Quercetin Alleviates Lipopolysaccharide-Induced Cell Oxidative Stress and Inflammatory Responses via Regulation of the TLR4-NF-κB Signaling Pathway in Bovine Rumen Epithelial Cells
Abstract
:1. Introduction
2. Results
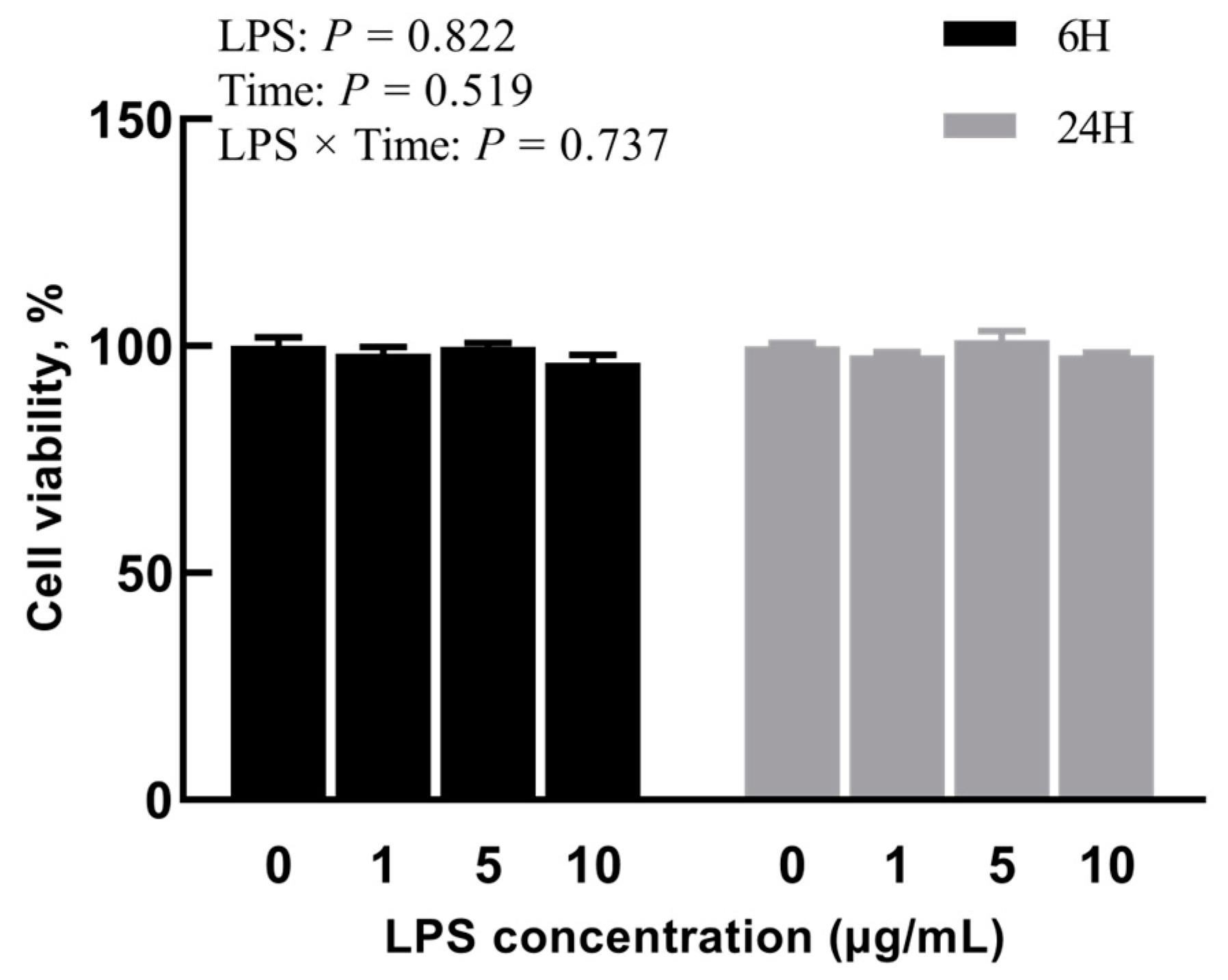
2.1. Experiment 1: Effects of Different Concentrations of LPS on BREC
2.2. Experiment 2: Effect of Quercetin on LPS-Induced BREC Inflammatory Response
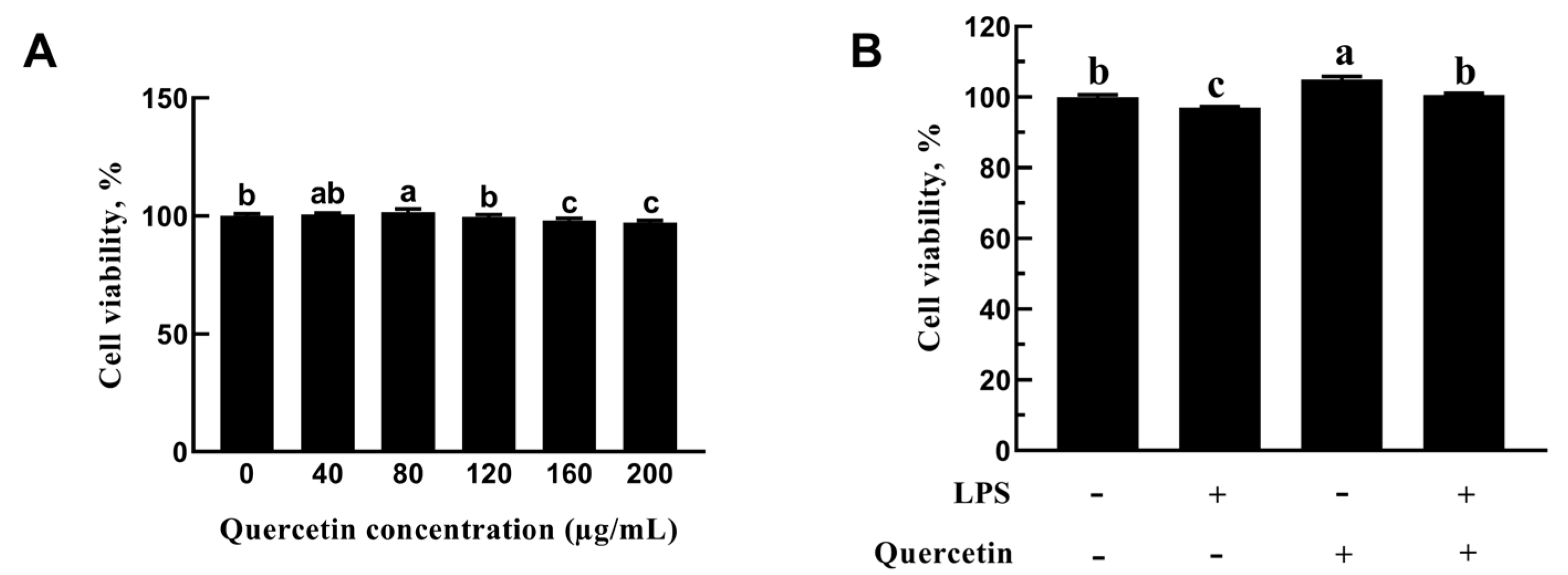
2.2.1. Role of Quercetin in the Cell Viability of BREC
2.2.2. Effect of Quercetin on Oxidative Characteristics of BREC after LPS Stimulation
2.2.3. Effect of Quercetin on Pro-Inflammation Factors of BREC after LPS Stimulation
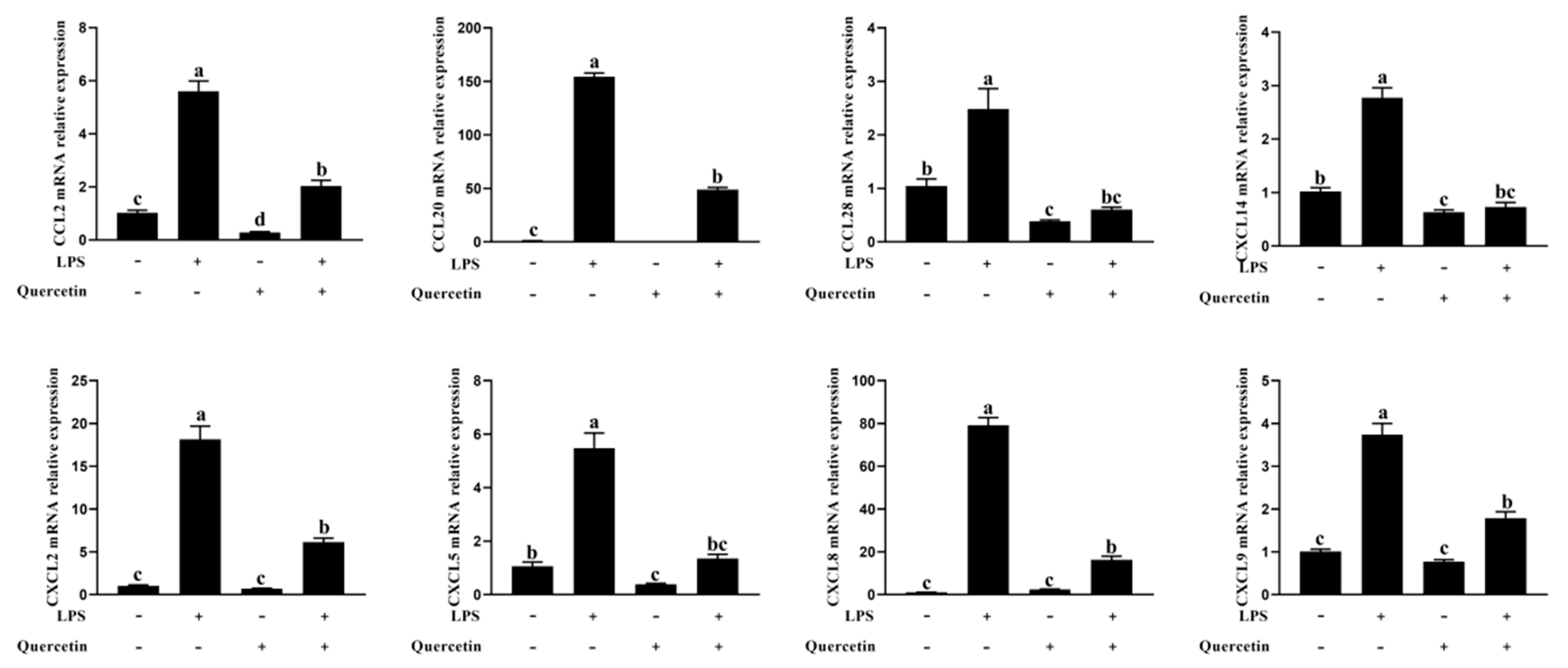
2.2.4. Effect of Quercetin on the Immune Response of BREC after LPS Stimulation
2.2.5. Effect of Quercetin on TLR4 Signaling Pathway of BREC after LPS Stimulation
2.2.6. Effect of Quercetin on ERK1/2 and NF-κB Signaling Pathways of BREC after LPS Stimulation
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cell Culture
5.2. Experiment 1: Establishment of an LPS-Induced BREC Inflammatory Model
5.2.1. Effects of Different Dosage/Concentrations of LPS on BREC
5.2.2. Effects of LPS on mRNA Levels of Inflammatory Factors in BREC
5.3. Experiment 2: Effect of Quercetin on LPS-Induced BREC Inflammatory Response
5.3.1. Proliferative Activity Analysis
5.3.2. Experimental Design and Treatment
5.3.3. RNA Isolation and cDNA Synthesis
5.3.4. Antioxidant Analysis
5.3.5. Immunocy to Fluorescence
5.3.6. Western Blotting
5.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khafipour, E.; Krause, D.O.; Plaizier, J.C. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J. Dairy Sci. 2009, 92, 1060–1070. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lelliott, C.; Hakansson, P.; Ploj, K.; Tuneld, A.; Verolin-Johansson, M.; Benthem, L.; Carlsson, B.; Storlien, L.; Michaelsson, E. Intestinal, adipose, and liver inflammation in diet-induced obese mice. Metab. Clin. Exp. 2008, 57, 1704–1710. [Google Scholar] [CrossRef] [PubMed]
