The Impact of Storage Temperature and Time on Ergot Alkaloid Concentrations
Abstract
1. Introduction
2. Results
2.1. Total Ergot Concentration
2.2. Total R and S-epimer Concentration
2.3. Temperature Effects on Total, Total R, and Total S-epimer Concentration
2.4. Individual Ergot Epimer Concentration
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sample Preparation
5.2. Pilot Study
5.3. Quantification
5.4. Experimental Design and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tittlemier, S.A.; Drul, D.; Roscoe, M.; Mckendry, T. Occurrence of Ergot and Ergot Alkaloids in Western Canadian Wheat and Other Cereals. J. Agric. Food Chem. 2015, 63, 6644–6650. [Google Scholar] [CrossRef] [PubMed]
- Walkowiak, S.; Taylor, D.; Fu, X.; Drul, D.; Pleskach, K.; Tittlemier, S.A. Ergot in Canadian Cereals-Relevance, Occurrence, and Current Status. Can. J. Plant Pathol. 2022, 44, 793–805. [Google Scholar] [CrossRef]
- Poapolathep, S.; Klangkaew, N.; Zhang, Z.; Giorgi, M.; Logrieco, A.F.; Poapolathep, A. Simultaneous Determination of Ergot Alkaloids in Swine and Dairy Feeds Using Ultra High-Performance Liquid Chromatography-Tandem Mass Spectrometry. Toxins 2021, 13, 724. [Google Scholar] [CrossRef] [PubMed]
- Agriopoulou, S. Ergot Alkaloids Mycotoxins in Cereals and Cereal-Derived Food Products: Characteristics, Toxicity, Prevalence, and Control Strategies. Agronomy 2021, 11, 931. [Google Scholar] [CrossRef]
- Babič, J.; Tavčar-Kalcher, G.; Celar, F.A.; Kos, K.; Červek, M.; Jakovac-Strajn, B. Ergot and Ergot Alkaloids in Cereal Grains Intended for Animal Feeding Collected in Slovenia: Occurrence, Pattern and Correlations. Toxins 2020, 12, 730. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.C. A Critical Review of Analytical Methods for Ergot Alkaloids in Cereals and Feed and in Particular Suitability of Method Performance for Regulatory Monitoring and Epimer-Specific Quantification. Food Addit. Contam. A 2021, 38, 997–1012. [Google Scholar] [CrossRef] [PubMed]
