Slowly Making Sense: A Review of the Two-Step Venom System within Slow (Nycticebus spp.) and Pygmy Lorises (Xanthonycticebus spp.)
Abstract
1. Introduction
- The morphology and the two components (BGE and saliva) of the slow loris venom system.
- Functional usages of the slow loris venom system.
- The toxicity and mechanisms behind the slow loris venom system.
- The possible selection pressures that have caused slow lorises to evolve venom.
- The proposal of a research agenda for future research into the slow loris venom system to aid in providing focused and structured research.
2. Morphology and Components of the Slow Loris Venom System
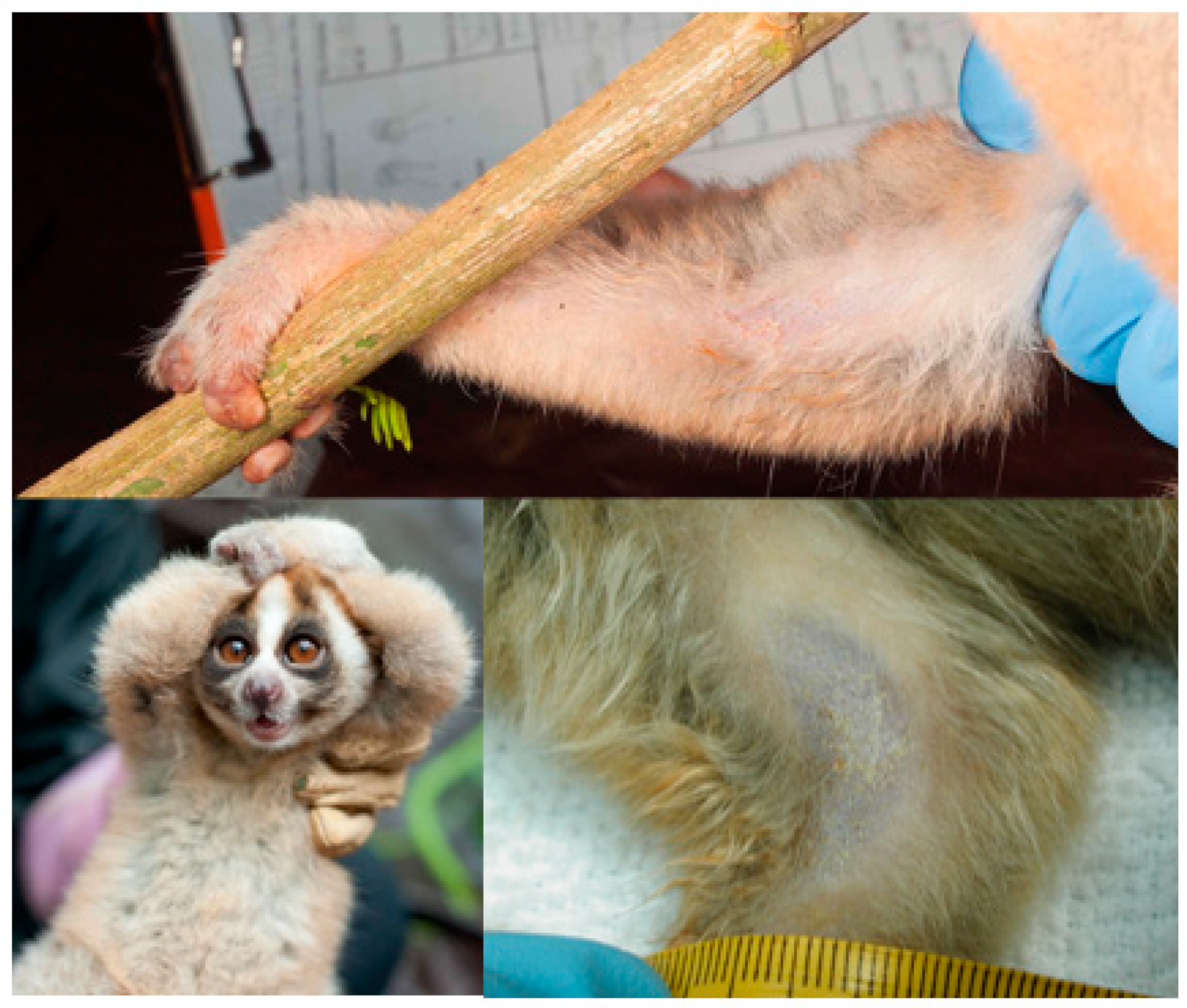
2.1. Brachial Gland
2.2. Components of the Brachial Gland
| Molecule | MW | Consensus Log P | Ali Class | Predicted LD50 (mg/kg) | Predicted Tox Class Toxicity | Potential Toxicity Endpoint | Examples Found in Other Mammal Olfactory Glands |
|---|---|---|---|---|---|---|---|
| acetic acid | 6005 | −9 | Very soluble | 333 | 1 | None | Yes, short-beaked echidna (Tachyglossus aculeatus setosus) [78] |
| benzaldehyde | 10,612 | 157 | Very soluble | 28 | 2 | Carcinogenicity | Yes, ring-tailed lemur (Lemur catta), Iberian red deer (Cervus elaphus hispanicus) and tamarins (Saguinus imperator and Leontocebus weddelli) [72,74,79] |
| m-cresol | 10,814 | 177 | Soluble | 242 | 3 | None | Yes, African bush elephant (Loxodonta africana) and Iberian red deer (Cervus elaphus hispanicus) [79,80] |
| phenol | 9411 | 141 | Very soluble | 270 | 3 | None | Yes, ring-tailed lemur (Lemur catta), African bush elephant (Loxodonta africana), Iberian red deer (Cervus elaphus hispanicus) and short-beaked echidna (Tachyglossus aculeatus setosus) [72,78,79,80] |
