Abstract
The pooled incidences of treatment-emergent adverse events (TEAEs) were examined by indication using the integrated clinical database of Merz-sponsored, placebo-controlled, or repeat-dose studies of incobotulinumtoxinA in adults with cervical dystonia, blepharospasm, limb spasticity, sialorrhea, or essential tremor of the upper limb. Overall incidences of TEAEs, serious TEAEs, TEAEs leading to discontinuation, fatal TEAEs, TEAEs of special interest (TEAESIs; indicating possible toxin spread), and treatment-related (TR) events were determined for incobotulinumtoxinA and placebo after a single injection and for repeated dose cycles of incobotulinumtoxinA. The most frequent events after a single dose of incobotulinumtoxinA are summarized. After a single cycle, incidences of overall TEAEs were similar between incobotulinumtoxinA and the placebo in most indications, although between-indication differences were observed. Few TEAEs led to incobotulinumtoxinA discontinuation; there were no fatal TEAEs with incobotulinumtoxinA. In general, repeated cycles did not increase the incidence of any event. The most frequent TR-TEAEs were indication-dependent, including dysphagia for indications affecting the head or neck. The TR-TEAESIs across all indications were most commonly muscular weakness, dysphagia and dry mouth. Overall, the results of this pooled analysis support and extend the favorable safety and tolerability profile of incobotulinumtoxinA for the treatment of adult neurological disorders established by individual clinical studies.
Keywords:
incobotulinumtoxinA; cervical dystonia; blepharospasm; spasticity; sialorrhea; essential tremor; neurological disorders; motor disorders; safety; immunogenicity Key Contribution:
As many patients with neurological disorders require long-term treatment with botulinum toxin type A (BoNT-A), it is important to ensure that the product used is safe and well tolerated. Analysis of adverse event data collected for up to 121 weeks after treatment initiation and pooled by indication from sponsored clinical trials of incobotulinumtoxinA support and extend the favorable safety and tolerability profile of this BoNT-A for the treatment of adult neurological disorders (cervical dystonia, blepharospasm, limb spasticity, sialorrhea, or essential tremor of the upper limb) seen in individual clinical trials.
1. Introduction
IncobotulinumtoxinA (Xeomin; Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany) is a formulation of botulinum toxin type A (BoNT-A) that is purified to contain only the active neurotoxin and no accessory proteins or other bacterial proteins [1]. It is approved for use in adults in the United States for the treatment of cervical dystonia, blepharospasm, upper limb spasticity and chronic sialorrhea [1] and in Europe for cervical dystonia of a predominantly rotational form (spasmodic torticollis), blepharospasm, and hemifacial spasm, spasticity of the upper limb, and chronic sialorrhea due to neurological disorders [2]. Multiple clinical studies support the safety and efficacy of incobotulinumtoxinA in these indications [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20], as well as evaluating the safety and efficacy of the biologic in subjects with lower limb spasticity [21] and essential tremor of the upper limb [22]. Additional indications, including some for pediatric populations, are also approved but are not the focus of this analysis [1,2].
As with any treatment, the nature and incidence of adverse events (AEs) can vary by trial and indication. A comprehensive, pooled assessment of data across the studies for these indications would provide additional insights into the safety of incobotulinumtoxinA and could identify any differences in the frequency of AEs across indications. Such an analysis has been conducted using incobotulinumtoxinA to treat different types of facial lines (glabellar frown lines, crow’s feet, and upper facial lines) [23]. That analysis revealed differences in the AE profile of the biologic across these indications and a possible decrease in the frequency of AEs with repeated injection cycles.
The objective of this analysis was to further assess the incidence of treatment-related AEs across Merz-sponsored prospective placebo-controlled or repeat-dose incobotulinumtoxinA studies in adult subjects in cervical dystonia, blepharospasm, upper limb spasticity, lower limb spasticity, sialorrhea, and essential tremor of the upper limb. The studies included in these analyses all pre-dated the COVID-19 pandemic.
2. Results
The patient demographics varied by indication and study (Table 1). Similarly, the dose of incobotulinumtoxinA was indication-specific, being lowest in patients receiving treatment for blepharospasm and highest for those with spasticity. Across all studies, the planned duration of follow-up ranged from 6 to 121 weeks (Table 1).

Table 1.
Summary of prospective clinical trials included in the safety analysis of incobotulinumtoxinA in patients with neurological disorders.
2.1. Safety
2.1.1. Overall Frequency of Adverse Events
After a single dose, the overall occurrence of treatment-emergent AEs (TEAEs) did not differ greatly between incobotulinumtoxinA and the placebo in most indications, although between-indication differences were observed (Table 2). In general, the incidences of TEAEs and TEAEs of special interest (TEAESIs; listed in Table 3), including those that were treatment-related, were highest in subjects receiving incobotulinumtoxinA or placebo for cervical dystonia or blepharospasm. Subjects receiving treatment for these indications also showed the greatest differences between incobotulinumtoxinA and the placebo in the overall incidences of TEAEs, particularly treatment-related TEAEs. Few serious TEAEs (SAEs) or TEAEs leading to discontinuation of incobotulinumtoxinA occurred, and only one subject each, both of whom were receiving on-label treatment only for lower limb spasticity, experienced a treatment-related SAE or a treatment-related TEAE that led to discontinuation of treatment. There were no fatal TEAEs in subjects receiving incobotulinumtoxinA.

Table 2.
Pooled incidence of TEAEs after a single dose in PBO-controlled studies evaluating incobotulinumtoxinA in the treatment of adults with neurological disorders by indication.

Table 3.
Summary of adverse events of special interest that may potentially indicate toxin spread.
