The Clostridium botulinum C2 Toxin Subunit C2IIa Delivers Enzymes with Positively Charged N-Termini into the Cytosol of Target Cells
Abstract
1. Introduction
2. Results
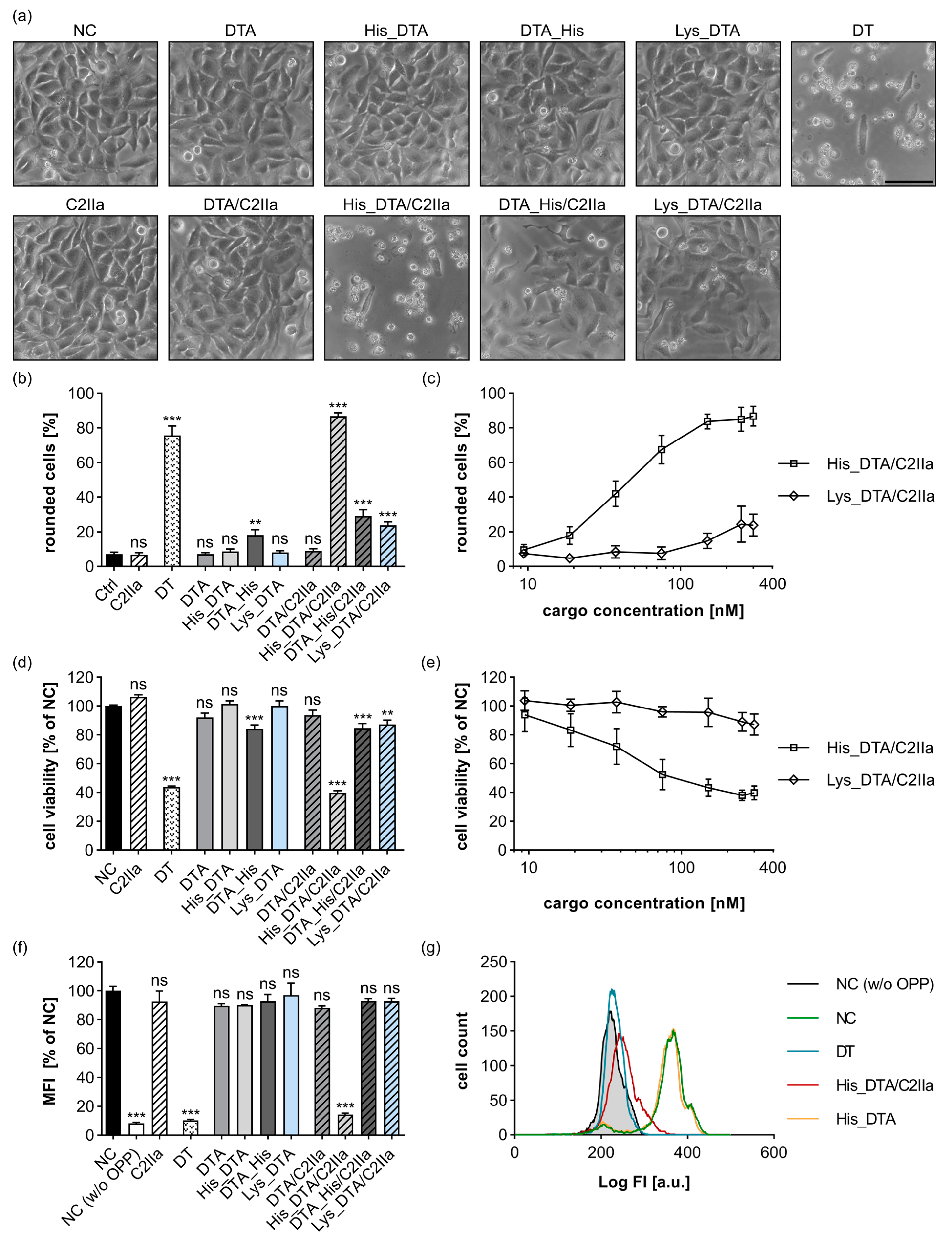
2.1. N-Terminal Polyhistidine-Tagging of DTA Mediates Cytosolic Delivery via C2IIa
2.2. Delivery of His_DTA Is Dependent on C2IIa-Mediated Internalization, Endosomal Acidification, and Translocation through the C2IIa Pore
2.3. Stably Folded Cargo Proteins Such as eGFP Are Not Translocated into the Cytosol
2.4. Transport Evaluation with the Glucosyltransferase Domain of Toxin B
2.5. Transport of Untagged Proteins with Natively Positively Charged N-Termini
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Molecular Cloning and Mutagenesis
5.2. Recombinant Protein Expression and Cell Lysis
5.3. Purification of His-Tagged Proteins
5.4. Purification of Proteins via GST-Tag and Tag Removal
5.5. Cell Culture
5.6. Phase-Contrast Microscopy
5.7. STED Super-Resolution Microscopy
5.8. Cell Viability/Proliferation Assay
5.9. OPP-Based Protein Synthesis Assay
5.10. SDS-PAGE and Western Blot
5.11. Probing of Intracellular Rac1-Glucosylation Status in Intact Cells
5.12. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aktories, K.; Bärmann, M.; Ohishi, I.; Tsuyama, S.; Jakobs, K.H.; Habermann, E. Botulinum C2 Toxin ADP-Ribosylates Actin. Nature 1986, 322, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Barth, H.; Blöcker, D.; Aktories, K. The Uptake Machinery of Clostridial Actin ADP-Ribosylating Toxins--a Cell Delivery System for Fusion Proteins and Polypeptide Drugs. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2002, 366, 501–512. [Google Scholar] [CrossRef]
- Ohishi, I.; Iwasaki, M.; Sakaguchi, G. Purification and Characterization of Two Components of Botulinum C2 Toxin. Infect. Immun. 1980, 30, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, I. Activation of Botulinum C2 Toxin by Trypsin. Infect. Immun. 1987, 55, 1461–1465. [Google Scholar] [CrossRef]
- Barth, H.; Blöcker, D.; Behlke, J.; Bergsma-Schutter, W.; Brisson, A.; Benz, R.; Aktories, K. Cellular Uptake of Clostridium Botulinum C2 Toxin Requires Oligomerization and Acidification. J. Biol. Chem. 2000, 275, 18704–18711. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, M.; Barth, H.; Blöcker, D.; Aktories, K. Binding of Clostridium Botulinum C2 Toxin to Asparagine-Linked Complex and Hybrid Carbohydrates. J. Biol. Chem. 2000, 275, 2328–2334. [Google Scholar] [CrossRef]
- Ohishi, I.; Yanagimoto, A. Visualizations of Binding and Internalization of Two Nonlinked Protein Components of Botulinum C2 Toxin in Tissue Culture Cells. Infect. Immun. 1992, 60, 4648–4655. [Google Scholar] [CrossRef]
- Nagahama, M.; Hagiyama, T.; Kojima, T.; Aoyanagi, K.; Takahashi, C.; Oda, M.; Sakaguchi, Y.; Oguma, K.; Sakurai, J. Binding and Internalization of Clostridium Botulinum C2 Toxin. Infect. Immun. 2009, 77, 5139–5148. [Google Scholar] [CrossRef]
