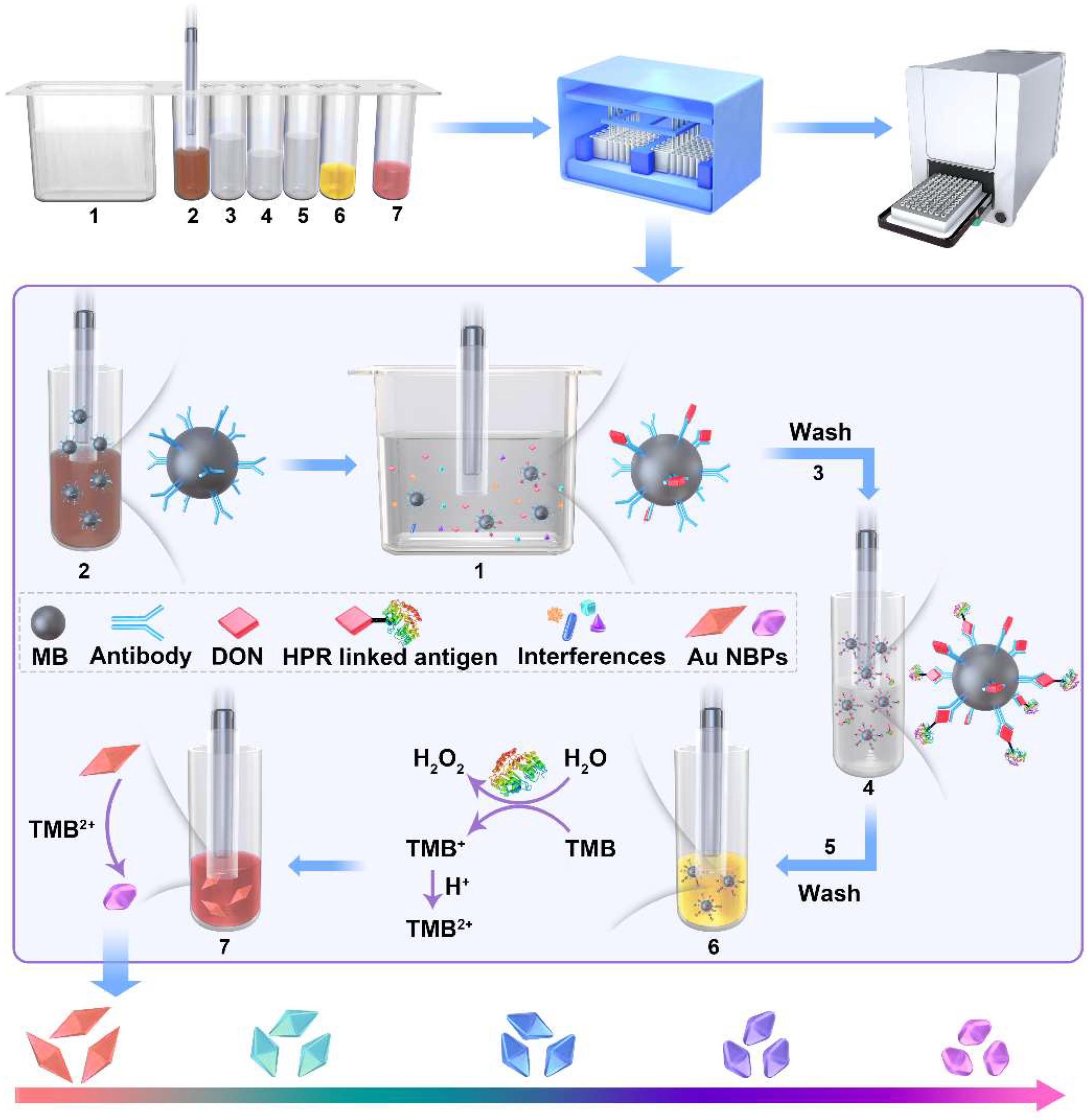
2.1. Principle of Multicolor Visual Strategy for DON Detection
2.2. Characterization of Au NBPs
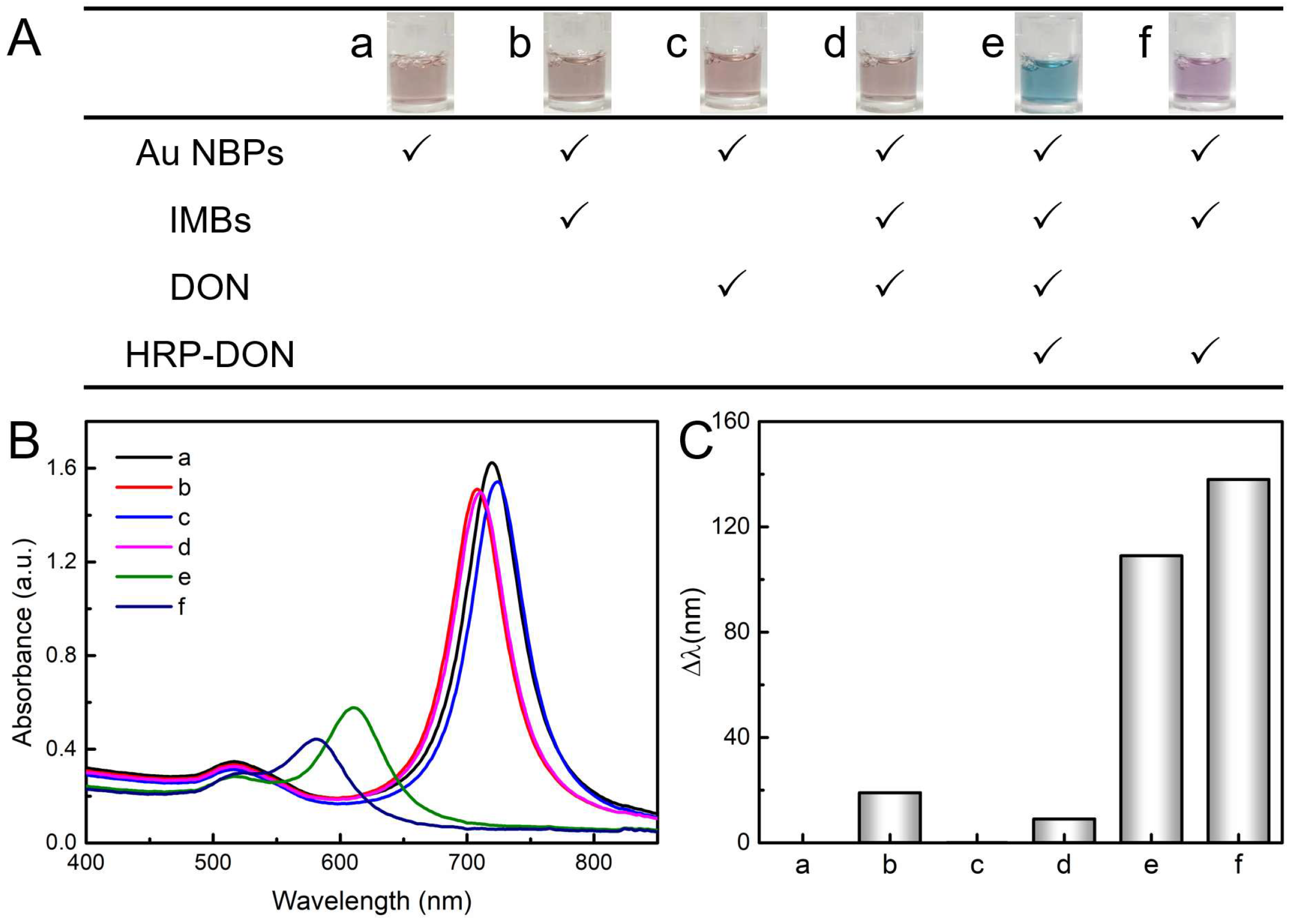
2.3. Feasibility of the DON Detection
2.4. Optimization of Experimental Conditions
2.5. Performance Evaluation of the Proposed Method
2.6. Analytical Application for Actual Samples
3. Conclusions
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Synthesis of Au NBPs
4.3. Preparation of IMBs
4.4. Principle of HRP Catalyzed Etching of Au NBPs
4.5. Visual Detection of DON
4.6. Selectivity of the Assay
4.7. Validation of the Method
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ganesan, A.R.; Mohan, K.; Karthick Rajan, D.; Pillay, A.A.; Palanisami, T.; Sathishkumar, P.; Conterno, L. Distribution, toxicity, interactive effects, and detection of ochratoxin and deoxynivalenol in food: A review. Food Chem. 2022, 378, 131978. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Ansari, K.M.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Occurrence of deoxynivalenol in cereals and exposure risk assessment in Indian population. Food Control 2013, 30, 549–555. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Wan, D.; Huang, L.; Pan, Y.; Wu, Q.; Chen, D.; Tao, Y.; Wang, X.; Liu, Z.; Li, J.; Wang, L.; et al. Metabolism, distribution, and excretion of deoxynivalenol with combined techniques of radiotracing, high-performance liquid chromatography ion trap time-of-flight mass spectrometry, and online radiometric detection. J. Agric. Food Chem. 2014, 62, 288–296. [Google Scholar] [CrossRef]
- Golge, O.; Kabak, B. Occurrence of deoxynivalenol and zearalenone in cereals and cereal products from Turkey. Food Control 2020, 110, 106982. [Google Scholar] [CrossRef]
- Karami-Osboo, R.; Maham, M.; Miri, R.; AliAbadi, M.H.S.; Mirabolfathy, M.; Javidnia, K. Evaluation of dispersive liquid–liquid microextraction–HPLC–UV for determination of deoxynivalenol (DON) in wheat flour. Food Anal. Method. 2012, 6, 176–180. [Google Scholar] [CrossRef]
- Warth, B.; Parich, A.; Bueschl, C.; Schoefbeck, D.; Neumann, N.K.; Kluger, B.; Schuster, K.; Krska, R.; Adam, G.; Lemmens, M.; et al. GC-MS based targeted metabolic profiling identifies changes in the wheat metabolome following deoxynivalenol treatment. Metabolomics 2015, 11, 722–738. [Google Scholar] [CrossRef] [PubMed]