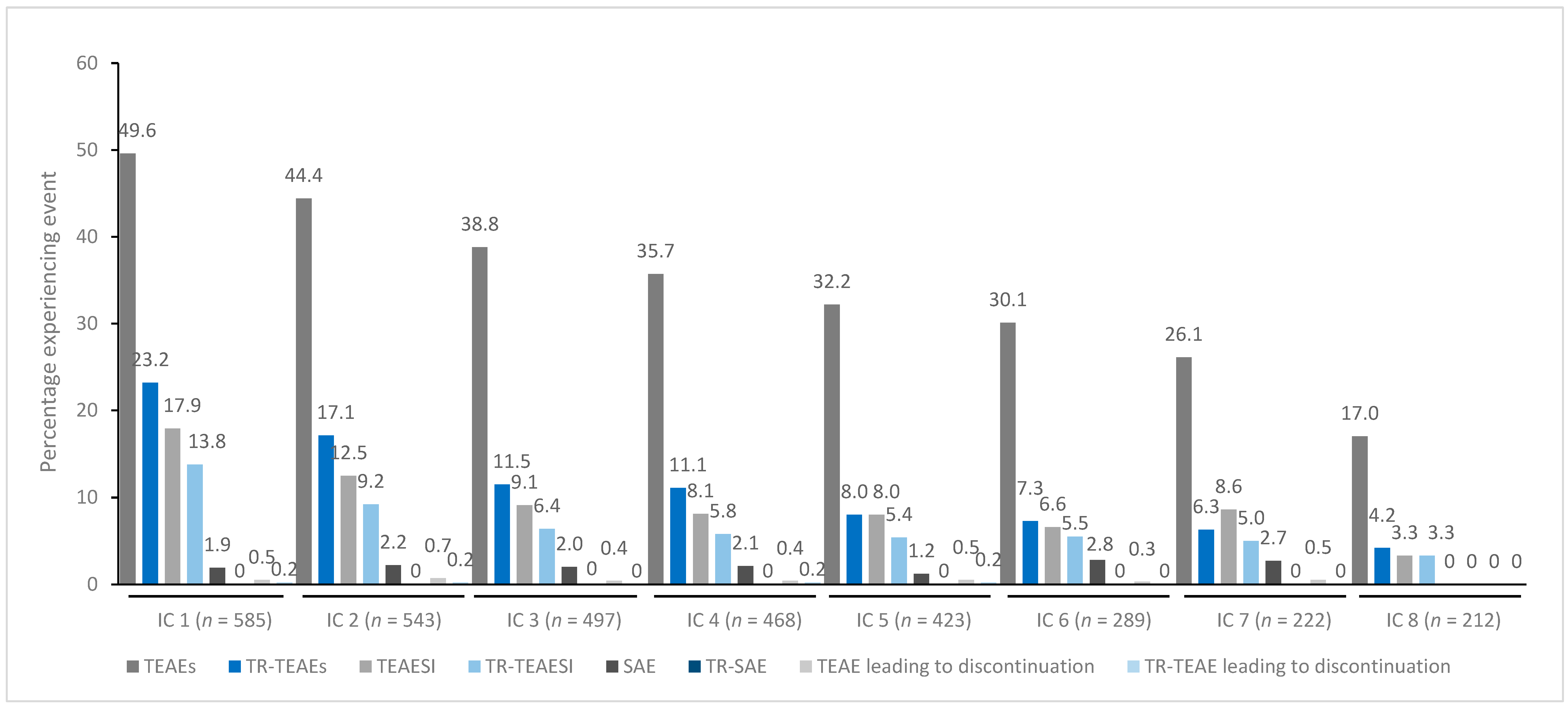
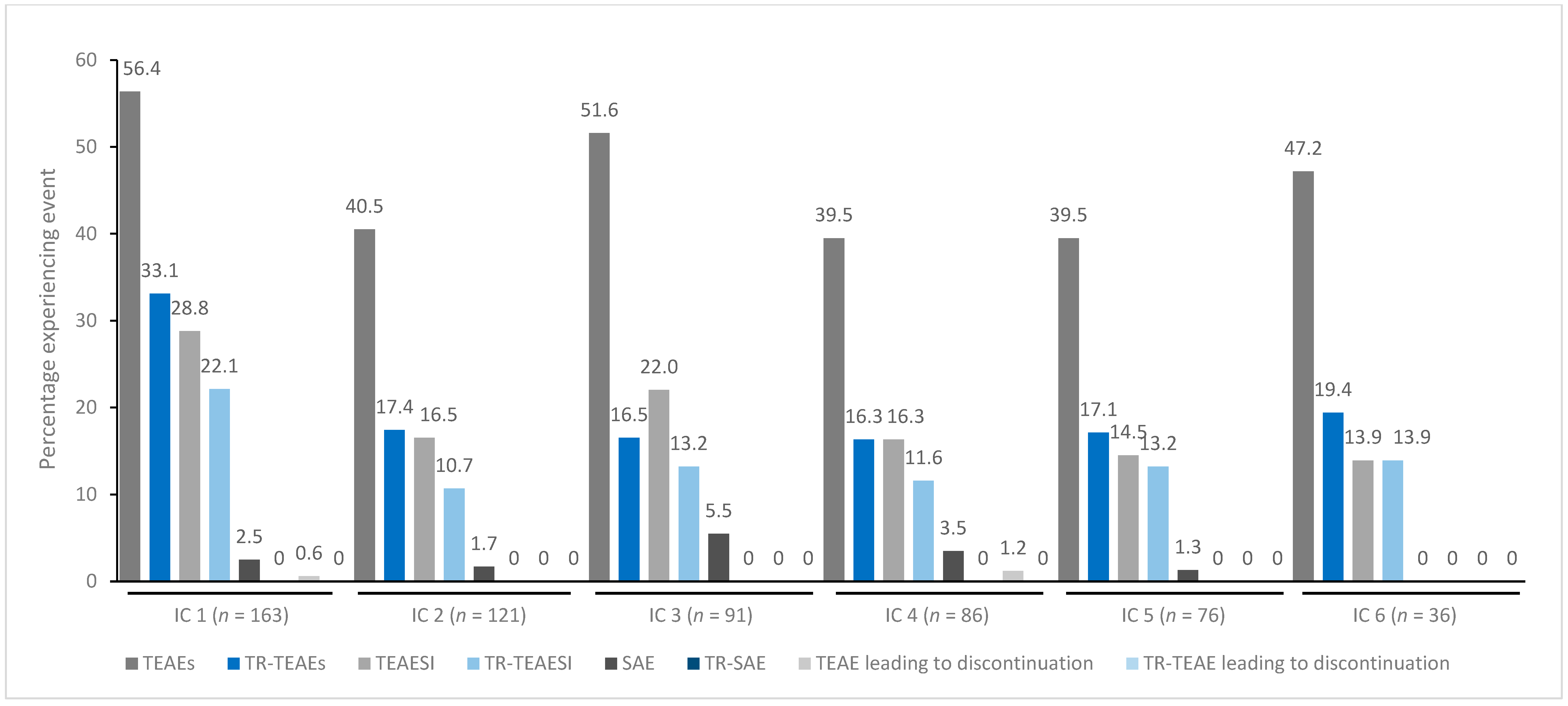
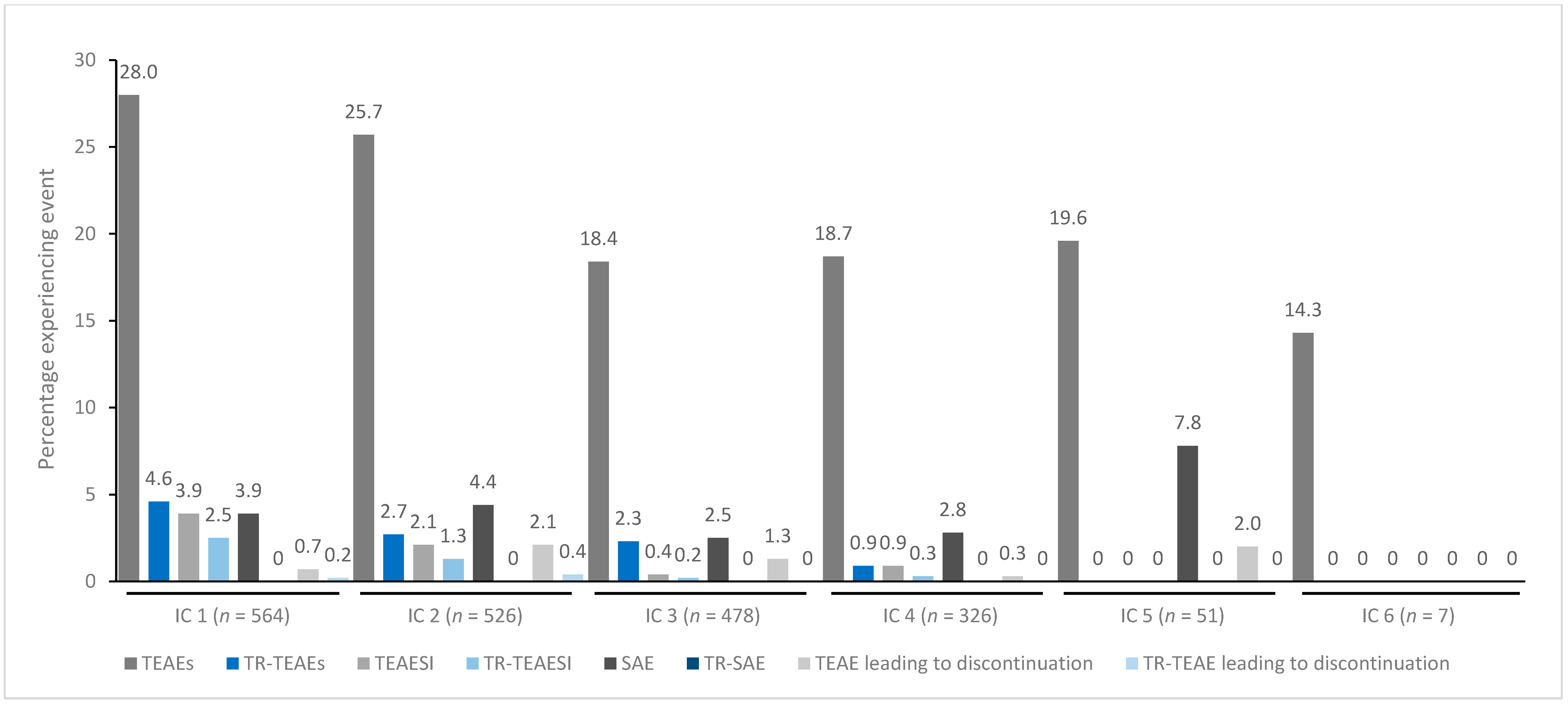
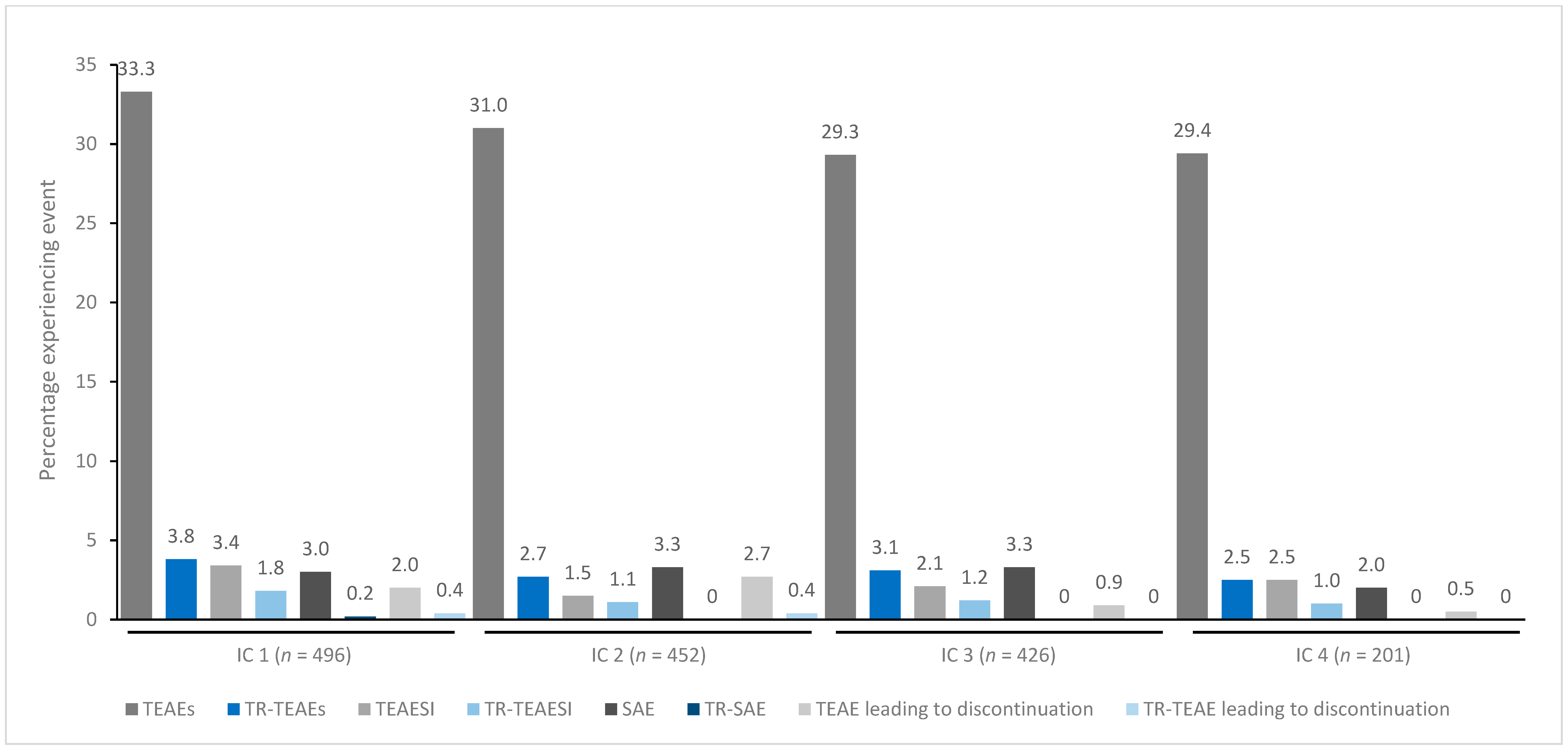
In general, treatment for up to eight repeated cycles did not increase the incidences of any TEAE category (Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5). Although