- Lea, K.; Smith, L.; Gaskill, C.; Coleman, R.; Smith, S.R. Ergovaline Stability in Tall Fescue Based on Sample Handling and Storage Methods. Front. Chem. 2014, 2, 76. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.A.; Davis, D.K.; Looper, M.L.; Kallenbach, R.L.; Rottinghaus, G.E.; Hill, N.S. Ergot Alkaloid Concentrations in High-and Low-Moisture Tall Fescue Silage. Crop. Sci. 2014, 54, 1887–1892. [Google Scholar] [CrossRef]
- Roberts, C.A.; Kallenbach, R.L.; Rottinghaus, G.E.; Hill, N.S. Ergovaline and Ergot Alkaloid Concentrations Change in Conserved Tall Fescue. Forage Grazinglands 2011, 9, 1–9. [Google Scholar] [CrossRef]
- Roberts, C.A.; Kallenbach, R.L.; Hill, N.S.; Rottinghaus, G.E.; Evans, T.J. Ergot Alkaloid Concentrations in Tall Fescue Hay during Production and Storage. Crop. Sci. 2009, 49, 1496–1502. [Google Scholar] [CrossRef]
- Tkachenko, A.; Benson, K.; Mostrom, M.; Guag, J.; Reimschuessel, R.; Webb, B. Extensive Evaluation via Blinded Testing of an UHPLC-MS/MS Method for Quantitation of Ten Ergot Alkaloids in Rye and Wheat Grains. J. AOAC Int. 2021, 104, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Coufal-Majewski, S.; Stanford, K.; McAllister, T.; Blakley, B.; McKinnon, J.; Chaves, A.V.; Wang, Y. Impacts of Cereal Ergot in Food Animal Production. Front. Vet. Sci. 2016, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Young, J.C.; Chen, Z.J.; Marquardt, R.R. Reduction in Alkaloid Content of Ergot Sclerotia by Chemical and Physical Treatment. J. Agric. Food Chem. 1983, 31, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Hafner, M.; Sulyok, M.; Schuhmacher, R.; Crews, C.; Krska, R. Stability and Epimerisation Behaviour of Ergot Alkaloids in Various Solvents. World Mycotoxin J. 2008, 1, 67–78. [Google Scholar] [CrossRef]
- Crews, C. Analysis of Ergot Alkaloids. Toxins 2015, 7, 2024–2050. [Google Scholar] [CrossRef]
- Schummer, C.; Zandonella, I.; van Nieuwenhuyse, A.; Moris, G. Epimerization of Ergot Alkaloids in Feed. Heliyon 2020, 6, e04336. [Google Scholar] [CrossRef]