- Mackay, N.; Marley, E.; Leeman, D.; Poplawski, C.; Donnelly, C. Analysis of aflatoxins, fumonisins, deoxynivalenol, ochratoxin A, zearalenone, HT-2, and T-2 toxins in animal feed by LC-MS/MS using cleanup with a multi-antibody immunoaffinity column. J. AOAC Int. 2022, 105, 1330–1340. [Google Scholar] [CrossRef]
- Trakselyte-Rupsiene, K.; Juodeikiene, G.; Hajnal, E.J.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Cernauskas, D.; Lele, V.; Zadeike, D.; Bartkiene, E. Challenges of Lactobacillus fermentation in combination with acoustic screening for deoxynivalenol and deoxynivalenol conjugates reduction in contaminated wheat-based products. Food Control 2022, 134, 108699. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Lu, Y.; Ma, D.-Y.; Qi, M.G.; Wang, S. A competitive direct enzyme-linked immunosorbent assay for the rapid detection of deoxynivalenol: Development and application in agricultural products and feedstuff. Food Agric. Immunol. 2017, 28, 516–527. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Z.; He, Q.; Deng, S.; Li, L.; Li, Y. Development of an immunochromatographic strip test for the rapid detection of deoxynivalenol in wheat and maize. Food Chem. 2010, 119, 834–839. [Google Scholar] [CrossRef]
- Hou, S.; Ma, J.; Cheng, Y.; Wang, H.; Sun, J.; Yan, Y. Quantum dot nanobead-based fluorescent immunochromatographic assay for simultaneous quantitative detection of fumonisin B1, dexyonivalenol, and zearalenone in grains. Food Control 2020, 117, 107331. [Google Scholar] [CrossRef]
- Meneely, J.; Fodey, T.; Armstrong, L.; Sulyok, M.; Krska, R.; Elliott, C. Rapid surface plasmon resonance immunoassay for the determination of deoxynivalenol in wheat, wheat products, and maize-based baby food. J. Agric. Food Chem. 2010, 58, 8936–8941. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Ren, P.; Huang, L.; Ouyang, Z.; Wang, Z.; Kong, X.; Li, T.; Yin, Y.; Wu, Y.; He, Q. Simultaneous detection of aflatoxin B1, ochratoxin A, zearalenone and deoxynivalenol in corn and wheat using surface plasmon resonance. Food Chem. 2019, 300, 125176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tang, S.; Jin, Y.; Yang, C.; He, L.; Wang, J.; Chen, Y. Multiplex SERS-based lateral flow immunosensor for the detection of major mycotoxins in maize utilizing dual Raman labels and triple test lines. J. Hazard. Mater. 2020, 393, 122348. [Google Scholar] [CrossRef]