some subjects who received incobotulinumtoxinA for cervical dystonia received additional cycles (up to 13), subject numbers were very small (≤15) relative to earlier cycles; therefore, analyses for these would not lead to meaningful results.
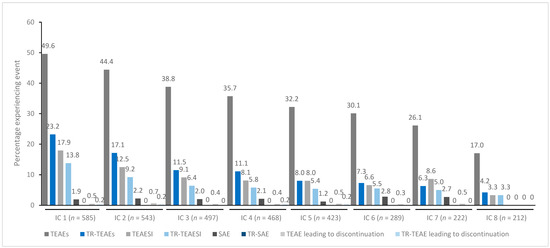
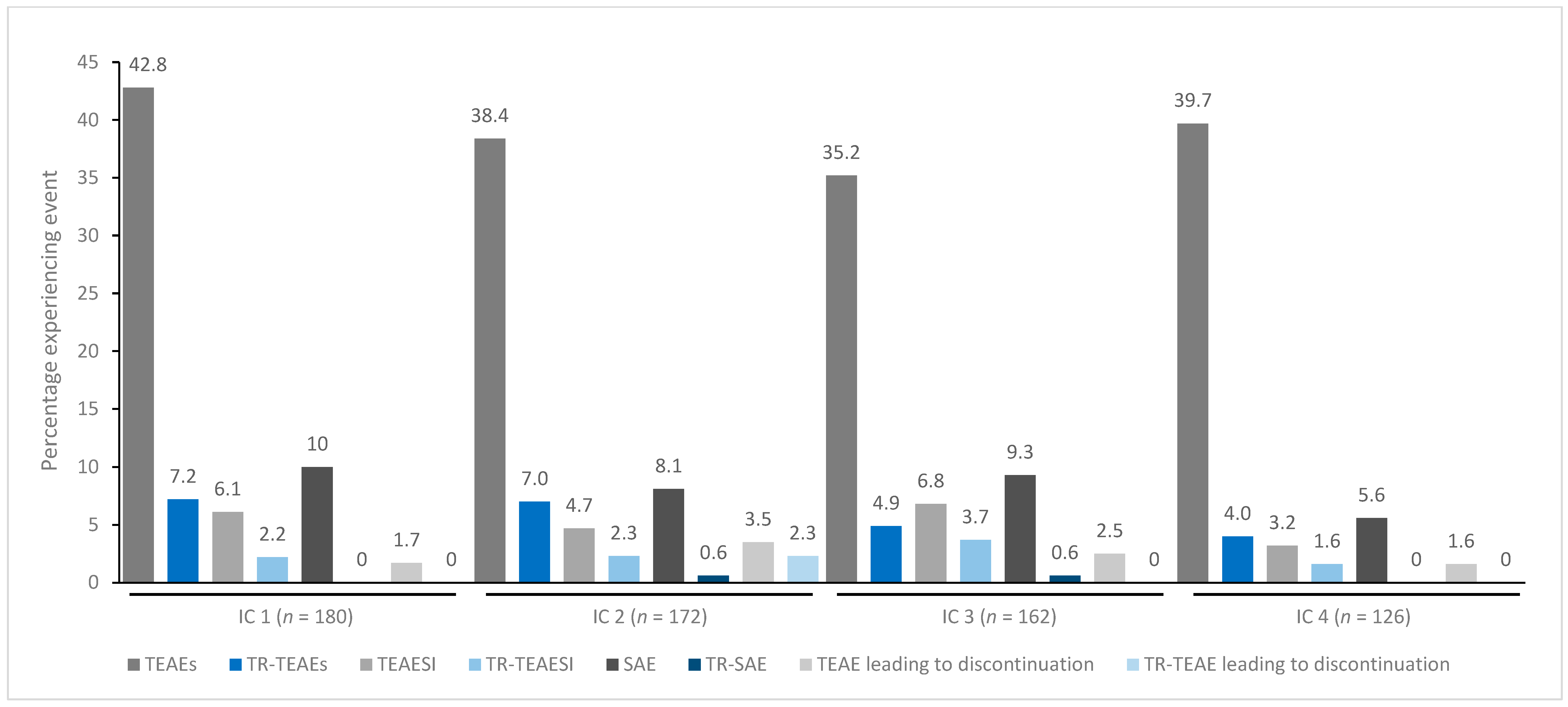
Figure 1.