- Kleen, J.L.; Hooijer, G.A.; Rehage, J.; Noordhuizen, J.P.T.M. Subacute ruminal acidosis in Dutch dairy herds. Vet. Rec. 2009, 164, 681–684. [Google Scholar] [CrossRef]
- Kleen, J.L.; Upgang, L.; Rehage, J. Prevalence and consequences of subacute ruminal acidosis in German dairy herds. Acta Vet. Scand. 2013, 55, 48. [Google Scholar] [CrossRef]
- Gozho, G.N.; Krause, D.O.; Plaizier, J.C. Ruminal lipopolysaccharide concentration and inflammatory response during grain-induced subacute ruminal acidosis in dairy cows. J. Dairy Sci. 2007, 90, 856–866. [Google Scholar] [CrossRef]
- Khafipour, E.; Krause, D.O.; Plaizier, J.C. Alfalfa pellet-induced subacute ruminal acidosis in dairy cows increases bacterial endotoxin in the rumen without causing inflammation. J. Dairy Sci. 2009, 92, 1712–1724. [Google Scholar] [CrossRef]
- Besle, J.M.; Viala, D.; Martin, B.; Pradel, P.; Meunier, B.; Berdague, J.L.; Fraisse, D.; Lamaison, J.L.; Coulon, J.B. Ultraviolet-absorbing compounds in milk are related to forage polyphenols. J. Dairy Sci. 2010, 93, 2846–2856. [Google Scholar] [CrossRef]
- Berger, L.M.; Wein, S.; Blank, R.; Metges, C.C.; Wolffram, S. Bioavailability of the flavonol quercetin in cows after intraruminal application of quercetin aglycone and rutin. J. Dairy Sci. 2012, 95, 5047–5055. [Google Scholar] [CrossRef]
- Berger, L.M.; Blank, R.; Zorn, F.; Wein, S.; Metges, C.C.; Wolffram, S. Ruminal degradation of quercetin and its influence on fermentation in ruminants. J. Dairy Sci. 2015, 98, 5688–5698. [Google Scholar] [CrossRef]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Cermak, R.; Landgraf, S.; Wolffram, S. The bioavailability of quercetin in pigs depends on the glycoside moiety and on dietary factors. J. Nutr. 2003, 133, 2802–2807. [Google Scholar] [CrossRef]
- Reinboth, M.; Wolffram, S.; Abraham, G.; Ungemach, F.R.; Cermak, R. Oral bioavailability of quercetin from different quercetin glycosides in dogs. Br. J. Nutr. 2010, 104, 198–203. [Google Scholar] [CrossRef]