- Carbonell-Rozas, L.; Mahdjoubi, C.K.; Arroyo-Manzanares, N.; García-Campaña, A.M.; Gámiz-Gracia, L. Occurrence of Ergot Alkaloids in Barley and Wheat from Algeria. Toxins 2021, 13, 316. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Drul, D.; Roscoe, M.; Turnock, D.; Taylor, D.; Fu, B.X. Fate of Ergot Alkaloids during Laboratory Scale Durum Processing and Pasta Production. Toxins 2019, 11, 195. [Google Scholar] [CrossRef]
- Kodisch, A.; Oberforster, M.; Raditschnig, A.; Rodemann, B.; Tratwal, A.; Danielewicz, J.; Korbas, M.; Schmiedchen, B.; Eifler, J.; Gordillo, A.; et al. Covariation of Ergot Severity and Alkaloid Content Measured by HPLC and One ELISA Method in Inoculated Winter Rye across Three Isolates and Three European Countries. Toxins 2020, 12, 676. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2021/1399 of 24 August 2021 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Ergot Sclerotia and Ergot Alkaloids in Certain Foodstuffs. Official Journal of the European Union 2021. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32021R1399 (accessed on 1 October 2022).
- CFIA RG-8 Regulatory Guidance: Contaminants in Feed (Formerly RG-1, Chapter 7). Available online: https://www.inspection.gc.ca/animalhealth/%0Alivestock-feeds/regulatory-guidance/rg-8/eng/%0A1347383943203/1347384015909?chap=0 (accessed on 13 December 2022).
- Mulac, D.; Hüwel, S.; Galla, H.J.; Humpf, H.U. Permeability of Ergot Alkaloids across the Blood-Brain Barrier in Vitro and Influence on the Barrier Integrity. Mol. Nutr. Food Res. 2012, 56, 475–485. [Google Scholar] [CrossRef]
- Cherewyk, J.E.; Parker, S.E.; Blakley, B.R.; Al-Dissi, A.N. Assessment of the Vasoactive Effects of the (S)-Epimers of Ergot Alkaloids in Vitro. J. Anim. Sci. 2020, 98, skaa203. [Google Scholar] [CrossRef] [PubMed]
- Cherewyk, J.E.; Parker, S.E.; Blakley, B.R.; Al-Dissi, A.N. Sustained Vascular Contractile Response Induced by an R-and S-Epimer of the Ergot Alkaloid Ergocristine and Attenuation by a Noncompetitive Antagonist. J. Anim. Sci. 2022, 100, skac235. [Google Scholar] [CrossRef] [PubMed]
- Ensley, S.M.; Radke, S.L. Mycotoxins in Grains and Feeds. Dis. Swine 2019, 11, 1055–1071. [Google Scholar]