TEAE incidence by treatment cycle in patients with cervical dystonia treated with incobotulinumtoxinA. Note: In addition, 15 patients received a ninth IC (n = 2 TR-TEAE, n = 1 TR-TEAESI, no other events), eight patients received a tenth IC (n = 2 TEAE, n = 1 SAE, no other events), five patients received an eleventh IC (n = 1 TEAE, no other events), and one patient received a twelfth and thirteenth IC (no events). IC, injection cycle; INCO, incobotulinumtoxinA; TEAE, treatment-emergent adverse event; TEAESI, treatment-emergent adverse event of special interest (potentially indicative of toxin spread); SAE, serious TEAE; TR, treatment-related.
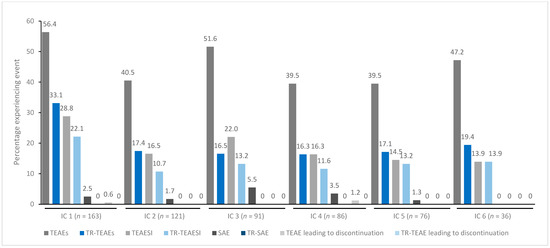
Figure 2.
TEAE incidence by treatment cycle in patients with blepharospasm treated with incobotulinumtoxinA. IC, injection cycle; INCO, incobotulinumtoxinA; TEAE, treatment-emergent adverse event; TEAESI, treatment-emergent adverse event of special interest (potentially indicative of toxin spread); SAE, serious TEAE; TR, treatment-related.
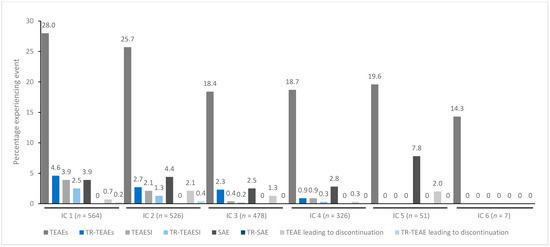
Figure 3.
TEAE incidence by treatment cycle in patients with UL spasticity treated with incobotulinumtoxinA. IC, injection cycle; INCO, incobotulinumtoxinA; TEAE, treatment-emergent adverse event; TEAESI, treatment-emergent adverse event of special interest (potentially indicative of toxin spread); SAE, serious TEAE; TR, treatment-related; UL, upper limb.
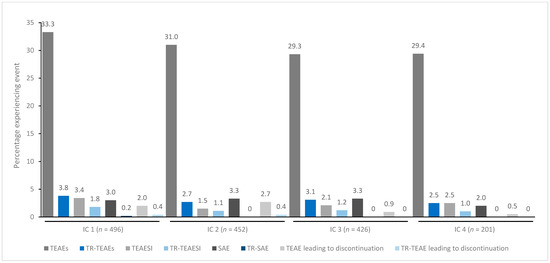
Figure 4.
TEAE incidence by treatment cycle in patients with LL spasticity treated with incobotulinumtoxinA. IC, injection cycle; INCO, incobotulinumtoxinA; LL, lower limb; TEAE, treatment-emergent adverse event; TEAESI, treatment-emergent adverse event of special interest (potentially indicative of toxin spread); SAE, serious TEAE; TR, treatment-related.
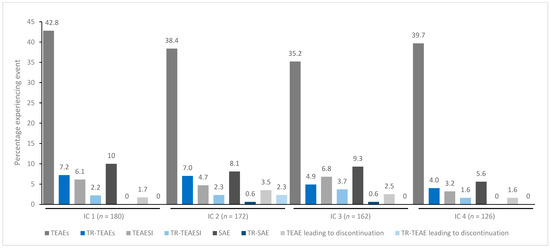
Figure 5.
TEAE incidence by treatment cycle in patients with sialorrhea treated with incobotulinumtoxinA. IC, injection cycle; INCO, incobotulinumtoxinA; TEAE, treatment-emergent adverse event; TEAESI, treatment-emergent adverse event of special interest (potentially indicative of toxin spread); SAE, serious TEAE; TR, treatment-related.
2.1.2. Most Common Adverse Events after a Single-Dose
After a single dose, the most frequent TEAEs and treatment-related TEAEs were indication-dependent (Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9), although injection site pain was reported by a small proportion of subjects across the indications. The most frequently reported TEAEs in subjects with cervical dystonia included dysphagia (in 15.7% of those treated with incobotulinumtoxinA and 4.1% of those who received a placebo), neck pain (10.1% vs. 4.1%), and muscular weakness (8.8% vs. 1.4%). These were also the most frequently reported treatment-related TEAEs in subjects with cervical dystonia (Table 4). In subjects with blepharospasm, eyelid ptosis (16.5% vs. 3.7%), dry eye (12.2% vs. 11.1%), and dry mouth (9.6% vs. 1.9%) were the most frequently reported TEAEs and treatment-related TEAEs (Table 5).

Table 4.
Summary of the most common treatment-emergent adverse events overall and of special interest in single-dose placebo-controlled studies of incobotulinumtoxinA in patients with cervical dystonia.

Table 5.
Summary of the most common treatment-emergent adverse events overall and of special interest in single-dose placebo-controlled studies of incobotulinumtoxinA in patients with blepharospasm.

Table 6.
Summary of the most common treatment-emergent adverse events overall and of special interest in single-dose placebo-controlled studies of incobotulinumtoxinA in patients with upper limb spasticity.

Table 7.
Summary of the most common treatment-emergent adverse events overall and of special interest in single-dose placebo-controlled studies of incobotulinumtoxinA in patients with lower limb spasticity.

Table 8.
Summary of the most common treatment-emergent adverse events overall and of special interest in single-dose placebo-controlled studies of incobotulinumtoxinA in patients with sialorrhea.

Table 9.
Summary of the most common treatment-emergent adverse events overall and of special interest in single-dose placebo-controlled studies of incobotulinumtoxinA in patients with essential tremor of the upper limb.