- Askar, M.A.; El-Nashar, H.A.; Al-Azzawi, M.A.; Rahman, S.S.A.; Elshawi, O.E. Synergistic Effect of Quercetin Magnetite Nanoparticles and Targeted Radiotherapy in Treatment of Breast Cancer. Breast Cancer Basic Clin. Res. 2022, 16, 11782234221086728. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; El-Din, M.I.G.; Hritcu, L.; Eldahshan, O.A. Insights on the Inhibitory Power of Flavonoids on Tyrosinase Activity: A Survey from 2016 to 2021. Molecules 2021, 26, 7546. [Google Scholar] [CrossRef]
- Abdelghffar, E.A.; El-Nashar, H.A.S.; Al-Mohammadi, A.G.A.; Eldahshan, O.A. Orange fruit (Citrus sinensis) peel extract attenuates chemotherapy-induced toxicity in male rats. Food Funct. 2021, 12, 9443–9455. [Google Scholar] [CrossRef]
- Batiha, G.E.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; Abd El-Hack, M.E.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.Y.; Han, C.Y.; Yang, J.X.; Chaudhry, M.T.; Wang, S.N.; Liu, H.N.; Yin, Y.L. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Xiong, G.; Ji, W.S.; Wang, F.; Zhang, F.X.; Xue, P.; Cheng, M.; Sun, Y.S.; Wang, X.; Zhang, T.L. Quercetin Inhibits Inflammatory Response Induced by LPS from Porphyromonas gingivalis in Human Gingival Fibroblasts via Suppressing NF-kappa B Signaling Pathway. Biomed. Res. Int. 2019, 2019, 6282635. [Google Scholar] [CrossRef]
- Ekstrom, A.M.; Serafini, M.; Nyren, O.; Wolk, A.; Bosetti, C.; Bellocco, R. Dietary quercetin intake and risk of gastric cancer: Results from a population-based study in Sweden. Ann. Oncol. 2011, 22, 438–443. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of Tight Junction Permeability by Intestinal Bacteria and Dietary Components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef]
- Biasi, F.; Astegiano, M.; Maina, M.; Leonarduzzi, G.; Poli, G. Polyphenol Supplementation as a Complementary Medicinal Approach to Treating Inflammatory Bowel Disease. Curr. Med. Chem. 2011, 18, 4851–4865. [Google Scholar] [CrossRef]