| 1-heptanol | 11,620 | 208 | Soluble | 1000 | 4 | None | Yes, domestic sheep (Ovis aries) [81] |
| 2-heptanol | 11,620 | 199 | Soluble | 1000 | 4 | None | N/A |
| anti-2-methyl-butyraldehyde-oxime | 10,115 | 126 | Very soluble | 2000 | 4 | Mutagenicity, Carcinogenicity | N/A |
| syn-2-methyl-butyraldehyde-oxime | 10,115 | 123 | Very soluble | 2000 | 4 | Mutagenicity, Carcinogenicity | N/A |
| n-butane | 5812 | 205 | Soluble | 2000 | 4 | none | N/A |
| dodecyl-aldehyde | 18,432 | 394 | Moderately soluble | 5000 | 5 | Estrogen Receptor Alpha (ER) activation | N/A |
| 3-octanone | 12,821 | 233 | Soluble | 5000 | 5 | Estrogen Receptor Alpha (ER) activation | N/A |
| 6-methyl-hepten-2-one | 12,620 | 207 | Very soluble | 2400 | 5 | none | N/A |
| 2-methyl-butyraldehyde | 8613 | 120 | Very soluble | 2490 | 5 | Carcinogenicity | N/A |
2.3. BGE Secretion Protein (BGEsp) Only
2.4. Saliva
3. The Functional Usage of Venom in Slow Lorises
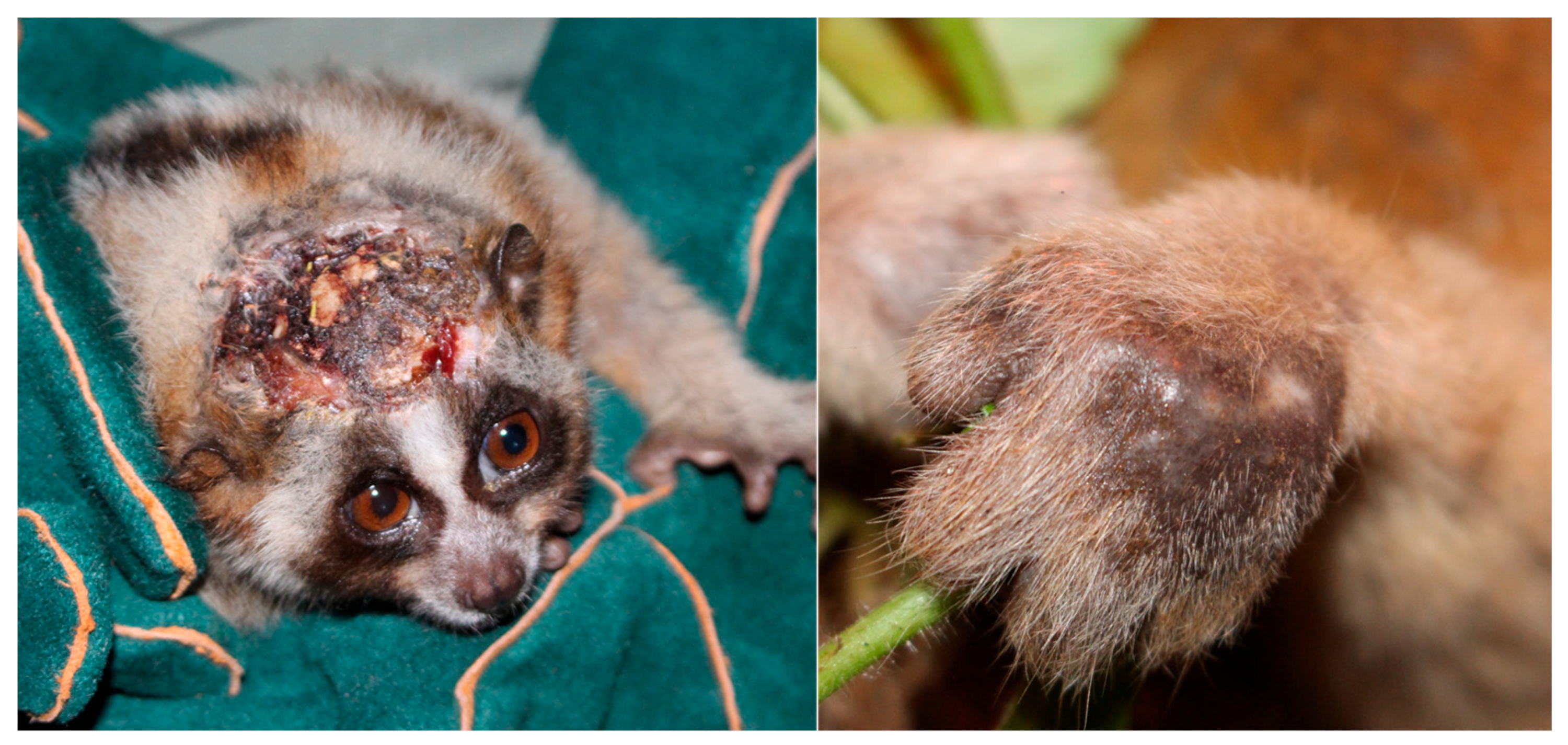
3.1. Ectoparasitic Defence
3.2. Intraspecific Competition
3.3. Intra- and Interspecific Chemical Communication Rather Than Defence
- The strong negative reaction to BGE by olfactory predators, the smell described by humans in both bite cases and research, the similarity in non-venomous slender lorises, the lack of descriptions to suggest human bite cases have venom involved and that BGE has many components found in non-venomous species (see Table 1) do suggest that venom has no role in antipredator strategies. Similar to animals like mustelids, slow loris brachial glands could produce olfactory communication that wards off predators and may communicate fitness or presence to other slow lorises.