In subjects with upper limb spasticity, nasopharyngitis was the most frequently reported TEAE (in 4.6% of incobotulinumtoxinA- and 0.9% of placebo-treated subjects), whereas dry mouth was the most frequent treatment-related TEAE (in 0.9% vs. 0.5% of subjects; Table 6). Similarly, in patients with lower limb spasticity, nasopharyngitis was the most frequently reported TEAE (6.5% vs. 4.8%), but muscular weakness was the most frequently reported treatment-related TEAE (Table 7).
Falling (5.4% vs. 2.8%) and dry mouth (4.7% vs. 0) were the most frequently reported TEAEs in subjects treated for sialorrhea, with dry mouth being the most frequent treatment-related TEAE (4.1% vs. 0; Table 8). The number of patients treated for essential tremor of the upper limb was small (N = 30 in one single-dose study), limiting the conclusions that could be drawn. In these subjects, muscular weakness was a common TEAE and treatment-related TEAE (Table 9).
In general, TEAESIs across all indications were most frequently muscular weakness, dysphagia, and/or dry mouth (Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9). Similarly, these events were also the most frequent treatment-related TEAESIs, although subjects with blepharospasm also reported eyelid ptosis and blurred vision, and those with upper limb spasticity reported dysarthria.
2.2. Immunogenicity
In patients enrolled in repeat-dose studies, which were reported as treatment-naïve at baseline (n = 815), there was no observable pattern of hemidiaphragm assay (HDA) positivity at the study end (Table 10). Of the 20 patients who were treatment-naïve at baseline and were HDA positive at the study end, 50% were HDA positive at screening, and an additional 5% had a positive fluorescence immunoassay (FIA; but a missing HDA test) at screening. HDA positivity appeared more frequently in patients treated for spasticity or cervical dystonia (Table 10). The vast majority of positive HDA values were only marginally above the threshold of positivity, and the evolution of HDA values in individual patients did not correlate with the investigator’s judgment of efficacy, with no patients demonstrating a secondary lack of treatment response due to neutralizing antibodies (NAb).

Table 10.
Summary of immunogenicity with incobotulinumtoxinA, overall and by botulinum toxin pre-treatment status.
3. Discussion
In this pooled analysis of placebo-controlled trials reporting data across a variety of neurological disorders in adults from a range of countries, incobotulinumtoxinA had a good safety profile, with no new or unexpected safety findings, no fatalities, and few SAEs. This analysis has helped to define and allow comparison of the safety profile of incobotulinumtoxinA administered over a range of doses for up to 13 injection cycles in subjects with cervical dystonia, blepharospasm, upper limb spasticity, lower limb spasticity, sialorrhea, and essential tremor of the upper limb.
Findings were consistent with those of the individual trials included in this analysis [3,4,5,6,7,8,9,11,12,13,14,15,16,17,21,22], with TEAEs generally specific to each indication. The most common TEAEs by indication reported for incobotulinumtoxinA were comparable to those reported for all available BoNT-A formulations. Reviews of botulinum toxin therapy reveal that dysphagia, weakness, and upper respiratory complications are the main TEAEs to occur during the treatment of cervical dystonia; ptosis (usually temporary and self-resolving), vision changes, and dry eye are the most common complications of treatment of blepharospasm; viscous saliva, dry mouth, and dysphagia can occur with treatment for sialorrhea; and the treatment of spasticity can result in weakness or paralysis of off-target musculature [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. There is potential for off-target muscle weakness or paralysis to lead to falls. However, our findings agree with the broader literature that demonstrates BoNT-A is well tolerated [24,25,52,53] and, when correctly administered, is considered very safe [54].
Analysis of up to eight repeated cycles of incobotulinumtoxinA revealed that, although fluctuations were observed, the incidences of all TEAE categories trended downwards over time. These findings are consistent with those of Coleman et al. [23], who confirmed the favorable safety and tolerability of incobotulinumtoxinA in patients with facial lines. In that analysis, investigators found that the frequency of treatment-related AEs with incobotulinumtoxinA was low and generally decreased with repeated injection cycles.
IncobotulinumtoxinA was associated with a very low rate of NAb formation over up to 13 injection cycles in the subjects evaluated, many of whom had received previous treatment with a botulinum toxin, and no patient demonstrated a secondary lack of treatment response due to NAb. Low rates of NAb formation have also been observed in a pooled analysis of clinical trials conducted in children with upper or lower limb spasticity or sialorrhea [55]. A recent cross-sectional study in 465 patients receiving either incobotulinumtoxinA or a complex protein-containing BoNT-A formulation (abobotulinumtoxinA or onabotulinumtoxinA) for periods of up to 10 years revealed that incobotulinumtoxinA reduces the risk of NAb induction compared with the complex protein-containing BoNT-A formulations [56], which may translate into a lower risk of secondary non-response with incobotulinumtoxinA. The findings of the current analyses showed the highest rates of positive HDA tests in patients with cervical dystonia or lower limb spasticity, and the lowest rates in those with blepharospasm or sialorrhea. Similar differences have been seen with other BoNT-A formulations [57,58,59,60,61] and, in part, may be related to the toxin dose administered [61,62,63,64] or the site of injection; for example, lymph node-rich regions, such as the neck, are more likely to produce an immune response [54,65]. Patients receiving treatment for sialorrhea or, in particular, blepharospasm also generally received lower doses of incobotulinumtoxinA than patients receiving treatment for the other indications.
This analysis has limitations in that data for some indications were derived from single studies only, and the numbers of eligible subjects varied between indications. Nevertheless, findings were generally consistent across indications. Other limitations are that we could not analyze whether the development of TEAEs was related to injection technique and, in general, doses administered in the clinical trials included do not reflect the wide variability of dosing used in real-world clinical practice. Similarly, the follow-up duration (up to 104 weeks) is short relative to the required duration of treatment for many subjects in the real world, limiting the interpretation of both long-term safety and the immunogenicity data. Finally, all data were obtained from Merz-sponsored trials, which may have introduced some bias. It is possible that the administration of incobotulinumtoxinA in the controlled setting of a clinical trial may have reduced the risk for some TEAEs, but conversely, such settings increase the chances that all TEAEs will be collected.
4. Conclusions
Overall, the results of this pooled analysis support and extend the favorable safety and tolerability profile of incobotulinumtoxinA for the treatment of adult neurological disorders established by individual clinical studies.
5. Materials and Methods
Studies were obtained from the integrated clinical database containing data from all Merz-sponsored clinical trials of incobotulinumtoxinA (Supplementary Figure S1). The studies included were those that were placebo-controlled or were repeat-dose studies of incobotulinumtoxinA in adults with cervical dystonia, blepharospasm, upper limb spasticity, lower limb spasticity, sialorrhea, or essential tremor of the upper limb (Table 1).
Single-dose and repeat-dose data were considered separately, although individual studies contributed to either or both analyses, depending on their design. Single-dose data were defined as those obtained from a study with a single treatment of incobotulinumtoxinA in a placebo-controlled setting or studies in which the first injection session of a repeat-dose study was placebo-controlled. Repeat-dose data were defined as those from studies in which subjects were intended to receive repeated treatments with incobotulinumtoxinA over ≥2 cycles; however, subjects who, for any reason, received only one incobotulinumtoxinA treatment in a repeat-dose study were also included in the safety analyses. Only subjects who received treatment with incobotulinumtoxinA were included in the repeat-dose analysis (i.e., subjects who received a placebo only were excluded).
5.1. Participants
All the participants were enrolled in the prospective clinical trials of incobotulinumtoxinA, summarized in Table 1. Subjects in all studies provided informed consent; all studies were institutional review board approved and conducted in accordance with the Declaration of Helsinki.