- Andrade, A.I.; Chavez, E.G.; Bautista, C.R.; Ovalle, C.O.; Cabrera, M.A.R.; Lagunes, A.G. Influence of prebiotic activity of agave salmiana fructans on mucus production and morphology changes in colonic epithelium cell of healthy wistar rats. Front. Plant Sci. 2021, 12, 717460. [Google Scholar] [CrossRef]
- Cornejo, O.E.; Lefebure, T.; Bitar, P.D.P.; Lang, P.; Richards, V.P.; Eilertson, K.; Do, T.; Beighton, D.; Zeng, L.; Ahn, S.J.; et al. Evolutionary and Population Genomics of the Cavity Causing Bacteria Streptococcus mutans. Mol. Biol. Evol. 2013, 30, 881–893. [Google Scholar] [CrossRef]
- Khafipour, E.; Plaizier, J.C.; Aikman, P.C.; Krause, D.O. Population structure of rumen Escherichia coli associated with subacute ruminal acidosis (SARA) in dairy cattle. J. Dairy Sci. 2011, 94, 351–360. [Google Scholar] [CrossRef]
- Whitt, J.; Woo, V.; Lee, P.; Moncivaiz, J.; Haberman, Y.; Denson, L.; Tso, P.; Alenghat, T. Disruption of Epithelial HDAC3 in Intestine Prevents Diet-Induced Obesity in Mice. Gastroenterology 2018, 155, 501–513. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Zhu, W.Y.; Mao, S.Y. High-concentrate feeding upregulates the expression of inflammation-related genes in the ruminal epithelium of dairy cattle. J. Anim. Sci. Biotechnol. 2016, 7, 42. [Google Scholar] [CrossRef]
- Kent-Dennis, C.; Aschenbach, J.R.; Griebel, P.J.; Penner, G.B. Effects of lipopolysaccharide exposure in primary bovine ruminal epithelial cells. J. Dairy Sci. 2020, 103, 9587–9603. [Google Scholar] [CrossRef]
- Gohlke, A.; Ingelmann, C.J.; Nurnberg, G.; Starke, A.; Wolffram, S.; Metges, C.C. Bioavailability of quercetin from its aglycone and its glucorhamnoside rutin in lactating dairy cows after intraduodenal administration. J. Dairy Sci. 2013, 96, 2303–2313. [Google Scholar] [CrossRef]
- Oleszek, M.; Pecio, Ł.; Kozachok, S.; Lachowska-Filipiuk, Z.; Oszust, K.; Frąc, M. Phytochemicals of Apple Pomace as Prospect Bio-Fungicide Agents against Mycotoxigenic Fungal Species-In Vitro Experiments. Toxins 2019, 11, 361. [Google Scholar] [CrossRef]
