Microcystin-LR-Exposure-Induced Kidney Damage by Inhibiting MKK6-Mediated Mitophagy in Mice
Abstract
:1. Introduction
2. Results
2.1. Characteristics of MC-LR-Exposed Mice
2.2. Effects of MC-LR on Parameters Related to Kidney Function in Mice
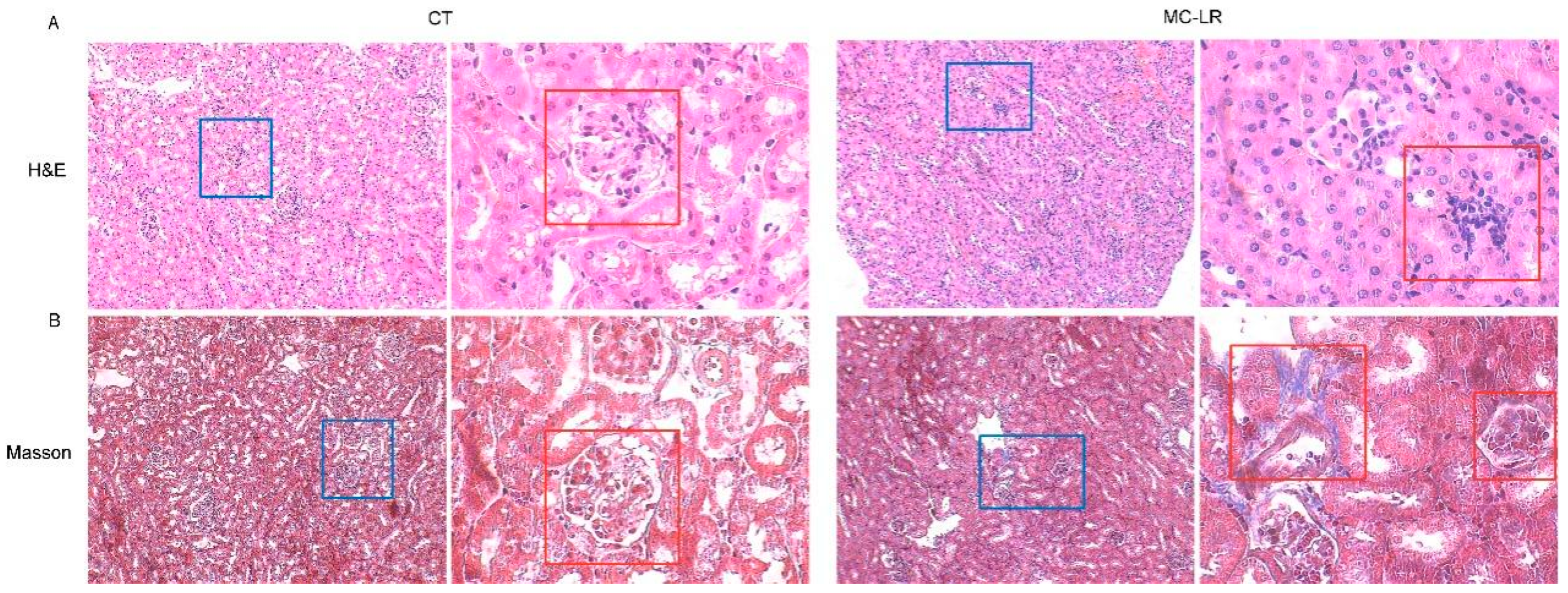
2.3. Histopathology in Kidney
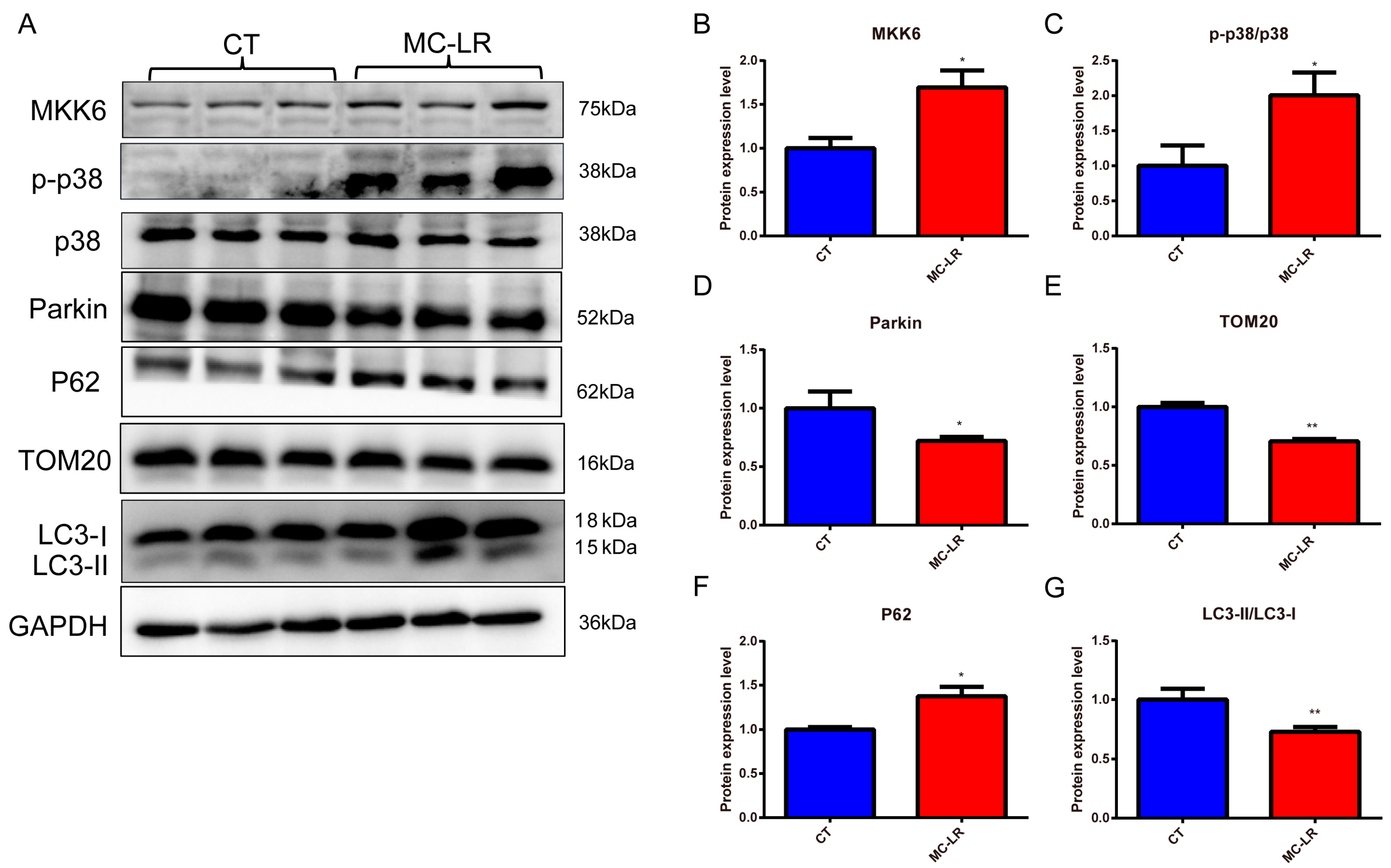
2.4. Effect of MC-LR on Mitophagy-Related Protein Expressions in the Kidneys of Mice
2.5. Viability of HEK293 Cells Exposed to MC-LR
2.6. Effect of MC-LR on Mitophagy-Related Protein Expressions in HEK293 Cells
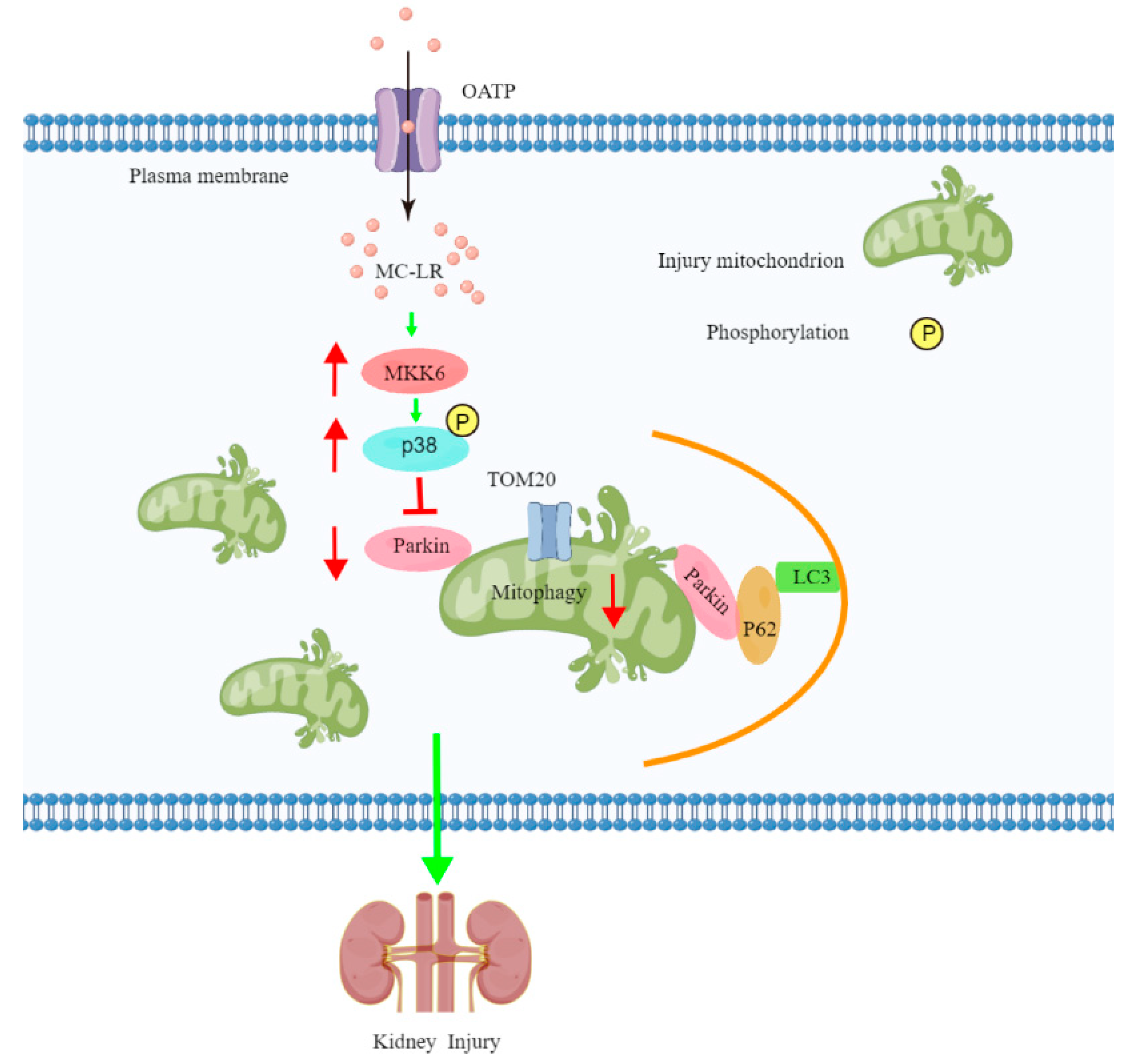
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals and Experimental Design
5.2. Renal Function Indicators
5.3. Histology and Morphometry
5.4. Cell Culture
5.5. CCK8
5.6. Western Blotting
5.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Wei, J.; Pengji, Z.; Zhang, J.; Peng, T.; Luo, J.; Yang, F. Biodegradation of MC-LR and its key bioactive moiety Adda by Sphingopyxis sp. YF1: Comprehensive elucidation of the mechanisms and pathways. Water Res. 2023, 229, 119397. [Google Scholar] [CrossRef]
- Wei, J.; Xie, X.; Huang, F.; Xiang, L.; Wang, Y.; Han, T.; Massey, I.Y.; Liang, G.; Pu, Y.; Yang, F. Simultaneous Microcystis algicidal and microcystin synthesis inhibition by a red pigment prodigiosin. Environ. Pollut. 2020, 256, 113444. [Google Scholar] [CrossRef]
- Zurawell, R.; Chen, H.; Burke, J.; Prepas, E. Hepatotoxic cyanobacteria: A review of the biological importance of microcystins in freshwater environments. J. Toxicol. Environ. Health Part B 2005, 8, 1–37. [Google Scholar] [CrossRef]
- Massey, I.Y.; Yang, F.; Ding, Z.; Yang, S.; Guo, J.; Tezi, C.; Al-Osman, M.; Kamegni, R.B.; Zeng, W. Exposure routes and health effects of microcystins on animals and humans: A mini-review. Toxicon 2018, 151, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Massey, I.Y.; Feng, H.; Yang, F. A Review of Cardiovascular Toxicity of Microcystins. Toxins 2019, 11, 507. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Chen, J.; Fan, H.; Xie, P.; He, J. A review of neurotoxicity of microcystins. Environ. Sci. Pollut. Res. Int. 2016, 23, 7211–7219. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, J.; Zhang, X.; Xie, P. A review of reproductive toxicity of microcystins. J. Hazard. Mater. 2016, 301, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Zegura, B.; Straser, A.; Filipic, M. Genotoxicity and potential carcinogenicity of cyanobacterial toxins—A review. Mutat. Res. 2011, 727, 16–41. [Google Scholar] [CrossRef]