5.2. Data Extraction and Statistical Analysis
Subjects were pooled according to indication, and the single-dose and repeat-dose data were analyzed separately. The single-dose safety analysis set included all subjects who received one cycle of study medication or placebo. The repeat-dose safety analysis set included all subjects who received at least one cycle of study medication in a study that evaluated repeated doses of incobotulinumtoxinA (patients initially treated with placebo who subsequently received incobotulinumtoxinA in an open-label extension phase were included with the first dose of incobotulinumtoxinA considered injection cycle 1).
The incidence of TEAEs was summarized descriptively using counts and percentages; calculations were performed using statistical analysis system software (SAS) version 9.4 (SAS Institute, Cary, NC, USA). All TEAEs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) dictionary version 22.0.
Specific analyses for single-dose data included: the overall incidence of TEAEs and the categories of SAEs, TEAEs leading to discontinuation, fatal TEAEs, as well as treatment-related events in all categories; the most frequent TEAEs (≥2% frequency in any group) and the most frequent treatment-related TEAEs (≥2 subjects in any group) were also summarized. TEAESIs, defined by regulatory authorities as potentially indicating toxin spread (Table 3), and treatment-related TEAESIs, were also analyzed for the single-dose data. In each study, subjects were asked to report TEAESIs, and in most studies, detailed active questioning for symptoms was used.
For the repeat-dose data, the overall frequency of TEAEs, treatment-related TEAEs and SAEs were analyzed according to the treatment cycle.
NAb testing was also performed in most repeat-dose studies and is reported for all subjects who received at least one cycle of incobotulinumtoxinA across all studies with testing. In general, antibody samples were collected before the first treatment injection at the screening or baseline visit and the final individual visit of each trial. Blood samples for immunogenicity testing were screened using FIA to detect any binding antibodies against botulinum toxin in the first step. In case of a positive FIA finding, further testing with the highly sensitive mouse ex vivo HDA for the final confirmation of NAb presence and respective determination of titers was performed as a second step. Descriptive results are presented with no statistical testing. Immunogenicity data were evaluated by indication and previous treatment (previously treated—defined as pre-treatment with botulinum toxin for any indication before study entry—or treatment naïve).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxins15060353/s1, Figure S1: Selection of clinical trials to be included in the pooled analysis.
Author Contributions
Conceptualization, M.A.H., A.H. and M.A.; Validation, W.H.J., P.K. and A.T.P.; Formal analysis, A.H.; Resources, M.A.H.; Writing–review & editing, W.H.J., P.K., M.A.H., A.H., M.A. and A.T.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Merz Pharmaceuticals, LLC, Raleigh, NC, USA.
Institutional Review Board Statement
All studies included in these analyses were conducted in accordance with the Declaration of Helsinki and approved by the appropriate Institutional Review Boards, as previously reported.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the studies included in this analysis.
Data Availability Statement
Data are not publicly available.
Acknowledgments
The authors would like to acknowledge Caroline Spencer and Deirdre Elmhirst (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this manuscript.
Conflicts of Interest
Wolfgang H. Jost is an advisor and speaker for Abbvie, Ipsen, and Merz Therapeutics. Petr Kaňovský has received speaker’s honoraria from Desitin, Ipsen Biopharmaceuticals, Merz Thera-peutics, and Medtronic. Michael A. Hast is an employee of Merz Pharmaceuticals, LLC. Angelika Hanschmann and Michael Althaus are employees of Merz Therapeutics GmbH. Atul T. Patel has received research grant support from Allergan plc, Revance, and Ipsen, and speaking honoraria from Ipsen and Allergan plc.
References
- Merz Pharmaceuticals GmbH, Xeomin Prescribing Information. May 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125360s074lbl.pdf (accessed on 8 August 2022).
- Merz Pharma UK Ltd. Xeomin 200 Units Powder for Solution for Injection. 4 October 2021. Available online: https://www.medicines.org.uk/emc/product/2162/smpc (accessed on 8 August 2022).
- Comella, C.L.; Jankovic, J.; Truong, D.D.; Hanschmann, A.; Grafe, S.U.S. XEOMIN Cervical Dystonia Study Group. Efficacy and safety of incobotulinumtoxinA (NT 201, XEOMIN®, botulinum neurotoxin type A, without accessory proteins) in patients with cervical dystonia. J. Neurol. Sci. 2011, 308, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Comella, C.; Hauser, R.A.; Isaacson, S.H.; Truong, D.; Oguh, O.; Hui, J.; Molho, E.S.; Brodsky, M.; Furr-Stimming, E.; Comes, G.; et al. Efficacy and safety of two incobotulinumtoxinA injection intervals in cervical dystonia patients with inadequate benefit from standard injection intervals of botulinum toxin: Phase 4, open-label, randomized, noninferiority study. Clin. Park Relat. Disord. 2022, 6, 100142. [Google Scholar] [CrossRef] [PubMed]
- Evidente, V.G.; Fernandez, H.H.; LeDoux, M.S.; Brashear, A.; Grafe, S.; Hanschmann, A.; Comella, C.L. A randomized, double-blind study of repeated incobotulinumtoxinA (Xeomin(®)) in cervical dystonia. J. Neural. Transm. 2013, 120, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Dressler, D.; Paus, S.; Seitzinger, A.; Gebhardt, B.; Kupsch, A. Long-term efficacy and safety of incobotulinumtoxin A injections in patients with cervical dystonia. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1014–1019. [Google Scholar] [CrossRef]
- Jankovic, J.; Comella, C.; Hanschmann, A.; Grafe, S. Efficacy and safety of incobotulinumtoxinA (NT 201, Xeomin) in the treatment of blepharospasm-a randomized trial. Mov. Disord. 2011, 26, 1521–1528. [Google Scholar] [CrossRef]
- Truong, D.D.; Gollomp, S.M.; Jankovic, J.; LeWitt, P.A.; Marx, M.; Hanschmann, A.; Fernandez, H.H. Xeomin US Blepharospasm Study Group. Sustained efficacy and safety of repeated incobotulinumtoxinA (Xeomin(®)) injections in blepharospasm. J. Neural. Transm. 2013, 120, 1345–1353. [Google Scholar] [CrossRef]
- Mitsikostas, D.D.; Dekundy, A.; Sternberg, K.; Althaus, M.; Pagan, F. IncobotulinumtoxinA for the treatment of blepharospasm in toxin-naïve subjects: A multi-center, double-blind, randomized, placebo-controlled trial. Adv Ther. 2020, 37, 4249–4265. [Google Scholar] [CrossRef]
- Barnes, M.; Schnitzler, A.; Medeiros, L.; Aguilar, M.; Lehnert-Batar, A.; Minnasch, P. Efficacy and safety of NT 201 for upper limb spasticity of various etiologies--a randomized parallel-group study. Acta Neurol. Scand. 2010, 122, 295–302. [Google Scholar] [CrossRef]