- Gao, H.; Ta, L.; Li, Z.; Zhu, B.; Lu, H.; Ding, T.; Deng, X.; Li, X.; Han, F.; Xu, D. Simultaneous detection of four mycotoxins in cereals and edible oils by using a colorimetric protein microarray. ACS Food Sci. Technol. 2022, 2, 993–999. [Google Scholar] [CrossRef]
- Jiang, Q.; Wu, J.; Yao, K.; Yin, Y.; Gong, M.M.; Yang, C.; Lin, F. Paper-based microfluidic device (DON-Chip) for rapid and low-cost deoxynivalenol quantification in food, feed, and feed ingredients. ACS Sens. 2019, 4, 3072–3079. [Google Scholar] [CrossRef]
- Che Sulaiman, I.S.; Chieng, B.W.; Osman, M.J.; Ong, K.K.; Rashid, J.I.A.; Wan Yunus, W.M.Z.; Noor, S.A.M.; Kasim, N.A.M.; Halim, N.A.; Mohamad, A. A review on colorimetric methods for determination of organophosphate pesticides using gold and silver nanoparticles. Microchim. Acta 2020, 187, 131. [Google Scholar] [CrossRef]
- Zarlaida, F.; Adlim, M. Gold and silver nanoparticles and indicator dyes as active agents in colorimetric spot and strip tests for mercury(II) ions: A review. Microchim. Acta 2017, 184, 45–58. [Google Scholar] [CrossRef]
- Mauriz, E. Recent Progress in Plasmonic Biosensing Schemes for Virus Detection. Sensors 2020, 20, 4745. [Google Scholar] [CrossRef]
- Kristensen, A.; Yang, J.; Bozhevolnyi, S.; Link, S.; Nordlander, P.; Halas, N.; Mortensen, N. Plasmonic colour generation. Nat. Rev. Mater. 2017, 2, 16088. [Google Scholar] [CrossRef]
- Rathnakumar, S.; Bhaskar, S.; Rai, A.; Saikumar, D.; Kambhampati, N.; Sivaramakrishnan, V.; Ramamurthy, S. Plasmon-coupled silver nanoparticles for mobile phone-based attomolar sensing of mercury ions. ACS Appl. Nano Mater. 2021, 4, 8066–8080. [Google Scholar] [CrossRef]
- Wang, D.; Guo, R.; Wei, Y.; Zhang, Y.; Zhao, X.; Xu, Z. Sensitive multicolor visual detection of telomerase activity based on catalytic hairpin assembly and etching of Au nanorods. Biosens. Bioelectron. 2018, 122, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Y.; Zhao, X.; Xu, Z. Plasmonic colorimetric biosensor for visual detection of telomerase activity based on horseradish peroxidase-encapsulated liposomes and etching of Au nanobipyramids. Sens. Actuators B Chem. 2019, 296, 126646. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Ye, X.; Huang, C.; Yang, W.; Zhang, L.; Wu, Z.; Fu, F. Multicolor visual screening of total dithiocarbamate pesticides in foods based on sulfydryl-mediated growth of gold nanobipyramids. Anal. Chim. Acta 2020, 1139, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Jiang, L.; Liu, Y.; Liu, P.; Wang, W.; Luo, X. A morphology-based ultrasensitive multicolor colorimetric assay for detection of blood glucose by enzymatic etching of plasmonic gold nanobipyramids. Anal. Chim. Acta 2019, 1071, 53–58. [Google Scholar] [CrossRef]
- Xu, S.; Ouyang, W.; Xie, P.; Lin, Y.; Qiu, B.; Lin, Z.; Chen, G.; Guo, L. Highly uniform gold nanobipyramids for ultrasensitive colorimetric detection of influenza virus. Anal. Chem. 2017, 89, 1617–1623. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, C.; Liu, X.; Quan, Z.; Liu, W.; Liu, Y. A multi-colorimetric immunosensor for visual detection of ochratoxin A by mimetic enzyme etching of gold nanobipyramids. Microchim. Acta 2021, 188, 62. [Google Scholar] [CrossRef] [PubMed]
- Chateau, D.; Liotta, A.; Vadcard, F.; Navarro, J.R.; Chaput, F.; Lerme, J.; Lerouge, F.; Parola, S. From gold nanobipyramids to nanojavelins for a precise tuning of the plasmon resonance to the infrared wavelengths: Experimental and theoretical aspects. Nanoscale 2015, 7, 1934–1943. [Google Scholar] [CrossRef]
- Liu, H.; Xuan, Z.; Ye, J.; Chen, J.; Wang, M.; Freitag, S.; Krska, R.; Liu, Z.; Li, L.; Wu, Y.; et al. An automatic immunoaffinity pretreatment of deoxynivalenol coupled with UPLC–UV analysis. Toxins 2022, 14, 93. [Google Scholar] [CrossRef]
- Xuan, Z.; Wu, Y.; Liu, H.; Li, L.; Ye, J.; Wang, S. Copper oxide nanoparticle-based immunosensor for zearalenone analysis by combining automated sample pre-processing and high-throughput terminal detection. Sensors 2021, 21, 6538. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ye, J.; Li, L.; Wu, Y.; Liu, H.; Xuan, Z.; Chen, M.; Wang, S. One-step automatic sample pretreatment for rapid, simple, sensitive, and efficient determination of aflatoxin M1 in milk by immunomagnetic beads coupled to liquid chromatography-tandem mass spectrometry. Food Control 2022, 137, 108927. [Google Scholar] [CrossRef]