- Du, C.; Zheng, S.; Yang, Y.; Feng, X.; Chen, J.; Tang, Y.; Wang, H.; Yang, F. Chronic exposure to low concentration of MC-LR caused hepatic lipid metabolism disorder. Ecotoxicol. Environ. Saf. 2022, 239, 113649. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for drinking-water quality. In Volume 2—Health Criteria and Other Supporting Information, 2nd ed.; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Sedan, D.; Giannuzzi, L.; Rosso, L.; Marra, C.A.; Andrinolo, D. Biomarkers of prolonged exposure to microcystin-LR in mice. Toxicon 2013, 68, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Giesy, J.P.; Adamovsky, O.; Svircev, Z.; Meriluoto, J.; Codd, G.A.; Mijovic, B.; Shi, T.; Tuo, X.; Li, S.C.; et al. Challenges of using blooms of Microcystis spp. in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Sci. Total Environ. 2021, 764, 142319. [Google Scholar] [CrossRef]
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar] [PubMed]
- Codd, G.A.; Ward, C.J.; Bell, S.G. Cyanobacterial Toxins: Occurrence, Modes of Action, Health Effects and Exposure Routes. Archives of Toxicology. In Applied Toxicology: Approaches Through Basic Science: Proceedings of the 1996 EUROTOX Congress Meeting, Alicante, Spain, 22–25 September 1996; Springer Science & Business Media: Berlin, Germany, 1997; Volume 19, pp. 399–410. [Google Scholar]
- Lu, H.; Choudhuri, S.; Ogura, K.; Csanaky, I.; Lei, X.; Cheng, X.; Song, P.; Klaassen, C. Characterization of organic anion transporting polypeptide 1b2-null mice: Essential role in hepatic uptake/toxicity of phalloidin and microcystin-LR. Toxicol. Sci. Off. J. Soc. Toxicol. 2008, 103, 35–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feurstein, D.; Holst, K.; Fischer, A.; Dietrich, D. Oatp-associated uptake and toxicity of microcystins in primary murine whole brain cells. Toxicol. Appl. Pharmacol. 2009, 234, 247–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, X.; Xu, S.; Huang, F.; Wen, C.; Zheng, S.; Feng, H.; Guo, J.; Chen, J.; Feng, X.; Yang, A. Effects of Chronic Exposure to Microcystin-LR on Kidney in Mice. Int. J. Environ. Res. Public Health 2019, 16, 5030. [Google Scholar] [CrossRef] [Green Version]