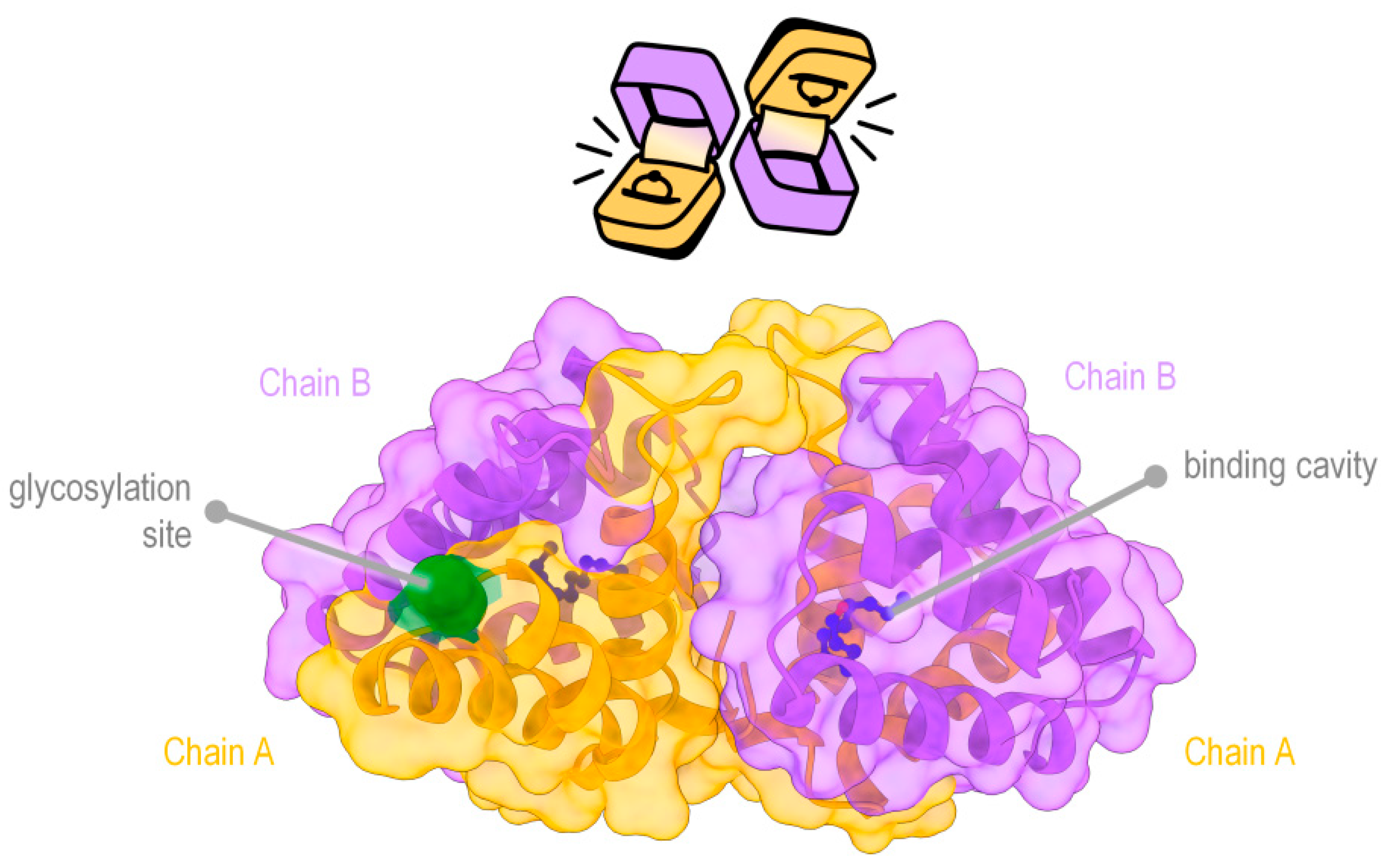
- Venom does play a role in antipredator defences, but it would be better described as intra- and interspecific communication. Referring to the similarity of BGEsp to Fel-d-1, post-transcript modification of Fel-d-1 has been observed in cats. With different types of glycosylation (a process in which different carbohydrate molecules can be bound to another molecule) of the protein depending not only on breed, age or sex but even under high levels of cortisol present within an individual cat [93]. This modification of Fel-d-1 not only alters the amount produced, but also produces different isoforms of the protein, with glycosylation being able to alter the ligand-binding cavity volume, providing additional layers of plasticity. These are assumed to have different signals, such as alerting other nearby individuals of potential stress or marking territory. It is possible that grooming near predators and licking of conspecifics in slow lorises may still be using venom in an additional functional way by using posttranslational modifications on BGEsp.