- Lourenco, M.; Cardozo, P.W.; Calsamiglia, S.; Fievez, V. Effects of saponins, quercetin, eugenol, and cinnamaldehyde on fatty acid biohydrogenation of forage polyunsaturated fatty acids in dual-flow continuous culture fermenters. J. Anim. Sci. 2008, 86, 3045–3053. [Google Scholar] [CrossRef]
- Maciej, J.; Schaff, C.T.; Kanitz, E.; Tuchscherer, A.; Bruckmaier, R.M.; Wolffram, S.; Hammon, H.M. Bioavailability of the flavonol quercetin in neonatal calves after oral administration of quercetin aglycone or rutin. J. Dairy Sci. 2015, 98, 3906–3917. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.Y.; Wan, Y.; Qi, M.L.; Chen, Q.H.; Sun, Y.; Sun, X.L.; Fang, J.; Fu, L.; Xu, L.; et al. Quercetin-Loaded Ceria Nanocomposite Potentiate Dual-Directional Immunoregulation via Macrophage Polarization against Periodontal Inflammation. Small 2021, 17, e2101505. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.J.; Riffelmacher, T.; Braas, D.; Cornall, R.J.; Simon, A.K. B1a B cells require autophagy for metabolic homeostasis and self-renewal. J. Exp. Med. 2018, 215, 399–413. [Google Scholar] [CrossRef]
- Furman, D.; Chang, J.L.; Lartigue, L.; Bolen, C.R.; Haddad, F.; Gaudilliere, B.; Ganio, E.A.; Fragiadakis, G.K.; Spitzer, M.H.; Douchet, I.; et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat. Med. 2017, 23, 174–184. [Google Scholar] [CrossRef]
- Jiang, M.C.; Lv, Z.Y.; Huang, Y.H.; Cheng, Z.Q.; Meng, Z.T.; Yang, T.Y.; Yan, Q.; Lin, M.; Zhan, K.; Zhao, G.Q. Quercetin Alleviates Lipopolysaccharide-Induced Inflammatory Response in Bovine Mammary Epithelial Cells by Suppressing TLR4/NF-kappa B Signaling Pathway. Front. Vet. Sci. 2022, 9, 915726. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, G.F.; Lu, S.E.; Kipen, H.; Wang, Y.D.; Hu, M.; Lin, W.W.; Rich, D.; Ohman-Strickland, P.; Diehl, S.R.; et al. Inflammatory and Oxidative Stress Responses of Healthy Young Adults to Changes in Air Quality during the Beijing Olympics. Am. J. Respir. Crit. Care 2012, 186, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.B.; Yang, M.Z.; Ju, D.H.; Jiang, H.; Zheng, J.P.; Xu, Z.H.; Li, L. Disruption of SM22 Promotes Inflammation After Artery Injury via Nuclear Factor kappa B Activation. Circ. Res. 2010, 106, 1351–1362. [Google Scholar] [CrossRef]