- Vandekerckhove, J.; Schering, B.; Barmann, M.; Aktories, K. Botulinum C2 Toxin ADP-Ribosylates Cytoplasmic β/γ-Actin in Arginine 177. J. Biol. Chem. 1988, 263, 696–700. [Google Scholar] [CrossRef]
- Reuner, K.H.; Presek, P.; Boschek, C.B.; Aktories, K. Botulinum C2 Toxin ADP-Ribosylates Actin and Disorganizes the Microfilament Network in Intact Cells. Eur. J. Cell Biol. 1987, 43, 134–140. [Google Scholar]
- Ohishi, I.; Miyake, M.; Ogura, H.; Nakamura, S. Cytopathic Effect of Botulinum C 2 Toxin on Tissue-Culture Cells. FEMS Microbiol. Lett. 1984, 23, 281–284. [Google Scholar] [CrossRef]
- Heine, K.; Pust, S.; Enzenmüller, S.; Barth, H. ADP-Ribosylation of Actin by the Clostridium Botulinum C2 Toxin in Mammalian Cells Results in Delayed Caspase-Dependent Apoptotic Cell Death. Infect. Immun. 2008, 76, 4600–4608. [Google Scholar] [CrossRef] [PubMed]
- Schleberger, C.; Hochmann, H.; Barth, H.; Aktories, K.; Schulz, G.E. Structure and Action of the Binary C2 Toxin from Clostridium Botulinum. J. Mol. Biol. 2006, 364, 705–715. [Google Scholar] [CrossRef]
- Milne, J.C.; Furlong, D.; Hanna, P.C.; Wall, J.S.; Collier, R.J. Anthrax Protective Antigen Forms Oligomers during Intoxication of Mammalian Cells. J. Biol. Chem. 1994, 269, 20607–20612. [Google Scholar] [CrossRef] [PubMed]
- Petosa, C.; Collier, R.J.; Klimpel, K.R.; Leppla, S.H.; Liddington, R.C. Crystal Structure of the Anthrax Toxin Protective Antigen. Nature 1997, 385, 833–838. [Google Scholar] [CrossRef]
- Blöcker, D.; Barth, H.; Maier, E.; Benz, R.; Barbieri, J.T.; Aktories, K. The C Terminus of Component C2II of Clostridium Botulinum C2 Toxin Is Essential for Receptor Binding. Infect. Immun. 2000, 68, 4566–4573. [Google Scholar] [CrossRef]
- Zhao, J.; Milne, J.C.; Collier, R.J. Effect of Anthrax Toxin’s Lethal Factor on Ion Channels Formed by the Protective Antigen. J. Biol. Chem. 1995, 270, 18626–18630. [Google Scholar] [CrossRef]
- Barth, H.; Stiles, B.G. Binary Actin-ADP-Ribosylating Toxins and Their Use as Molecular Trojan Horses for Drug Delivery into Eukaryotic Cells. Curr. Med. Chem. 2008, 15, 459–469. [Google Scholar] [CrossRef]
- Lang, A.E.; Neumeyer, T.; Sun, J.; Collier, R.J.; Benz, R.; Aktories, K. Amino Acid Residues Involved in Membrane Insertion and Pore Formation of Clostridium Botulinum C2 Toxin. Biochemistry 2008, 47, 8406–8413. [Google Scholar] [CrossRef]
- Blöcker, D.; Bachmeyer, C.; Benz, R.; Aktories, K.; Barth, H. Channel Formation by the Binding Component of Clostridium Botulinum C2 Toxin: Glutamate 307 of C2II Affects Channel Properties in Vitro and PH-Dependent C2I Translocation in Vivo. Biochemistry 2003, 42, 5368–5377. [Google Scholar] [CrossRef]