- Kotak, B.; Semalulu, S.; Fritz, D.; Prepas, E.; Hrudey, S.; Coppock, R. Hepatic and renal pathology of intraperitoneally administered microcystin-LR in rainbow trout (Oncorhynchus mykiss). Toxicon Off. J. Int. Soc. Toxinology 1996, 34, 517–525. [Google Scholar] [CrossRef]
- Atencio, L.; Moreno, I.; Jos, A.; Pichardo, S.; Moyano, R.; Blanco, A.; Cameán, A. Dose-dependent antioxidant responses and pathological changes in tenca (Tinca tinca) after acute oral exposure to Microcystis under laboratory conditions. Toxicon Off. J. Int. Soc. Toxinol. 2008, 52, 1–12. [Google Scholar] [CrossRef]
- Nobre, A.; Coêlho, G.; Coutinho, M.; Silva, M.; Angelim, E.; Menezes, D.; Fonteles, M.; Monteiro, H. The role of phospholipase A (2) and cyclooxygenase in renal toxicity induced by microcystin-LR. Toxicon Off. J. Int. Soc. Toxinol. 2001, 39, 721–724. [Google Scholar] [CrossRef]
- Li, L.; Xie, P.; Lei, H.; Zhang, X. Renal accumulation and effects of intraperitoneal injection of extracted microcystins in omnivorous crucian carp (Carassius auratus). Toxicon Off. J. Int. Soc. Toxinol. 2013, 70, 62–69. [Google Scholar] [CrossRef]
- Lin, H.; Liu, W.; Zeng, H.; Pu, C.; Zhang, R.; Qiu, Z.; Chen, J.; Wang, L.; Tan, Y.; Zheng, C.; et al. Determination of Environmental Exposure to Microcystin and Aflatoxin as a Risk for Renal Function Based on 5493 Rural People in Southwest China. Environ. Sci. Technol. 2016, 50, 5346–5356. [Google Scholar] [CrossRef]
- Feng, S.; Deng, S.; Tang, Y.; Liu, Y.; Yang, Y.; Xu, S.; Tang, P.; Lu, Y.; Duan, Y.; Wei, J.; et al. Microcystin-LR Combined with Cadmium Exposures and the Risk of Chronic Kidney Disease: A Case-Control Study in Central China. Environ. Sci. Technol. 2022, 56, 15818–15827. [Google Scholar] [CrossRef]
- He, L.; Liu, L.; Lin, C.; Ruan, J.; Liang, X.; Zhou, Y.; Wei, L. Effects of MC-LR on histological structure and cell apoptosis in the kidney of grass carp (Ctenopharyngodon idella). Fish Physiol. Biochem. 2020, 46, 2005–2014. [Google Scholar] [CrossRef]