4. The Toxicity and Mechanisms behind the Slow Venom System
4.1. The Type of Toxicity
4.2. Activation of the Toxicity
4.2.1. Diet and Its Influence on Venom
4.2.2. Complement Component 1r Found within Slow Loris Saliva
4.3. Modulation or Multiple Venom Systems?
5. Why Have Slow Loris Evolved Venom?
5.1. Competition and Fitness
5.2. Coevolution with Snakes
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex Cocktails: The Evolutionary Novelty of Venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Schendel, V.; Rash, L.D.; Jenner, R.A.; Undheim, E.A.B. The Diversity of Venom: The Importance of Behavior and Venom System Morphology in Understanding Its Ecology and Evolution. Toxins 2019, 11, 666. [Google Scholar] [CrossRef] [PubMed]
- Zancolli, G.; Reijnders, M.; Waterhouse, R.M.; Robinson-Rechavi, M. Convergent Evolution of Venom Gland Transcriptomes across Metazoa. Proc. Natl. Acad. Sci. USA 2022, 119, e2111392119. [Google Scholar] [CrossRef]
- Walker, A.A. The Evolutionary Dynamics of Venom Toxins Made by Insects and Other Animals. Biochem. Soc. Trans. 2020, 48, 1353–1365. [Google Scholar] [CrossRef]
- Surm, J.M.; Moran, Y. Insights into How Development and Life-History Dynamics Shape the Evolution of Venom. EvoDevo 2021, 12, 1. [Google Scholar] [CrossRef]
- O’Connell, L.A.; LS50: Integrated Science Laboratory Course; O’Connell, J.D.; Paulo, J.A.; Trauger, S.A.; Gygi, S.P.; Murray, A.W. Rapid Toxin Sequestration Modifies Poison Frog Physiology. J. Exp. Biol. 2021, 224, jeb230342. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Arakawa, O.; Takatani, T. Toxicity of Pufferfish Takifugu rubripes Cultured in Netcages at Sea or Aquaria on Land. Comp. Biochem. Physiol. Part D Genom. Proteom. 2006, 1, 153–157. [Google Scholar] [CrossRef]
- Arbuckle, K. Evolutionary Context of Venom in Animals. In Evolution of Venomous Animals and Their Toxins; Gopalakrishnakone, P., Malhotra, A., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 1–23. ISBN 978-94-007-6727-0. [Google Scholar]
- Kazandjian, T.D.; Petras, D.; Robinson, S.D.; van Thiel, J.; Greene, H.W.; Arbuckle, K.; Barlow, A.; Carter, D.A.; Wouters, R.M.; Whiteley, G.; et al. Convergent Evolution of Pain-Inducing Defensive Venom Components in Spitting Cobras. Science 2021, 371, 386–390. [Google Scholar] [CrossRef]
- Brighton Ndandala, C.; Mustapha, U.F.; Wang, Y.; Assan, D.; Zhao, G.; Huang, C.; Mkuye, R.; Huang, H.; Li, G.; Chen, H. The Perspective of Fish Venom: An Overview of the Physiology, Evolution, Molecular and Genetics. Front. Mar. Sci. 2023, 10, 1085669. [Google Scholar] [CrossRef]
- Dashevsky, D.; Rodriguez, J. A Short Review of the Venoms and Toxins of Spider Wasps (Hymenoptera: Pompilidae). Toxins 2021, 13, 744. [Google Scholar] [CrossRef]
- Snakebite Envenoming: A Strategy for Prevention and Control; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151564-1.