- Mogridge, J.; Mourez, M.; Collier, R.J. Involvement of Domain 3 in Oligomerization by the Protective Antigen Moiety of Anthrax Toxin. J. Bacteriol. 2001, 183, 2111–2116. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.S.; Lovell, S.; Anbanandam, A.; El-Chami, R.; Bann, J.G. Domain 4 of the Anthrax Protective Antigen Maintains Structure and Binding to the Host Receptor CMG2 at Low PH. Protein Sci. 2009, 18, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Rosovitz, M.J.; Schuck, P.; Varughese, M.; Chopra, A.P.; Mehra, V.; Singh, Y.; McGinnis, L.M.; Leppla, S.H. Alanine-Scanning Mutations in Domain 4 of Anthrax Toxin Protective Antigen Reveal Residues Important for Binding to the Cellular Receptor and to a Neutralizing Monoclonal Antibody. J. Biol. Chem. 2003, 278, 30936–30944. [Google Scholar] [CrossRef] [PubMed]
- Scobie, H.M.; Rainey, G.J.A.; Bradley, K.A.; Young, J.A.T. Human Capillary Morphogenesis Protein 2 Functions as an Anthrax Toxin Receptor. Proc. Natl. Acad. Sci. USA 2003, 100, 5170–5174. [Google Scholar] [CrossRef]
- Wigelsworth, D.J.; Krantz, B.A.; Christensen, K.A.; Lacy, D.B.; Juris, S.J.; Collier, R.J. Binding Stoichiometry and Kinetics of the Interaction of a Human Anthrax Toxin Receptor, CMG2, with Protective Antigen. J. Biol. Chem. 2004, 279, 23349–23356. [Google Scholar] [CrossRef]
- Bradley, K.A.; Mogridge, J.; Mourez, M.; Collier, R.J.; Young, J.A. Identification of the Cellular Receptor for Anthrax Toxin. Nature 2001, 414, 225–229. [Google Scholar] [CrossRef]
- Rabideau, A.E.; Pentelute, B.L. Delivery of Non-Native Cargo into Mammalian Cells Using Anthrax Lethal Toxin. ACS Chem. Biol. 2016, 11, 1490–1501. [Google Scholar] [CrossRef]
- Fahrer, J.; Rausch, J.; Barth, H. A Cell-Permeable Fusion Protein Based on Clostridium Botulinum C2 Toxin for Delivery of P53 Tumorsuppressor into Cancer Cells. PLoS ONE 2013, 8, e72455. [Google Scholar] [CrossRef]
- Barth, H.; Hofmann, F.; Olenik, C.; Just, I.; Aktories, K. The N-Terminal Part of the Enzyme Component (C2I) of the Binary Clostridium Botulinum C2 Toxin Interacts with the Binding Component C2II and Functions as a Carrier System for a Rho ADP-Ribosylating C3-like Fusion Toxin. Infect. Immun. 1998, 66, 1364–1369. [Google Scholar] [CrossRef]
- Fahrer, J.; Funk, J.; Lillich, M.; Barth, H. Internalization of Biotinylated Compounds into Cancer Cells Is Promoted by a Molecular Trojan Horse Based upon Core Streptavidin and Clostridial C2 Toxin. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 383, 263–273. [Google Scholar] [CrossRef]
- Arora, N.; Leppla, S.H. Fusions of Anthrax Toxin Lethal Factor with Shiga Toxin and Diphtheria Toxin Enzymatic Domains Are Toxic to Mammalian Cells. Infect. Immun. 1994, 62, 4955–4961. [Google Scholar] [CrossRef] [PubMed]
- Wesche, J.; Elliott, J.L.; Falnes, P.O.; Olsnes, S.; Collier, R.J. Characterization of Membrane Translocation by Anthrax Protective Antigen. Biochemistry 1998, 37, 15737–15746. [Google Scholar] [CrossRef] [PubMed]
- Antic, I.; Biancucci, M.; Satchell, K.J.F. Cytotoxicity of the Vibrio Vulnificus MARTX Toxin Effector DUF5 Is Linked to the C2A Subdomain. Proteins 2014, 82, 2643–2656. [Google Scholar] [CrossRef]