- Ni, B.; Ye, J.; Chen, J.; Li, L.; Liu, H.; Wu, Y.; Wang, S. Surfactant-enhanced and automated pretreatment based on immunoaffinity magnetic beads coupled with ultra-performance liquid chromatography with fluorescence detection for the determination of aflatoxins in peanut oils. J. Agric. Food Chem. 2022, 70, 10654–10661. [Google Scholar] [CrossRef] [PubMed]




| Sample | State Value/(μg/kg) | Detected Average/(μg/kg) | Recovery/% | RSD/% |
|---|---|---|---|---|
| Wheat | 200 ± 30 | 211.4 | 105.7 | 11.80 |
| 400 ± 60 | 418.0 | 104.5 | 11.61 | |
| 610 ± 92 | 622.1 | 101.9 | 9.59 | |
| 820 ± 98.4 | 820.7 | 100.1 | 8.67 | |
| 1023 ± 153 | 1002 | 97.98 | 4.10 | |
| 1033 ± 199 | 1263 | 94.74 | 9.15 | |
| 1606 ± 241 | 1604 | 99.85 | 9.61 | |
| 1845 ± 227 | 1759 | 95.33 | 4.06 | |
| Maize | 231 ± 34 | 225.4 | 97.57 | 5.15 |
| 462 ± 43 | 443.9 | 96.09 | 10.03 | |
| 770 ± 115 | 816.8 | 106.1 | 8.70 | |
| 1077 ± 161 | 1157 | 107.5 | 7.12 | |
| 1577 ± 236 | 1477 | 93.65 | 6.56 | |
| 2076 ± 294 | 2073 | 99.85 | 9.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, R.; Ji, Y.; Chen, J.; Ye, J.; Ni, B.; Li, L.; Yang, Y. Multicolor Visual Detection of Deoxynivalenol in Grain Based on Magnetic Immunoassay and Enzymatic Etching of Plasmonic Gold Nanobipyramids. Toxins 2023, 15, 351. https://doi.org/10.3390/toxins15060351
Guo R, Ji Y, Chen J, Ye J, Ni B, Li L, Yang Y. Multicolor Visual Detection of Deoxynivalenol in Grain Based on Magnetic Immunoassay and Enzymatic Etching of Plasmonic Gold Nanobipyramids. Toxins. 2023; 15(6):351. https://doi.org/10.3390/toxins15060351
Chicago/Turabian StyleGuo, Rui, Yue Ji, Jinnan Chen, Jin Ye, Baoxia Ni, Li Li, and Yongtan Yang. 2023. "Multicolor Visual Detection of Deoxynivalenol in Grain Based on Magnetic Immunoassay and Enzymatic Etching of Plasmonic Gold Nanobipyramids" Toxins 15, no. 6: 351. https://doi.org/10.3390/toxins15060351
APA StyleGuo, R., Ji, Y., Chen, J., Ye, J., Ni, B., Li, L., & Yang, Y. (2023). Multicolor Visual Detection of Deoxynivalenol in Grain Based on Magnetic Immunoassay and Enzymatic Etching of Plasmonic Gold Nanobipyramids. Toxins, 15(6), 351. https://doi.org/10.3390/toxins15060351