- Bryan, J. From Snake Venom to ACE Inhibitor—The Discovery and Rise of Captopril. Pharm. J. 2009, 282, 455–456. [Google Scholar]
- Sukiran, N.A.; Pyati, P.; Willis, C.E.; Brown, A.P.; Readshaw, J.J.; Fitches, E.C. Enhancing the Oral and Topical Insecticidal Efficacy of a Commercialized Spider Venom Peptide Biopesticide via Fusion to the Carrier Snowdrop Lectin (Galanthus Nivalis Agglutinin). Pest Manag. Sci. 2023, 79, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, V.; Gubitosa, J.; Fini, P.; Cosma, P. Neurocosmetics in Skincare—The Fascinating World of Skin–Brain Connection: A Review to Explore Ingredients, Commercial Products for Skin Aging, and Cosmetic Regulation. Cosmetics 2021, 8, 66. [Google Scholar] [CrossRef]
- Pineda, S.S.; Chin, Y.K.-Y.; Undheim, E.A.B.; Senff, S.; Mobli, M.; Dauly, C.; Escoubas, P.; Nicholson, G.M.; Kaas, Q.; Guo, S.; et al. Structural Venomics Reveals Evolution of a Complex Venom by Duplication and Diversification of an Ancient Peptide-Encoding Gene. Proc. Natl. Acad. Sci. USA 2020, 117, 11399–11408. [Google Scholar] [CrossRef] [PubMed]
- Barua, A.; Koludarov, I.; Mikheyev, A.S. Co-Option of the Same Ancestral Gene Family Gave Rise to Mammalian and Reptilian Toxins. BMC Biol. 2021, 19, 268. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Petras, D.; Card, D.C.; Suranse, V.; Mychajliw, A.M.; Richards, D.; Koludarov, I.; Albulescu, L.-O.; Slagboom, J.; Hempel, B.-F.; et al. Solenodon Genome Reveals Convergent Evolution of Venom in Eulipotyphlan Mammals. Proc. Natl. Acad. Sci. USA 2019, 116, 25745–25755. [Google Scholar] [CrossRef]
- Folinsbee, K.E. Evolution of Venom across Extant and Extinct Eulipotyphlans. Comptes Rendus Palevol 2013, 12, 531–542. [Google Scholar] [CrossRef]
- Hargreaves, A.D.; Swain, M.T.; Hegarty, M.J.; Logan, D.W.; Mulley, J.F. Restriction and Recruitment—Gene Duplication and the Origin and Evolution of Snake Venom Toxins. Genome Biol. Evol. 2014, 6, 2088–2095. [Google Scholar] [CrossRef]
- Harris, R.J.; Arbuckle, K. Tempo and Mode of the Evolution of Venom and Poison in Tetrapods. Toxins 2016, 8, 193. [Google Scholar] [CrossRef]
- Phillips, C.D.; Baker, R.J. Secretory Gene Recruitments in Vampire Bat Salivary Adaptation and Potential Convergences with Sanguivorous Leeches. Front. Ecol. Evol. 2015, 3, 122. [Google Scholar] [CrossRef]
- Holding, M.L.; Biardi, J.E.; Gibbs, H.L. Coevolution of Venom Function and Venom Resistance in a Rattlesnake Predator and Its Squirrel Prey. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152841. [Google Scholar] [CrossRef] [PubMed]
- Verano-Braga, T.; Dutra, A.A.A.; León, I.R.; Melo-Braga, M.N.; Roepstorff, P.; Pimenta, A.M.C.; Kjeldsen, F. Moving Pieces in a Venomic Puzzle: Unveiling Post-Translationally Modified Toxins from Tityus Serrulatus. J. Proteome Res. 2013, 12, 3460–3470. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, T.B.; Clark, R.J. Advances in Venom Peptide Drug Discovery: Where Are We at and Where Are We Heading? Expert Opin. Drug Discov. 2021, 16, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Slagboom, J.; Kaal, C.; Arrahman, A.; Vonk, F.J.; Somsen, G.W.; Calvete, J.J.; Wüster, W.; Kool, J. Analytical Strategies in Venomics. Microchem. J. 2022, 175, 107187. [Google Scholar] [CrossRef]
- Calvete, J.J.; Lomonte, B.; Saviola, A.J.; Bonilla, F.; Sasa, M.; Williams, D.J.; Undheim, E.A.B.; Sunagar, K.; Jackson, T.N.W. Mutual Enlightenment: A Toolbox of Concepts and Methods for Integrating Evolutionary and Clinical Toxinology via Snake Venomics and the Contextual Stance. Toxicon X 2021, 9–10, 100070. [Google Scholar] [CrossRef]
- León, G.; Vargas, M.; Segura, Á.; Herrera, M.; Villalta, M.; Sánchez, A.; Solano, G.; Gómez, A.; Sánchez, M.; Estrada, R.; et al. Current Technology for the Industrial Manufacture of Snake Antivenoms. Toxicon 2018, 151, 63–73. [Google Scholar] [CrossRef]
- Rathore, A.S.; Kumar, R.; Tiwari, O.S. Recent Advancements in Snake Antivenom Production. Int. J. Biol. Macromol. 2023, 240, 124478. [Google Scholar] [CrossRef]