- Menezes, C.; Alverca, E.; Dias, E.; Sam-Bento, F.; Pereira, P. Involvement of endoplasmic reticulum and autophagy in microcystin-LR toxicity in Vero-E6 and HepG2 cell lines. Toxicol. Vitr. 2013, 27, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Stramucci, L.; Pranteda, A.; Bossi, G. Insights of Crosstalk between P53 Protein and the Mkk3/Mkk6/P38 Mapk Signaling Pathway in Cancer. Cancers 2018, 10, 131. [Google Scholar] [CrossRef] [Green Version]
- Glancy, B.; Kane, D.; Kavazis, A.; Goodwin, M.; Willis, W.; Gladden, L. Mitochondrial lactate metabolism: History and implications for exercise and disease. J. Physiol. 2021, 599, 863–888. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Livingston, M.J.; Liu, Z.; Dong, Z. Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 2020, 16, 489–508. [Google Scholar] [CrossRef]
- Tang, C.; Han, H.; Yan, M.; Zhu, S.; Liu, J.; Liu, Z.; He, L.; Tan, J.; Liu, Y.; Liu, H.; et al. PINK1-PRKN/PARK2 pathway of mitophagy is activated to protect against renal ischemia-reperfusion injury. Autophagy 2018, 14, 880–897. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Li, S.; Jiang, N.; Shao, X.; Zhang, M.; Jin, H.; Zhang, Z.; Shen, J.; Zhou, Y.; Zhou, W.; et al. PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. 2019, 26, 101254. [Google Scholar] [CrossRef]
- Su, L.; Zhang, J.; Gomez, H.; Kellum, J.; Peng, Z. Mitochondria Ros and Mitophagy in Acute Kidney Injury. Autophagy 2023, 19, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, C.; Cai, J.; Chen, G.; Zhang, D.; Zhang, Z.; Dong, Z. PINK1/Parkin-mediated mitophagy is activated in cisplatin nephrotoxicity to protect against kidney injury. Cell Death Dis. 2018, 9, 1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhu, J.; Liu, Z.; Shu, S.; Fu, Y.; Liu, Y.; Cai, J.; Tang, C.; Liu, Y.; Yin, X.; et al. The PINK1/PARK2/optineurin pathway of mitophagy is activated for protection in septic acute kidney injury. Redox Biol. 2021, 38, 101767. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhou, Q.; Huang, Z.; Xu, J.; Zhou, H.; Lv, D.; Lu, L.; Huang, S.; Tang, M.; Zhong, J.; et al. PINK1/Parkin-mediated mitophagy promotes apelin-13-induced vascular smooth muscle cell proliferation by AMPKα and exacerbates atherosclerotic lesions. J. Cell. Physiol. 2019, 234, 8668–8682. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, W.; Gao, G. Seasonal variations in microcystin concentrations in Lake Taihu, China. Environ. Monit. Assess. 2008, 145, 75–79. [Google Scholar] [CrossRef]