- Schummer, C.; Brune, L.; Moris, G. Development of a UHPLC-FLD Method for the Analysis of Ergot Alkaloids and Application to Different Types of Cereals from Luxembourg. Mycotoxin Res. 2018, 34, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Krska, R.; Stubbings, G.; MacArthur, R.; Crews, C. Simultaneous Determination of Six Major Ergot Alkaloids and Their Epimers in Cereals and Foodstuffs by LC-MS-MS. Anal. Bioanal. Chem. 2008, 391, 563–576. [Google Scholar] [CrossRef]
- Diana Di Mavungu, J.; Malysheva, S.V.; Sanders, M.; Larionova, D.; Robbens, J.; Dubruel, P.; Van Peteghem, C.; De Saeger, S. Development and Validation of a New LC-MS/MS Method for the Simultaneous Determination of Six Major Ergot Alkaloids and Their Corresponding Epimers. Application to Some Food and Feed Commodities. Food Chem. 2012, 135, 292–303. [Google Scholar] [CrossRef]
- Guo, Q.; Shao, B.; Du, Z.; Zhang, J. Simultaneous Determination of 25 Ergot Alkaloids in Cereal Samples by Ultraperformance Liquid Chromatography−Tandem Mass Spectrometry. J. Agric. Food Chem. 2016, 64, 7033–7039. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Manzanares, N.; De Ruyck, K.; Uka, V.; Gámiz-Gracia, L.; García-Campaña, A.M.; De Saeger, S.; Diana Di Mavungu, J. In-House Validation of a Rapid and Efficient Procedure for Simultaneous Determination of Ergot Alkaloids and Other Mycotoxins in Wheat and Maize. Anal. Bioanal. Chem. 2018, 410, 5567–5581. [Google Scholar] [CrossRef]
- van der Fels-Klerx, H.J.; Liu, C.; Focker, M.; Montero-Castro, I.; Rossi, V.; Manstretta, V.; Magan, N.; Krska, R. Decision Support System for Integrated Management of Mycotoxins in Feed and Food Supply Chains. World Mycotoxin J. 2022, 15, 119–133. [Google Scholar] [CrossRef]
- Craig, A.M.; Klotz, J.L.; Duringer, J.M. Cases of Ergotism in Livestock and Associated Ergot Alkaloid Concentrations in Feed. Front. Chem. 2015, 3, 8. [Google Scholar] [CrossRef]
- Koester, L.R.; Poole, D.H.; Serão, N.V.L.; Schmitz-Esser Id, S. Beef Cattle That Respond Differently to Fescue Toxicosis Have Distinct Gastrointestinal Tract Microbiota. PLoS ONE 2020, 15, e0229192. [Google Scholar] [CrossRef] [PubMed]