- Haney, R.A.; Ayoub, N.A.; Clarke, T.H.; Hayashi, C.Y.; Garb, J.E. Dramatic Expansion of the Black Widow Toxin Arsenal Uncovered by Multi-Tissue Transcriptomics and Venom Proteomics. BMC Genom. 2014, 15, 366. [Google Scholar] [CrossRef]
- Rao, W.; Kalogeropoulos, K.; Allentoft, M.E.; Gopalakrishnan, S.; Zhao, W.; Workman, C.T.; Knudsen, C.; Jiménez-Mena, B.; Seneci, L.; Mousavi-Derazmahalleh, M.; et al. The Rise of Genomics in Snake Venom Research: Recent Advances and Future Perspectives. GigaScience 2022, 11, giac024. [Google Scholar] [CrossRef]
- Lüddecke, T.; Paas, A.; Harris, R.J.; Talmann, L.; Kirchhoff, K.N.; Billion, A.; Hardes, K.; Steinbrink, A.; Gerlach, D.; Fry, B.G.; et al. Venom Biotechnology: Casting Light on Nature’s Deadliest Weapons Using Synthetic Biology. Front. Bioeng. Biotechnol. 2023, 11, 1166601. [Google Scholar] [CrossRef]
- Rogalski, A.; Himaya, S.W.A.; Lewis, R.J. Coordinated Adaptations Define the Ontogenetic Shift from Worm- to Fish-Hunting in a Venomous Cone Snail. Nat. Commun. 2023, 14, 3287. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Jin, P.; Zhang, Y.; Shen, Y.; Wang, W.; Li, S. Genomic and Transcriptomic Analyses Support a Silk Gland Origin of Spider Venom Glands. BMC Biol. 2023, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Tang, X.; Chen, W.; Jiang, X.; Chen, Z.; He, K.; Li, Q.; Duan, Z.; He, X.; Kamau, P.M.; et al. Shrew’s Venom Quickly Causes Circulation Disorder, Analgesia and Hypokinesia. Cell. Mol. Life Sci. 2022, 79, 35. [Google Scholar] [CrossRef] [PubMed]
- Ligabue-Braun, R. Venom Use in Mammals: Evolutionary Aspects. In Evolution of Venomous Animals and Their Toxins; Gopalakrishnakone, P., Malhotra, A., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 1–23. ISBN 978-94-007-6727-0. [Google Scholar]
- Rode-Margono, J.E.; Nekaris, K.A.-I. Cabinet of Curiosities: Venom Systems and Their Ecological Function in Mammals, with a Focus on Primates. Toxins 2015, 7, 2639–2658. [Google Scholar] [CrossRef]
- Dufton, M.J. Venomous Mammals. Pharmacol. Ther. 1992, 53, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Barua, A.; Mikheyev, A.S. An Ancient, Conserved Gene Regulatory Network Led to the Rise of Oral Venom Systems. Proc. Natl. Acad. Sci. USA 2021, 118, e2021311118. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, L.L.J.; Nijman, V.; Ligabue-Braun, R.; Nekaris, K.A.-I. The Fast and the Furriest: Investigating the Rate of Selection on Mammalian Toxins. Toxins 2022, 14, 842. [Google Scholar] [CrossRef]
- Kowalski, K.; Rychlik, L. Venom Use in Eulipotyphlans: An Evolutionary and Ecological Approach. Toxins 2021, 13, 231. [Google Scholar] [CrossRef]
- Hanf, Z.R.; Chavez, A.S. A Comprehensive Multi-Omic Approach Reveals a Relatively Simple Venom in a Diet Generalist, the Northern Short-Tailed Shrew. Blarina Brevicauda Genome Biol. Evol. 2020, 12, 1148–1166. [Google Scholar] [CrossRef]
- Grigorev, K.; Kliver, S.; Dobrynin, P.; Komissarov, A.; Wolfsberger, W.; Krasheninnikova, K.; Afanador-Hernández, Y.M.; Brandt, A.L.; Paulino, L.A.; Carreras, R.; et al. Innovative Assembly Strategy Contributes to Understanding the Evolution and Conservation Genetics of the Endangered Solenodon Paradoxus from the Island of Hispaniola. GigaScience 2018, 7, giy025. [Google Scholar] [CrossRef]
- Rychlik, L. Changes in Prey Size Preferences during Successive Stages of Foraging in the Mediterranean Water Shrew Neomys anomalus. Behaviour 1999, 136, 345–365. [Google Scholar] [CrossRef]
- Kowalski, K.; Marciniak, P.; Rosiński, G.; Rychlik, L. Evaluation of the Physiological Activity of Venom from the Eurasian Water Shrew Neomys fodiens. Front. Zool. 2017, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, K.; Marciniak, P.; Rychlik, L. A New, Widespread Venomous Mammal Species: Hemolytic Activity of Sorex Araneus Venom Is Similar to That of Neomys fodiens Venom. Zool. Lett. 2022, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Low, D.H.W.; Sunagar, K.; Undheim, E.A.B.; Ali, S.A.; Alagon, A.C.; Ruder, T.; Jackson, T.N.W.; Pineda Gonzalez, S.; King, G.F.; Jones, A.; et al. Dracula’s Children: Molecular Evolution of Vampire Bat Venom. J. Proteom. 2013, 89, 95–111. [Google Scholar] [CrossRef]