- Rao, P.V.; Gupta, N.; Jayaraj, R.; Bhaskar, A.S.; Jatav, P.C. Age-dependent effects on biochemical variables and toxicity induced by cyclic peptide toxin microcystin-LR in mice. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 140, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Giesy, J.P.; Xie, P. The dose makes the poison. Sci. Total Environ. 2018, 621, 649–653. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zuo, X.; Ding, N.; Zeng, H.; Zou, X.; Han, X. Microcystin (-LR) induced testicular cell apoptosis via up-regulating apoptosis-related genes in vivo. Food Chem. Toxicol. 2013, 60, 309–317. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Wang, X.; Chen, L.; Liu, W.; Cai, D.; Deng, S.; Chu, H.; Liu, Y.; Feng, X.; et al. Long-term environmental levels of microcystin-LR exposure induces colorectal chronic inflammation, fibrosis and barrier disruption via CSF1R/Rap1b signaling pathway. J. Hazard. Mater. 2022, 440, 129793. [Google Scholar] [CrossRef]
- Wang, Z.; Li, G.; Wu, Q.; Liu, C.; Shen, J.; Yan, W. Microcystin-LR exposure induced nephrotoxicity by triggering apoptosis in female zebrafish. Chemosphere 2019, 214, 598–605. [Google Scholar] [CrossRef]
- Abdel-Daim, M.; Sayed, A.; Abdeen, A.; Aleya, L.; Ali, D.; Alkahtane, A.; Alarifi, S.; Alkahtani, S. Piperine Enhances the Antioxidant and Anti-Inflammatory Activities of Thymoquinone against Microcystin-LR-Induced Hepatotoxicity and Neurotoxicity in Mice. Oxid. Med. Cell. Longev. 2019, 2019, 1309175. [Google Scholar] [CrossRef]
- Du, X.; Liu, H.; Yuan, L.; Wang, Y.; Ma, Y.; Wang, R.; Chen, X.; Losiewicz, M.D.; Guo, H.; Zhang, H. The Diversity of Cyanobacterial Toxins on Structural Characterization, Distribution and Identification: A Systematic Review. Toxins 2019, 11, 530. [Google Scholar] [CrossRef] [Green Version]
- AlKahtane, A.; Abushouk, A.; Mohammed, E.; ALNasser, M.; Alarifi, S.; Ali, D.; Alessia, M.; Almeer, R.; AlBasher, G.; Alkahtani, S.; et al. Fucoidan alleviates microcystin-LR-induced hepatic, renal, and cardiac oxidative stress and inflammatory injuries in mice. Environ. Sci. Pollut. Res. Int. 2020, 27, 2935–2944. [Google Scholar] [CrossRef]