- Hirschenberger, M.; Stadler, N.; Fellermann, M.; Sparrer, K.M.J.; Kirchhoff, F.; Barth, H.; Papatheodorou, P. CRISPA: A Non-Viral, Transient Cas9 Delivery System Based on Reengineered Anthrax Toxin. Front. Pharmacol. 2021, 12, 770283. [Google Scholar] [CrossRef] [PubMed]
- Blanke, S.R.; Milne, J.C.; Benson, E.L.; Collier, R.J. Fused Polycationic Peptide Mediates Delivery of Diphtheria Toxin A Chain to the Cytosol in the Presence of Anthrax Protective Antigen. Proc. Natl. Acad. Sci. USA 1996, 93, 8437–8442. [Google Scholar] [CrossRef]
- Lang, A.E.; Ernst, K.; Lee, H.; Papatheodorou, P.; Schwan, C.; Barth, H.; Aktories, K. The Chaperone Hsp90 and PPIases of the Cyclophilin and FKBP Families Facilitate Membrane Translocation of Photorhabdus Luminescens ADP-Ribosyltransferases. Cell Microbiol. 2014, 16, 490–503. [Google Scholar] [CrossRef]
- Felix, I.; Lomada, S.K.; Barth, H.; Wieland, T. Bacillus Anthracis’ PA63 Delivers the Tumor Metastasis Suppressor Protein NDPK-A/NME1 into Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 3295. [Google Scholar] [CrossRef]
- Beitzinger, C.; Stefani, C.; Kronhardt, A.; Rolando, M.; Flatau, G.; Lemichez, E.; Benz, R. Role of N-Terminal His6-Tags in Binding and Efficient Translocation of Polypeptides into Cells Using Anthrax Protective Antigen (PA). PLoS ONE 2012, 7, e46964. [Google Scholar] [CrossRef]
- Collier, R.J.; Pappenheimer, A.M. Studies on the Mode of Action of Diphtheria Toxin: II. Effect of Toxin on Amino Acid Incorporation in Cell-Free Systems. J. Exp. Med. 1964, 120, 1019–1039. [Google Scholar] [CrossRef]
- Collier, R.J. Effect of Diphtheria Toxin on Protein Synthesis: Inactivation of One of the Transfer Factors. J. Mol. Biol. 1967, 25, 83–98. [Google Scholar] [CrossRef]
- Honjo, T.; Nishizuka, Y.; Hayaishi, O. Diphtheria Toxin-Dependent Adenosine Diphosphate Ribosylation of Aminoacyl Transferase II and Inhibition of Protein Synthesis. J. Biol. Chem. 1968, 243, 3553–3555. [Google Scholar] [CrossRef] [PubMed]
- Goor, R.S.; Pappenheimer, A.M.; Ames, E. Studies on the Mode of Action of Diphtheria Toxin. V. Inhibition of Peptide Bond Formation by Toxin and NAD in Cell-Free Systems and Its Reversal by Nicotinamide. J. Exp. Med. 1967, 126, 923–939. [Google Scholar] [CrossRef] [PubMed]
- Strauss, N.; Hendee, E.D. The Effect of Diphtheria Toxin on the Metabolism of HeLa Cells. J. Exp. Med. 1959, 109, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, B.; Hoffmann, C.; Aktories, K.; Backert, S.; Schmidt, G. The Cytotoxic Necrotizing Factors from Yersinia Pseudotuberculosis and from Escherichia Coli Bind to Different Cellular Receptors but Take the Same Route to the Cytosol. Infect. Immun. 2007, 75, 3344–3353. [Google Scholar] [CrossRef] [PubMed]