- Tellgren-Roth, Å.; Dittmar, K.; Massey, S.E.; Kemi, C.; Tellgren-Roth, C.; Savolainen, P.; Lyons, L.A.; Liberles, D.A. Keeping the Blood Flowing—Plasminogen Activator Genes and Feeding Behavior in Vampire Bats. Naturwissenschaften 2009, 96, 39–47. [Google Scholar] [CrossRef]
- Nekaris, K.A.-I.; Moore, R.S.; Rode, E.J.; Fry, B.G. Mad, Bad and Dangerous to Know: The Biochemistry, Ecology and Evolution of Slow Loris Venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 21. [Google Scholar] [CrossRef]
- Blair, M.E.; Cao, G.T.H.; López-Nandam, E.H.; Veronese-Paniagua, D.A.; Birchette, M.G.; Kenyon, M.; Md-Zain, B.M.; Munds, R.A.; Nekaris, K.A.-I.; Nijman, V.; et al. Molecular Phylogenetic Relationships and Unveiling Novel Genetic Diversity among Slow and Pygmy Lorises, Including Resurrection of Xanthonycticebus intermedius. Genes 2023, 14, 643. [Google Scholar] [CrossRef]
- Nekaris, K.A.I.; Jaffe, S. Unexpected Diversity of Slow Lorises (Nycticebus spp.) within the Javan Pet Trade: Implications for Slow Loris Taxonomy. Contrib. Zool. 2007, 76, 187–196. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Wilson, D.E. Handbook of the Mammals of the World: Volume 3. Primates. Lynx Edicions: Cerdanyola del Vallès, Spain, 2013. [Google Scholar]
- Munds, R.A.; Nekaris, K.A.I.; Ford, S.M. Taxonomy of the Bornean Slow Loris, with New Species Nycticebus kayan (Primates, Lorisidae). Am. J. Primatol. 2013, 75, 46–56. [Google Scholar] [CrossRef]
- Streicher, U.; Wilson, A.; Collins, R.L.; Nekaris, K.A.-I. Exudates and Animal Prey Characterize Slow Loris (Nycticebus pygmaeus, N. coucang and N. javanicus) Diet in Captivity and After Release into the Wild. In Leaping Ahead; Masters, J., Gamba, M., Génin, F., Eds.; Springer: New York, NY, USA, 2012; pp. 165–172. ISBN 978-1-4614-4510-4. [Google Scholar]
- Poindexter, S.A.; Nekaris, K.A.I. Vertical Clingers and Gougers: Rapid Acquisition of Adult Limb Proportions Facilitates Feeding Behaviours in Young Javan Slow Lorises (Nycticebus javanicus). Mamm. Biol. 2017, 87, 40–49. [Google Scholar] [CrossRef]
- Starr, C.; Nekaris, K.A.I. Obligate Exudativory Characterizes the Diet of the Pygmy Slow Loris Nycticebus pygmaeus: Pygmy Loris Diet in Cambodia. Am. J. Primatol. 2013, 75, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Nekaris, K.A.I. Extreme Primates: Ecology and Evolution of Asian Lorises. Evol. Anthropol. Issues News Rev. 2014, 23, 177–187. [Google Scholar] [CrossRef]
- Munds, R.A.; Titus, C.L.; Moreira, L.A.A.; Eggert, L.S.; Blomquist, G.E. Examining the Molecular Basis of Coat Color in a Nocturnal Primate Family (Lorisidae). Ecol. Evol. 2021, 11, 4442–4459. [Google Scholar] [CrossRef] [PubMed]
- Nekaris, K.A.-I.; Weldon, A.; Imron, M.A.; Maynard, K.Q.; Nijman, V.; Poindexter, S.A.; Morcatty, T.Q. Venom in Furs: Facial Masks as Aposematic Signals in a Venomous Mammal. Toxins 2019, 11, 93. [Google Scholar] [CrossRef]
- Nekaris, K.A.-I.; Campera, M.; Watkins, A.R.; Weldon, A.V.; Hedger, K.; Morcatty, T.Q. Aposematic Signaling and Seasonal Variation in Dorsal Pelage in a Venomous Mammal. Ecol. Evol. 2021, 11, 11387–11397. [Google Scholar] [CrossRef]
- Ruf, T.; Streicher, U.; Stalder, G.L.; Nadler, T.; Walzer, C. Hibernation in the Pygmy Slow Loris (Nycticebus pygmaeus): Multiday Torpor in Primates Is Not Restricted to Madagascar. Sci. Rep. 2015, 5, 17392. [Google Scholar] [CrossRef] [PubMed]
- Geerah, D.R.; O’Hagan, R.P.; Wirdateti, W.; Nekaris, K.A.I. The Use of Ultrasonic Communication to Maintain Social Cohesion in the Javan Slow Loris (Nycticebus javanicus). Folia Primatol. 2019, 90, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Alterman, L. Toxins and Toothcombs: Potential Allospecific Chemical Defenses in Nycticebus and Perodicticus. In Creatures of the Dark: The Nocturnal Prosimians; Alterman, L., Doyle, G.A., Izard, M.K., Eds.; Springer: Boston, MA, USA, 1995; pp. 413–424. ISBN 978-1-4757-2405-9. [Google Scholar]