- Sun, A.Z.; Nie, S.J.; Xing, D. Nitric Oxide-Mediated Maintenance of Redox Homeostasis Contributes to NPR1-Dependent Plant Innate Immunity Triggered by Lipopolysaccharides. Plant Physiol. 2012, 160, 1081–1096. [Google Scholar] [CrossRef]
- Holland, W.L.; Bikman, B.T.; Wang, L.P.; Yuguang, G.; Sargent, K.M.; Bulchand, S.; Knotts, T.A.; Shui, G.H.; Clegg, D.J.; Wenk, M.R.; et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Investig. 2011, 121, 1858–1870. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Wang, J.F.; Zhang, X.; He, X.J.; Yang, B.; Wang, H.; Shan, X.F.; Li, C.Q.; Sun, D.B.; Wu, R. LPS-induced reduction of triglyceride synthesis and secretion in dairy cow mammary epithelial cells via decreased SREBP1 expression and activity. J. Dairy Res. 2018, 85, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Cao, X.T. Regulation of Toll-like receptor signaling pathways in innate immune responses. Ann. N. Y. Acad. Sci. 2013, 1283, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lei, Q.; Ma, H.; Jiang, M.; Yang, T.; Ma, Q.; Datsomor, O.; Zhan, K.; Zhao, G. Phloretin Protects Bovine Rumen Epithelial Cells from LPS-Induced Injury. Toxins 2022, 14, 337. [Google Scholar] [CrossRef] [PubMed]





| Gene | Primer Sequence, 5′ to 3′ | Accession Number | Size (bp) |
|---|---|---|---|
| GAPDH | F: GGGTCATCATCTCTGCACCT R: GGTCATAAGTCCCTCCACGA | NM_001034034.2 | 176 |
| IL-1β | F: CAGTGCCTACGCACATGTCT R: AGAGGAGGTGGAGAGCCTTC | NM_174093.1 | 209 |
| IL-6 | F: CACCCCAGGCAGACTACTTC R: TCCTTGCTGCTTTCACACTC | NM_173923.2 | 129 |
| TNF-α | F: GCCCTCTGGTTCAGACACTC R: AGATGAGGTAAAGCCCGTCA | NM_173966.3 | 192 |
| CXCL5 | F: TGAGACTGCTATCCAGCCG R: AGATCACTGACCGTTTTGGG | NM_174300.2 | 193 |
| CCL2 | F: GCTCGCTCAGCCAGATGCAA R: GGACACTTGCTGCTGGTGACTC | NM_174006 | 171 |
| CXCL2 | F: CCCGTGGTCAACGAACTGCGCTGC R: CTAGTTTAGCATCTTATCGATGATT | NM_174299.3 | 204 |
| CXCL8 | F: TGGGCCACACTGTGAAAAT R: TCATGGATCTTGCTTCTCAGC | NM_173925.2 | 136 |