- Bazzano, T.; Restel, T.; Porfirio, L.; Souza, A.; Silva, I. Renal biomarkers of male and female Wistar rats (Rattus norvegicus) undergoing renal ischemia and reperfusion. Acta Cir. Bras. 2015, 30, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Arman, T.; Lynch, K.D.; Goedken, M.; Clarke, J.D. Sub-chronic microcystin-LR renal toxicity in rats fed a high fat/high cholesterol diet. Chemosphere 2021, 269, 128773. [Google Scholar] [CrossRef] [PubMed]
- Alverca, E.; Andrade, M.; Dias, E.; Bento, F.S.; Batoréu, M.; Jordan, P.; Silva, M.; Pereira, P. Morphological and ultrastructural effects of microcystin-LR from Microcystis aeruginosa extract on a kidney cell line. Toxicon Off. J. Int. Soc. Toxinol. 2009, 54, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, J.; Tang, C.; Dong, Z. Mitophagy in Acute Kidney Injury and Kidney Repair. Cells 2020, 9, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Chen, L.; Liu, W.; Qiao, Q.; Wu, K.; Wen, J.; Huang, C.; Tang, R.; Zhang, X. Involvement of oxidative stress and cytoskeletal disruption in microcystin-induced apoptosis in CIK cells. Aquat. Toxicol. 2015, 165, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Nikolic-Paterson, D.; Ma, F.; Ozols, E.; Thomas, M.; Flavell, R.; Davis, R.; Tesch, G. Role of Mkk3-P38 Mapk Signalling in the Development of Type 2 Diabetes and Renal Injury in Obese Db/Db Mice. Diabetologia 2009, 52, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; He, D.; Biswas, S.; Shafiquzzaman, M.; Zhou, X.; Charron, J.; Wang, Y.; Nayak, B.K.; Habib, S.L.; Liu, H.; et al. mTOR Activation Initiates Renal Cell Carcinoma Development by Coordinating ERK and p38MAPK. Cancer Res. 2021, 81, 3174–3186. [Google Scholar] [CrossRef]
- Sulkshane, P.; Ram, J.; Thakur, A.; Reis, N.; Kleifeld, O.; Glickman, M. Ubiquitination and receptor-mediated mitophagy converge to eliminate oxidation-damaged mitochondria during hypoxia. Redox Biol. 2021, 45, 102047. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhang, T.; Guo, J.; Chen, K.; Li, H.; Wang, J. PINK1 Activation and Translocation to Mitochondria-Associated Membranes Mediates Mitophagy and Protects Against Hepatic Ischemia/Reperfusion Injury. Shock 2020, 54, 783–793. [Google Scholar] [CrossRef]