- Caradus, J.R.; Card, S.D.; Finch, S.C.; Hume, D.E.; Johnson, L.J.; Mace, W.J.; Popay, A.J. Ergot Alkaloids in New Zealand Pastures and Their Impact. New Zeal. J. Agric. Res. 2020, 65, 1–41. [Google Scholar] [CrossRef]
- Krska, R.; Crews, C. Significance, Chemistry and Determination of Ergot Alkaloids: A Review. Food Addit. Contam. Part A Chem. Anal. Control. Expo Risk Assess 2008, 25, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Shappell, N.W. Technical Note: Epimerization of Ergopeptine Alkaloids in Organic and Aqueous Solvents. J. Anim. Sci. 2002, 80, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Komarova, E.L.; Tolkachev, O.N. The Chemistry of Peptide Ergot Alkaloids. Part 1. Classification and Chemistry of Ergot Peptides. Pharm. Chem. J. 2001, 35, 504–513. [Google Scholar] [CrossRef]
- Merkel, S.; Dib, B.; Maul, R.; Köppen, R.; Koch, M.; Nehls, I. Degradation and Epimerization of Ergot Alkaloids after Baking and in Vitro Digestion. Anal. Bioanal. Chem. 2012, 404, 2489–2497. [Google Scholar] [CrossRef]
- Grusie, T.; Cowan, V.; Singh, J.; McKinnon, J.; Blakley, B. Proportions of Predominant Ergot Alkaloids (Claviceps Purpurea) Detected in Western Canadian Grains from 2014 to 2016. World Mycotoxin J. 2018, 11, 259–264. [Google Scholar] [CrossRef]
- Coufal-Majewski, S.; Stanford, K.; McAllister, T.; Wang, Y.; Blakley, B.; McKinnon, J.; Swift, M.L.; Chaves, A.V. Effects of Continuously Feeding Diets Containing Cereal Ergot Alkaloids on Nutrient Digestibility, Alkaloid Recovery in Feces, and Performance Traits of Ram Lambs. Toxins 2017, 9, 405. [Google Scholar] [CrossRef]
- European Commission. Commission Recommendation (EU) No 691/2013 of 19 July 2013 Amending Regulation (EC) No 152/2009 as Regards Methods of Sampling and Analysis. Official Journal of the European Union 2013. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32013R0691 (accessed on 1 October 2022).
- Drakopoulos, D.; Sulyok, M.; Krska, R.; Logrieco, A.F.; Vogelgsang, S. Raised Concerns about the Safety of Barley Grains and Straw: A Swiss Survey Reveals a High Diversity of Mycotoxins and Other Fungal Metabolites. Food Control. 2021, 125, 107919. [Google Scholar] [CrossRef]