- Schnell, L.; Dmochewitz-Kück, L.; Feigl, P.; Montecucco, C.; Barth, H. Thioredoxin Reductase Inhibitor Auranofin Prevents Membrane Transport of Diphtheria Toxin into the Cytosol and Protects Human Cells from Intoxication. Toxicon 2016, 116, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kreidler, A.-M.; Benz, R.; Barth, H. Chloroquine Derivatives Block the Translocation Pores and Inhibit Cellular Entry of Clostridium Botulinum C2 Toxin and Bacillus Anthracis Lethal Toxin. Arch. Toxicol. 2017, 91, 1431–1445. [Google Scholar] [CrossRef]
- Eisele, J.; Schreiner, S.; Borho, J.; Fischer, S.; Heber, S.; Endres, S.; Fellermann, M.; Wohlgemuth, L.; Huber-Lang, M.; Fois, G.; et al. The Pore-Forming Subunit C2IIa of the Binary Clostridium Botulinum C2 Toxin Reduces the Chemotactic Translocation of Human Polymorphonuclear Leukocytes. Front. Pharmacol. 2022, 13, 810611. [Google Scholar] [CrossRef]
- Bowman, E.J.; Siebers, A.; Altendorf, K. Bafilomycins: A Class of Inhibitors of Membrane ATPases from Microorganisms, Animal Cells, and Plant Cells. Proc. Natl. Acad. Sci. USA 1988, 85, 7972–7976. [Google Scholar] [CrossRef]
- Zornetta, I.; Brandi, L.; Janowiak, B.; Dal Molin, F.; Tonello, F.; Collier, R.J.; Montecucco, C. Imaging the Cell Entry of the Anthrax Oedema and Lethal Toxins with Fluorescent Protein Chimeras. Cell Microbiol. 2010, 12, 1435–1445. [Google Scholar] [CrossRef]
- Just, I.; Selzer, J.; Wilm, M.; von Eichel-Streiber, C.; Mann, M.; Aktories, K. Glucosylation of Rho Proteins by Clostridium Difficile Toxin B. Nature 1995, 375, 500–503. [Google Scholar] [CrossRef]
- Heber, S.; Barthold, L.; Baier, J.; Papatheodorou, P.; Fois, G.; Frick, M.; Barth, H.; Fischer, S. Inhibition of Clostridioides Difficile Toxins TcdA and TcdB by Ambroxol. Front. Pharmacol. 2021, 12, 809595. [Google Scholar] [CrossRef] [PubMed]
- Campanella, J.J.; Bitincka, L.; Smalley, J. MatGAT: An Application That Generates Similarity/Identity Matrices Using Protein or DNA Sequences. BMC Bioinform. 2003, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zheng, W.; Pinkerton, N.; Hansen, J.; Tikunova, S.B.; Davis, J.P.; Heissler, S.M.; Kudryashova, E.; Egelman, E.H.; Kudryashov, D.S. Photorhabdus Luminescens TccC3 Toxin Targets the Dynamic Population of F-Actin and Impairs Cell Cortex Integrity. Int. J. Mol. Sci. 2022, 23, 7026. [Google Scholar] [CrossRef] [PubMed]
- Menacho-Melgar, R.; Decker, J.S.; Hennigan, J.N.; Lynch, M.D. A Review of Lipidation in the Development of Advanced Protein and Peptide Therapeutics. J. Control. Release 2019, 295, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Beilhartz, G.L.; Sugiman-Marangos, S.N.; Melnyk, R.A. Repurposing Bacterial Toxins for Intracellular Delivery of Therapeutic Proteins. Biochem. Pharmacol. 2017, 142, 13–20. [Google Scholar] [CrossRef]
- Ruschig, M.; Marschall, A.L.J. Targeting the Inside of Cells with Biologicals: Toxin Routes in a Therapeutic Context. BioDrugs 2023, 37, 181–203. [Google Scholar] [CrossRef]
- Machen, A.J.; Fisher, M.T.; Freudenthal, B.D. Anthrax Toxin Translocation Complex Reveals Insight into the Lethal Factor Unfolding and Refolding Mechanism. Sci. Rep. 2021, 11, 13038. [Google Scholar] [CrossRef]