- Kaňovský, P.; Slawek, J.; Denes, Z.; Platz, T.; Sassin, I.; Comes, G.; Grafe, S. Efficacy and safety of botulinum neurotoxin NT 201 in poststroke upper limb spasticity. Clin. Neuropharmacol. 2009, 32, 259–265. [Google Scholar] [CrossRef]
- Kaňovský, P.; Slawek, J.; Denes, Z.; Platz, T.; Comes, G.; Grafe, S.; Pulte, I. Efficacy and safety of treatment with incobotulinum toxin A (botulinum neurotoxin type A free from complexing proteins; NT 201) in post-stroke upper limb spasticity. J. Rehabil. Med. 2011, 43, 486–492. [Google Scholar] [CrossRef]
- Elovic, E.P.; Munin, M.C.; Kaňovský, P.; Hanschmann, A.; Hiersemenzel, R.; Marciniak, C. Randomized, placebo-controlled trial of incobotulinumtoxina for upper-limb post-stroke spasticity. Muscle Nerve 2016, 53, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, C.; Munin, M.C.; Brashear, A.; Rubin, B.S.; Patel, A.T.; Slawek, J.; Hanschmann, A.; Hiersemenzel, R.; Elovic, E.P. IncobotulinumtoxinA efficacy and safety in adults with upper-limb spasticity following stroke: Results from the open-label extension period of a phase 3 study. Adv. Ther. 2019, 36, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Masakado, Y.; Abo, M.; Kondo, K.; Saeki, S.; Saitoh, E.; Dekundy, A.; Hanschmann, A.; Kaji, R. J-PURE Study Group. Efficacy and safety of incobotulinumtoxinA in post-stroke upper-limb spasticity in Japanese subjects: Results from a randomized, double-blind, placebo-controlled study (J-PURE). J. Neurol. 2020, 267, 2029–2041. [Google Scholar] [CrossRef] [PubMed]
- Jost, W.H.; Friedman, A.; Michel, O.; Oehlwein, C.; Slawek, J.; Bogucki, A.; Ochudlo, S.; Banach, M.; Pagan, F.; Flatau-Baqué, B.; et al. SIAXI: Placebo-controlled, randomized, double-blind study of incobotulinumtoxinA for sialorrhea. Neurology 2019, 92, e1982–e1991. [Google Scholar] [CrossRef] [PubMed]
- Jost, W.H.; Friedman, A.; Michel, O.; Oehlwein, C.; Slawek, J.; Bogucki, A.; Ochudlo, S.; Banach, M.; Pagan, F.; Flatau-Baqué, B.; et al. Long-term incobotulinumtoxinA treatment for chronic sialorrhea: Efficacy and safety over 64 weeks. Parkinsonism. Relat. Disord. 2020, 70, 23–30. [Google Scholar] [CrossRef]
- Benecke, R.; Jost, W.H.; Kanovsky, P.; Ruzicka, E.; Comes, G.; Grafe, S. A new botulinum toxin type A free of complexing proteins for treatment of cervical dystonia. Neurology 2005, 64, 1949–1951. [Google Scholar] [CrossRef]
- Jankovic, J.; Kenney, C.; Grafe, S.; Goertelmeyer, R.; Comes, G. Relationship between various clinical outcome assessments in patients with blepharospasm. Mov Disord. 2009, 24, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Bensmail, D.; Ferreira, J.J.; Molteni, F.; Satkunam, L.; Moraleda, S.; Rekand, T.; McGuire, J.; Scheschonka, A.; Flatau-Baqué, B.; et al. TOWER study investigators. Safety and efficacy of incobotulinumtoxinA doses up to 800 U in limb spasticity: The TOWER study. Neurology 2017, 88, 1321–1328. [Google Scholar] [CrossRef]
- Masakado, Y.; Kagaya, H.; Kondo, K.; Otaka, Y.; Dekundy, A.; Hanschmann, A.; Geister, T.L.; Kaji, R. Efficacy and safety of IncobotulinumtoxinA in the treatment of lower limb spasticity in Japanese subjects. Front. Neurol. 2022, 13, 832937. [Google Scholar] [CrossRef]
- Jog, M.; Lee, J.; Scheschonka, A.; Chen, R.; Ismail, F.; Boulias, C.; Hobson, D.; King, D.; Althaus, M.; Simon, O.; et al. Tolerability and efficacy of customized incobotulinumtoxinA injections for essential tremor: A randomized, double-blind, placebo-controlled study. Toxins 2020, 12, 807. [Google Scholar] [CrossRef]
- Coleman, W.P., 3rd; Sattler, G.; Weissenberger, P.; Hast, M.A.; Hanschmann, A. Safety of incobotulinumtoxinA in the treatment of facial lines: Results from a pooled analysis of randomized, prospective, controlled clinical studies. Dermatol. Surg. 2017, 43 (Suppl. S3), S293–S303. [Google Scholar] [CrossRef] [PubMed]
- Bach, K.; Simman, R. The Multispecialty Toxin: A literature review of botulinum toxin. Plast Reconstr. Surg. Glob. Open 2022, 10, e4228. [Google Scholar] [CrossRef] [PubMed]
- Duarte, G.S.; Rodrigues, F.B.; Marques, R.E.; Castelão, M.; Ferreira, J.; Sampaio, C.; Moore, A.P.; Costa, J. Botulinum toxin type A therapy for blepharospasm. Cochrane Database Syst. Rev. 2020, 11, CD004900. [Google Scholar] [PubMed]
- Ruiz-Roca, J.A.; Pons-Fuster, E.; Lopez-Jornet, P. Effectiveness of the botulinum toxin for treating sialorrhea in patients with Parkinson’s disease: A systematic review. J. Clin. Med. 2019, 8, 317. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.C.; Chung, C.C.; Tu, Y.K.; Hong, C.T.; Chen, K.H.; Tam, K.W.; Kuan, Y.C. Efficacy and safety of botulinum toxin for treating sialorrhea: A systematic review and meta-analysis. Eur. J. Neurol. 2022, 29, 69–80. [Google Scholar] [CrossRef]
- Orsini, M.; Leite, M.A.; Chung, T.M.; Bocca, W.; de Souza, J.A.; de Souza, O.G.; Moreira, R.P.; Bastos, V.H.; Teixeira, S.; Oliveira, A.B.; et al. Botulinum Neurotoxin Type A in Neurology: Update. Neurol. Int. 2015, 7, 5886. [Google Scholar] [CrossRef]
- Petracca, M.; Guidubaldi, A.; Ricciardi, L.; Ialongo, T.; Del Grande, A.; Mulas, D.; Di Stasio, E.; Bentivoglio, A.R. Botulinum Toxin A and B in sialorrhea: Long-term data and literature overview. Toxicon 2015, 107, 129–140. [Google Scholar] [CrossRef]
- Stokholm, M.G.; Bisgård, C.; Vilholm, O.J. Safety and administration of treatment with botulinum neurotoxin for sialorrhoea in ALS patients: Review of the literature and a proposal for tailored treatment. Amyotroph Lateral Scler Front. Degener. 2013, 14, 516–520. [Google Scholar] [CrossRef]
- Naumann, M.; Dressler, D.; Hallett, M.; Jankovic, J.; Schiavo, G.; Segal, K.R.; Truong, D. Evidence-based review and assessment of botulinum neurotoxin for the treatment of secretory disorders. Toxicon 2013, 67, 141–152. [Google Scholar] [CrossRef]
- Walshe, M.; Smith, M.; Pennington, L. Interventions for drooling in children with cerebral palsy. Cochrane Database Syst. Rev. 2012, 11, CD008624. [Google Scholar] [CrossRef]
- Rodwell, K.; Edwards, P.; Ware, R.S.; Boyd, R. Salivary gland botulinum toxin injections for drooling in children with cerebral palsy and neurodevelopmental disability: A systematic review. Dev. Med. Child Neurol. 2012, 54, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.B.; Duarte, G.S.; Castelão, M.; Marques, R.E.; Ferreira, J.; Sampaio, C.; Moore, A.P.; Costa, J. Botulinum toxin type A versus anticholinergics for cervical dystonia. Cochrane Database Syst Rev. 2021, 4, CD004312. [Google Scholar] [CrossRef] [PubMed]