- Cherewyk, J.; Grusie-Ogilvie, T.; Blakley, B.; Al-Dissi, A. Validation of a New Sensitive Method for the Detection and Quantification of R and S-Epimers of Ergot Alkaloids in Canadian Spring Wheat Utilizing Deuterated Lysergic Acid Diethylamide as an Internal Standard. Toxins 2022, 14, 22. [Google Scholar] [CrossRef]
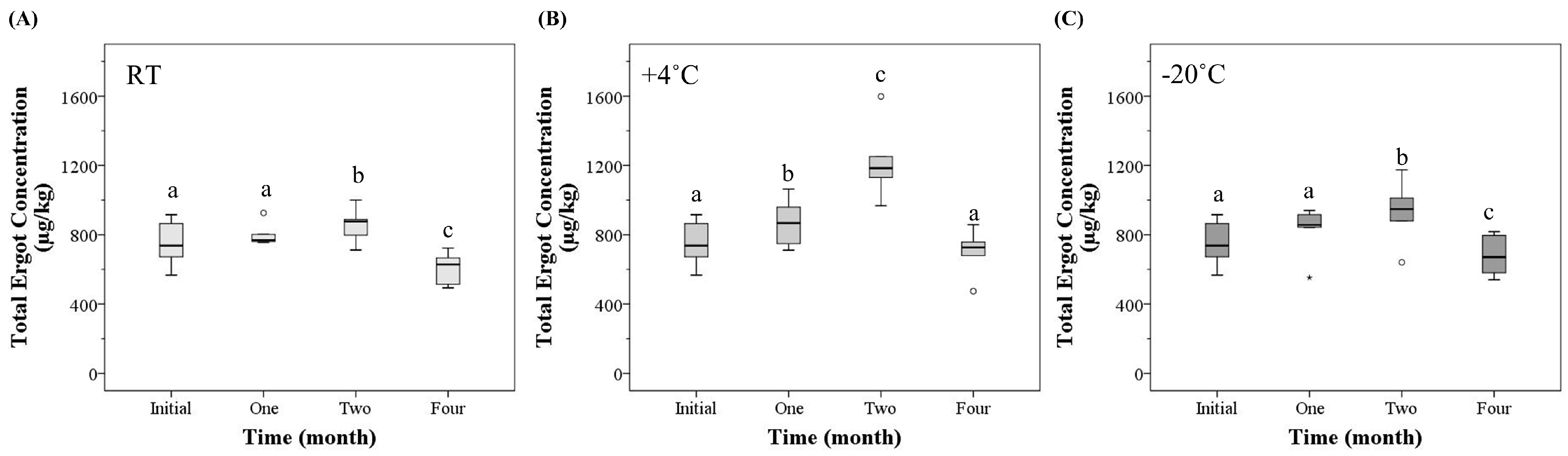
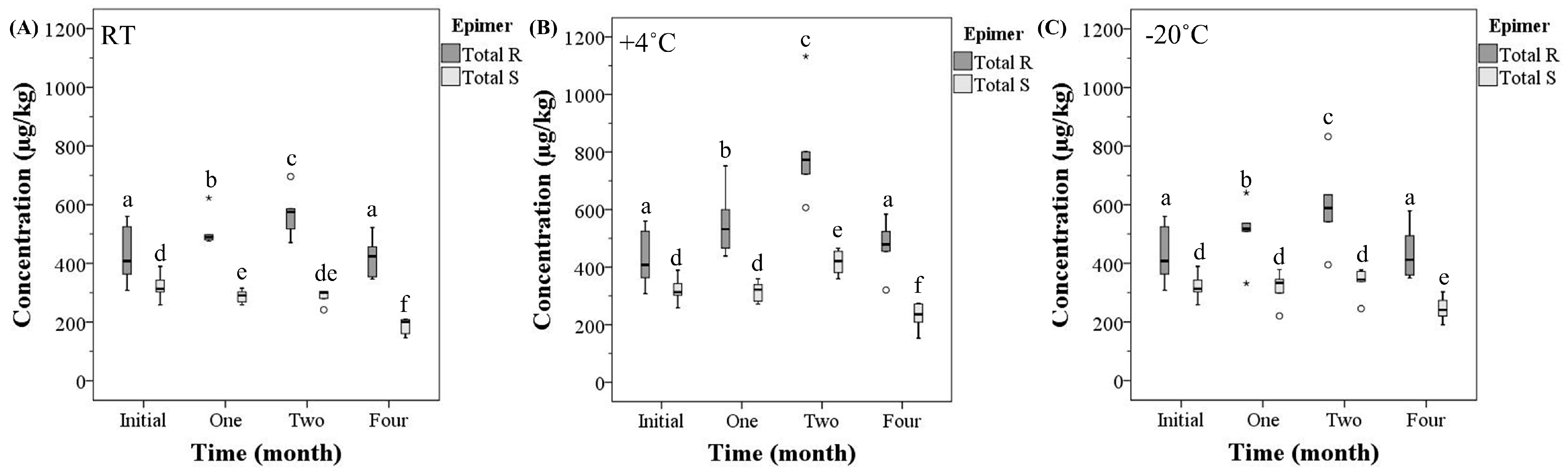
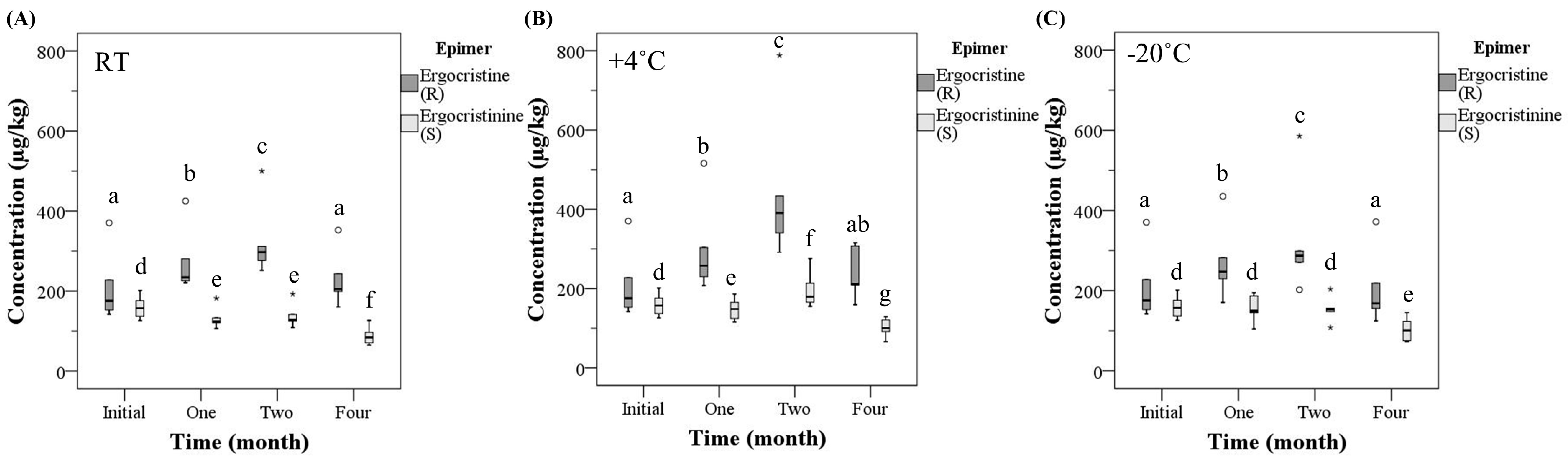
| Time (month) | Temperature (°C) | Mean Total Ergot Alkaloid Concentration | Mean Total R-Epimer Concentration | Mean Total S-Epimer Concentration |
|---|---|---|---|---|
| Initial | Initial | 749 ± 131 | 429 ± 99 | 320 ± 44 |
| 1 | Room | 797 ± 65 a | 509 ± 56 a | 288 ± 21 a |
| +4 | 870 ± 132 a | 554 ± 114 a | 316 ± 34 b | |
| −20 | 827 ± 140 a | 509± 100 a | 318 ± 54 ab | |
| 2 | Room | 859 ± 97 a | 570 ± 76 a | 289 ± 25 a |
| +4 | 1219 ± 210 b | 802 ± 177 b | 417 ± 41 b | |
| −20 | 934 ± 175 c | 597 ± 142 a | 337 ± 48 c | |
| 4 | Room | 609 ± 89 a | 421 ± 66 a | 188 ± 27 a |
| +4 | 704 ± 127 b | 474 ± 88 a | 230 ± 46 b | |
| −20 | 679 ± 113 b | 435 ± 88 a | 245 ± 39 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherewyk, J.E.; Grusie-Ogilvie, T.J.; Parker, S.E.; Blakley, B.R.; Al-Dissi, A.N. The Impact of Storage Temperature and Time on Ergot Alkaloid Concentrations. Toxins 2023, 15, 497. https://doi.org/10.3390/toxins15080497
Cherewyk JE, Grusie-Ogilvie TJ, Parker SE, Blakley BR, Al-Dissi AN. The Impact of Storage Temperature and Time on Ergot Alkaloid Concentrations. Toxins. 2023; 15(8):497. https://doi.org/10.3390/toxins15080497
Chicago/Turabian StyleCherewyk, Jensen E., Taylor J. Grusie-Ogilvie, Sarah E. Parker, Barry R. Blakley, and Ahmad N. Al-Dissi. 2023. "The Impact of Storage Temperature and Time on Ergot Alkaloid Concentrations" Toxins 15, no. 8: 497. https://doi.org/10.3390/toxins15080497
APA StyleCherewyk, J. E., Grusie-Ogilvie, T. J., Parker, S. E., Blakley, B. R., & Al-Dissi, A. N. (2023). The Impact of Storage Temperature and Time on Ergot Alkaloid Concentrations. Toxins, 15(8), 497. https://doi.org/10.3390/toxins15080497