- Kaushal, G.; Shah, S. Autophagy in acute kidney injury. Kidney Int. 2016, 89, 779–791. [Google Scholar] [CrossRef] [Green Version]
- Choi, M. Autophagy in Kidney Disease. Annu. Rev. Physiol. 2020, 82, 297–322. [Google Scholar] [CrossRef] [Green Version]
- Geisler, S.; Holmström, K.; Skujat, D.; Fiesel, F.; Rothfuss, O.; Kahle, P.; Springer, W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010, 12, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Andres-Manzano, M.J.; Andres, V.; Dorado, B. Oil Red O, and Hematoxylin and Eosin Staining for Quantification of Atherosclerosis Burden in Mouse Aorta and Aortic Root. Methods Mol. Biol. 2015, 1339, 85–99. [Google Scholar]
- Yao, X.; Liu, R.; Li, X.; Li, Y.; Zhang, Z.; Huang, S.; Ge, Y.; Chen, X.; Yang, X. Zinc, selenium, and chromium co-supplementation improves insulin resistance by preventing hepatic endoplasmic reticulum stress in diet-induced gestational diabetes rats. J. Nutr. Biochem. 2021, 96, 108810. [Google Scholar] [CrossRef] [PubMed]




| Parkin | Abcam, Cambridge, UK | 1:2000 |
| p38 | Cell Signaling Technology, UK | 1:1000 |
| p-p38 | Abcam, Cambridge, UK | 1:1000 |
| p62 | Proteintech, Wuhan, China | 1:1000 |
| LC3-II/LC3-I | Proteintech, Wuhan, China | 1:500 |
| TOM20 | Proteintech, Wuhan, China | 1:2000 |
| GAPDH | Proteintech, Wuhan, China | 1:50,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Liu, Y.; Yang, Y.; Li, Y.; Hu, N.; Song, F.; Yang, F. Microcystin-LR-Exposure-Induced Kidney Damage by Inhibiting MKK6-Mediated Mitophagy in Mice. Toxins 2023, 15, 404. https://doi.org/10.3390/toxins15060404
Yao X, Liu Y, Yang Y, Li Y, Hu N, Song F, Yang F. Microcystin-LR-Exposure-Induced Kidney Damage by Inhibiting MKK6-Mediated Mitophagy in Mice. Toxins. 2023; 15(6):404. https://doi.org/10.3390/toxins15060404
Chicago/Turabian StyleYao, Xueqiong, Ying Liu, Yue Yang, Yafang Li, Na Hu, Fengmei Song, and Fei Yang. 2023. "Microcystin-LR-Exposure-Induced Kidney Damage by Inhibiting MKK6-Mediated Mitophagy in Mice" Toxins 15, no. 6: 404. https://doi.org/10.3390/toxins15060404
APA StyleYao, X., Liu, Y., Yang, Y., Li, Y., Hu, N., Song, F., & Yang, F. (2023). Microcystin-LR-Exposure-Induced Kidney Damage by Inhibiting MKK6-Mediated Mitophagy in Mice. Toxins, 15(6), 404. https://doi.org/10.3390/toxins15060404