- Marsili, L.; Bologna, M.; Jankovic, J.; Colosimo, C. Long-term efficacy and safety of botulinum toxin treatment for cervical dystonia: A critical reappraisal. Expert Opin. Drug Saf. 2021, 20, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J. Towards flexible and tailored botulinum neurotoxin dosing regimens for focal dystonia and spasticity–Insights from recent studies. Toxicon 2018, 147, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Duarte, G.S.; Castelão, M.; Rodrigues, F.B.; Marques, R.E.; Ferreira, J.; Sampaio, C.; Moore, A.P.; Costa, J. Botulinum toxin type A versus botulinum toxin type B for cervical dystonia. Cochrane Database Syst. Rev. 2016, 10, CD004314. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Stevens, A.L.; Dashtipour, K.; Hauser, R.A.; Mari, Z. A mixed treatment comparison to compare the efficacy and safety of botulinum toxin treatments for cervical dystonia. J. Neurol. 2016, 263, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.R.; Pagan, F.L. Patient considerations in the treatment of cervical dystonia: Focus on botulinum toxin type A. Patient Prefer. Adherence 2015, 9, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Bonikowski, M.; Sławek, J. Safety and efficacy of Botulinum toxin type A preparations in cerebral palsy—An evidence-based review. Neurol. Neurochir. Pol. 2021, 55, 158–164. [Google Scholar] [CrossRef]
- Intiso, D.; Simone, V.; Bartolo, M.; Santamato, A.; Ranieri, M.; Gatta, M.T.; Di Rienzo, F. High dosage of botulinum toxin type A in adult subjects with spasticity following acquired central nervous system damage: Where are we at? Toxins 2020, 12, 315. [Google Scholar] [CrossRef]
- Santamato, A.; Micello, M.F.; Ranieri, M.; Valeno, G.; Albano, A.; Baricich, A.; Cisari, C.; Intiso, D.; Pilotto, A.; Logroscino, G.; et al. Employment of higher doses of botulinum toxin type A to reduce spasticity after stroke. J. Neurol. Sci. 2015, 350, 1–6. [Google Scholar] [CrossRef]
- Esquenazi, A.; Albanese, A.; Chancellor, M.B.; Elovic, E.; Segal, K.R.; Simpson, D.M.; Smith, C.P.; Ward, A.B. Evidence-based review and assessment of botulinum neurotoxin for the treatment of adult spasticity in the upper motor neuron syndrome. Toxicon 2013, 67, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Campbell, L.; Boyce, M.; Fung, V.S.; Cerebral Palsy Institute. Botulinum toxin assessment, intervention and aftercare for cervical dystonia and other causes of hypertonia of the neck: International consensus statement. Eur. J. Neurol. 2010, 17, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Elia, A.E.; Filippini, G.; Calandrella, D.; Albanese, A. Botulinum neurotoxins for post-stroke spasticity in adults: A systematic review. Mov. Disord. 2009, 24, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Mattler, W.J. Use of botulinum toxin A in adult neurological disorders: Efficacy, tolerability and safety. CNS Drugs. 2008, 22, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Sheean, G. Botulinum toxin treatment of adult spasticity: A benefit-risk assessment. Drug Saf. 2006, 29, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M.; Albanese, A.; Dressler, D.; Segal, K.R.; Simpson, D.M.; Truong, D.; Jankovic, J. Evidence-based review and assessment of botulinum neurotoxin for the treatment of movement disorders. Toxicon 2013, 67, 94–114. [Google Scholar] [CrossRef]
- Naumann, M.; Albanese, A.; Heinen, F.; Molenaers, G.; Relja, M. Safety and efficacy of botulinum toxin type A following long-term use. Eur. J. Neurol. 2006, 13, 35–40. [Google Scholar] [CrossRef]
- Charles, P.D. Botulinum neurotoxin serotype A: A clinical update on non-cosmetic uses. Am. J. Health Syst. Pharm. 2004, 61, S11–S23. [Google Scholar] [CrossRef]
- Rodrigues, F.B.; Duarte, G.S.; Marques, R.E.; Castelão, M.; Ferreira, J.; Sampaio, C.; Moore, A.P.; Costa, J. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst. Rev. 2020, 11, CD003633. [Google Scholar]
- Rosales, R.L.; Chua-Yap, A.S. Evidence-based systematic review on the efficacy and safety of botulinum toxin-A therapy in post-stroke spasticity. J. Neural. Transm. 2008, 115, 617–623. [Google Scholar] [CrossRef]
- Santamato, A.; Cinone, N.; Panza, F.; Letizia, S.; Santoro, L.; Lozupone, M.; Daniele, A.; Picelli, A.; Baricich, A.; Intiso, D.; et al. Botulinum toxin type A for the treatment of lower limb spasticity after stroke. Drugs 2019, 79, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum neurotoxins: Biology, pharmacology, and toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef] [PubMed]
- Berweck, S.; Banach, M.; Gaebler-Spira, D.; Chambers, H.G.; Schroeder, A.S.; Geister, T.L.; Althaus, M.; Hanschmann, A.; Vacchelli, M.; Bonfert, M.V.; et al. Safety profile and lack of immunogenicity of incobotulinumtoxinA in pediatric spasticity and sialorrhea: A pooled analysis. Toxins 2022, 14, 585. [Google Scholar] [CrossRef] [PubMed]
- Hefter, H.; Rosenthal, D.; Jansen, A.; Brauns, R.; Ürer, B.; Bigalke, H.; Hartung, H.P.; Meuth, S.G.; Lee, J.I.; Albrecht, P.; et al. Significantly lower antigenicity of incobotulinumtoxin than abo- or onabotulinumtoxin. J. Neurol. 2022, 270, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Naumann, M.; Boo, L.M.; Ackerman, A.H.; Gallagher, C.J. Immunogenicity of botulinum toxins. J. Neural Transm. 2013, 120, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Leodori, G.; Fernandes, R.M.; Bhidayasiri, R.; Marti, M.J.; Colosimo, C.; Ferreira, J.J. neutralizing antibody and botulinum toxin therapy: A systematic review and meta-analysis. Neurotox Res. 2016, 29, 105–117. [Google Scholar] [CrossRef]
- Rahman, E.; Alhitmi, H.K.; Mosahebi, A. Immunogenicity to botulinum toxin type A: A systematic review with meta-analysis across therapeutic indications. Aesthet. Surg. J. 2022, 42, 106–120. [Google Scholar] [CrossRef]
- Walter, U.; Mühlenhoff, C.; Benecke, R.; Dressler, D.; Mix, E.; Alt, J.; Wittstock, M.; Dudesek, A.; Storch, A.; Kamm, C. Frequency and risk factors of antibody-induced secondary failure of botulinum neurotoxin therapy. Neurology 2020, 94, e2109–e2120. [Google Scholar] [CrossRef]
- Carr, W.W.; Jain, N.; Sublett, J.W. Immunogenicity of botulinum toxin formulations: Potential therapeutic implications. Adv. Ther. 2021, 38, 5046–5064. [Google Scholar] [CrossRef]
- Albrecht, P.; Jansen, A.; Lee, J.I.; Moll, M.; Ringelstein, M.; Rosenthal, D.; Bigalke, H.; Aktas, O.; Hartung, H.P.; Hefter, H. High prevalence of neutralizing antibodies after long-term botulinum neurotoxin therapy. Neurology 2019, 92, e48–e54. [Google Scholar] [CrossRef]
- Bellows, S.; Jankovic, J. Immunogenicity associated with botulinum toxin treatment. Toxins 2019, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Hefter, H.; Samadzadeh, S. Clinical relevance of neutralizing antibodies in botulinum neurotoxin type A. In Botulinum Toxin—Recent Topics and Applications; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Brin, M.F.; Comella, C.L.; Jankovic, J.; Lai, F.; Naumann, M.; CD-017 BoNTA Study Group. Long-term treatment with botulinum toxin type A in cervical dystonia has low immunogenicity by mouse protection assay. Mov. Disord. 2008, 23, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).