- Haug, G.; Wilde, C.; Leemhuis, J.; Meyer, D.K.; Aktories, K.; Barth, H. Cellular Uptake of Clostridium Botulinum C2 Toxin: Membrane Translocation of a Fusion Toxin Requires Unfolding of Its Dihydrofolate Reductase Domain. Biochemistry 2003, 42, 15284–15291. [Google Scholar] [CrossRef]
- Dharmayanti, C.; Gillam, T.A.; Klingler-Hoffmann, M.; Albrecht, H.; Blencowe, A. Strategies for the Development of PH-Responsive Synthetic Polypeptides and Polymer-Peptide Hybrids: Recent Advancements. Polymers 2021, 13, 624. [Google Scholar] [CrossRef]
- Jiang, J.; Pentelute, B.L.; Collier, R.J.; Zhou, Z.H. Atomic Structure of Anthrax Protective Antigen Pore Elucidates Toxin Translocation. Nature 2015, 521, 545–549. [Google Scholar] [CrossRef]
- Fellermann, M.; Stemmer, M.; Noschka, R.; Wondany, F.; Fischer, S.; Michaelis, J.; Stenger, S.; Barth, H. Clostridium Botulinum C3 Toxin for Selective Delivery of Cargo into Dendritic Cells and Macrophages. Toxins 2022, 14, 711. [Google Scholar] [CrossRef] [PubMed]
- Fellermann, M.; Wondany, F.; Carle, S.; Nemeth, J.; Sadhanasatish, T.; Frick, M.; Barth, H.; Michaelis, J. Super-Resolution Microscopy Unveils Transmembrane Domain-Mediated Internalization of Cross-Reacting Material 197 into Diphtheria Toxin-Resistant Mouse J774A.1 Cells and Primary Rat Fibroblasts in Vitro. Arch. Toxicol. 2020, 94, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Osseforth, C.; Moffitt, J.R.; Schermelleh, L.; Michaelis, J. Simultaneous Dual-Color 3D STED Microscopy. Opt. Express 2014, 22, 7028–7039. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heber, S.; Borho, J.; Stadler, N.; Wondany, F.; König, I.; Michaelis, J.; Papatheodorou, P.; Barth, H.; Fellermann, M. The Clostridium botulinum C2 Toxin Subunit C2IIa Delivers Enzymes with Positively Charged N-Termini into the Cytosol of Target Cells. Toxins 2023, 15, 390. https://doi.org/10.3390/toxins15060390
Heber S, Borho J, Stadler N, Wondany F, König I, Michaelis J, Papatheodorou P, Barth H, Fellermann M. The Clostridium botulinum C2 Toxin Subunit C2IIa Delivers Enzymes with Positively Charged N-Termini into the Cytosol of Target Cells. Toxins. 2023; 15(6):390. https://doi.org/10.3390/toxins15060390
Chicago/Turabian StyleHeber, Sebastian, Joscha Borho, Nicole Stadler, Fanny Wondany, Irina König, Jens Michaelis, Panagiotis Papatheodorou, Holger Barth, and Maximilian Fellermann. 2023. "The Clostridium botulinum C2 Toxin Subunit C2IIa Delivers Enzymes with Positively Charged N-Termini into the Cytosol of Target Cells" Toxins 15, no. 6: 390. https://doi.org/10.3390/toxins15060390
APA StyleHeber, S., Borho, J., Stadler, N., Wondany, F., König, I., Michaelis, J., Papatheodorou, P., Barth, H., & Fellermann, M. (2023). The Clostridium botulinum C2 Toxin Subunit C2IIa Delivers Enzymes with Positively Charged N-Termini into the Cytosol of Target Cells. Toxins, 15(6), 390. https://doi.org/10.3390/toxins15060390