| CXCL9 | F: ACTGGAGTTCAAGGAGTTCCAGCA R: TCTCACAAGAAGGGCTTGGAGCAA | NM_001113172.1 | 127 |
| CCL20 | F: TTCGACTGCTGTCTCCGATA R: GCACAACTTGTTTCACCCACT | NM_174263.2 | 172 |
| CCL28 | F: GCTTCTGGAAAGAGTGACAACGT R: AGGATGACAGCAGCCAAGTC | NM_001101163.1 | 72 |
| CXCL14 | F: AATGGTACAACGCCTGGAAC R: GTTCCAGGCGTTGTACCATT | NM_001034410.2 | 153 |
| TLR2 | F: CAGGCTTCTTCTCTGTCTTGT R: CTGTTGCCGACATAGGTGATA | NM_174197.2 | 140 |
| TLR4 | F: GACCCTTGCGTACAGGTTGT R: GGTCCAGCATCTTGGTTGAT | NM_174198.6 | 103 |
| CD14 | F: CAGTATGCTGACACAATCAA R: AGTTCCTTGAGACGAGAGTA | NM_174008.1 | 122 |
| MD2 | F: GGAGAATCGTTGGGTCTGC R: GCTCAGAACGTATTGAAACAGGA | NM_001046517.1 | 92 |
| MyD88 | F: TCATTGAGAAGAGGTGCCGT R: TGGCTTGTACTTGATGGGGAT | NM_001014382.2 | 146 |
| IRF3 | F: TTGTGAACTCAGGAGTCAGG R: TGGGCTCAAGTCCATGTCAC | NM_001029845.3 | 125 |
| IRAK1 | F: CCTCAGCGACTGGACATCCT R: GGACGTTGGAACTCTTGACATCT | NM_001040555.1 | 103 |
| NF-κB | F: AACAACCCCTTCCAAGTTCC R: CTCCCAGAGTTCCGATTCAC | NM_001080242 | 203 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.; Wang, K.; Huang, Y.; Zhang, X.; Yang, T.; Zhan, K.; Zhao, G. Quercetin Alleviates Lipopolysaccharide-Induced Cell Oxidative Stress and Inflammatory Responses via Regulation of the TLR4-NF-κB Signaling Pathway in Bovine Rumen Epithelial Cells. Toxins 2023, 15, 512. https://doi.org/10.3390/toxins15080512
Jiang M, Wang K, Huang Y, Zhang X, Yang T, Zhan K, Zhao G. Quercetin Alleviates Lipopolysaccharide-Induced Cell Oxidative Stress and Inflammatory Responses via Regulation of the TLR4-NF-κB Signaling Pathway in Bovine Rumen Epithelial Cells. Toxins. 2023; 15(8):512. https://doi.org/10.3390/toxins15080512
Chicago/Turabian StyleJiang, Maocheng, Kexin Wang, Yinghao Huang, Xuelei Zhang, Tianyu Yang, Kang Zhan, and Guoqi Zhao. 2023. "Quercetin Alleviates Lipopolysaccharide-Induced Cell Oxidative Stress and Inflammatory Responses via Regulation of the TLR4-NF-κB Signaling Pathway in Bovine Rumen Epithelial Cells" Toxins 15, no. 8: 512. https://doi.org/10.3390/toxins15080512