- Scheib, H.; Nekaris, K.A.-I.; Rode-Margono, J.; Ragnarsson, L.; Baumann, K.; Dobson, J.S.; Wirdateti, W.; Nouwens, A.; Nijman, V.; Martelli, P.; et al. The Toxicological Intersection between Allergen and Toxin: A Structural Comparison of the Cat Dander Allergenic Protein Fel D1 and the Slow Loris Brachial Gland Secretion Protein. Toxins 2020, 12, 86. [Google Scholar] [CrossRef]
- Gardiner, M.; Weldon, A.; Poindexter, S.A.; Gibson, N.; Nekaris, K.A.I. Survey of Practitioners Handling Slow Lorises (Primates: Nycticebus): An Assessment of the Harmful Effects of Slow Loris Bites. J. Venom. Res. 2018, 9, 1–7. [Google Scholar]
- Grow, N.B.; Wirdateti; Nekaris, K.A.I. Does Toxic Defence in Nycticebus spp. Relate to Ectoparasites? The Lethal Effects of Slow Loris Venom on Arthropods. Toxicon 2015, 95, 1–5. [Google Scholar] [CrossRef]
- Nekaris, K.A.I.; Campera, M.; Nijman, V.; Birot, H.; Rode-Margono, E.J.; Fry, B.G.; Weldon, A.; Wirdateti, W.; Imron, M.A. Slow Lorises Use Venom as a Weapon in Intraspecific Competition. Curr. Biol. 2020, 30, R1252–R1253. [Google Scholar] [CrossRef] [PubMed]
- Drea, C.M. Design, Delivery and Perception of Condition-Dependent Chemical Signals in Strepsirrhine Primates: Implications for Human Olfactory Communication. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190264. [Google Scholar] [CrossRef] [PubMed]
- Nekaris, K.A.-I.; Pimley, E.R.; Ablard, K.M. Predator Defense by Slender Lorises and Pottos. In Primate Anti-Predator Strategies; Gursky, S.L., Nekaris, K.A.I., Eds.; Developments in Primatology: Progress and Prospects; Springer: Boston, MA, USA, 2007; pp. 222–240. ISBN 978-0-387-34810-0. [Google Scholar]
- Montagna, W.; Yasuda, K.; Ellis, R.A. The Skin of Primates. III. The Skin of the Slow Loris (Nycticebus coucang). Am. J. Phys. Anthropol. 1961, 19, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Montagna, W. The Skin of Nonhuman Primates. Am. Zool. 1972, 12, 109–124. [Google Scholar] [CrossRef]
- Scordato, E.S.; Dubay, G.; Drea, C.M. Chemical Composition of Scent Marks in the Ringtailed Lemur (Lemur catta): Glandular Differences, Seasonal Variation, and Individual Signatures. Chem. Senses 2007, 32, 493–504. [Google Scholar] [CrossRef]
- Laska, M.; Seibt, A.; Weber, A. ‘Microsmatic’ Primates Revisited: Olfactory Sensitivity in the Squirrel Monkey. Chem. Senses 2000, 25, 47–53. [Google Scholar] [CrossRef]
- Poirier, A.C.; Waterhouse, J.S.; Watsa, M.; Erkenswick, G.A.; Moreira, L.A.A.; Tang, J.; Dunn, J.C.; Melin, A.D.; Smith, A.C. On the Trail of Primate Scent Signals: A Field Analysis of Callitrichid Scent-gland Secretions by Portable Gas Chromatography-mass Spectrometry. Am. J. Primatol. 2021, 83, e23236. [Google Scholar] [CrossRef]
- Setchell, J.M.; Vaglio, S.; Moggi-Cecchi, J.; Boscaro, F.; Calamai, L.; Knapp, L.A. Chemical Composition of Scent-Gland Secretions in an Old World Monkey (Mandrillus sphinx): Influence of Sex, Male Status, and Individual Identity. Chem. Senses 2010, 35, 205–220. [Google Scholar] [CrossRef]
- Hagey, L.R.; Fry, B.G.; Fitch-Snyder, H. Talking Defensively, a Dual Use for the Brachial Gland Exudate of Slow and Pygmy Lorises. In Primate Anti-Predator Strategies; Gursky, S.L., Nekaris, K.A.I., Eds.; Developments in Primatology: Progress and Prospects; Springer: Boston, MA, USA, 2007; pp. 253–272. ISBN 978-0-387-34807-0. [Google Scholar]
- Ligabue-Braun, R.; Verli, H.; Carlini, C.R. Venomous Mammals: A Review. Toxicon 2012, 59, 680–695. [Google Scholar] [CrossRef]
- Harris, R.L.; Davies, N.W.; Nicol, S.C. Chemical Composition of Odorous Secretions in the Tasmanian Short-Beaked Echidna (Tachyglossus aculeatus setosus). Chem. Senses 2012, 37, 819–836. [Google Scholar] [CrossRef]
- De La Peña, E.; Martín, J.; Carranza, J. The Intensity of Male-Male Competition May Affect Chemical Scent Constituents in the Dark Ventral Patch of Male Iberian Red Deer. PLoS ONE 2019, 14, e0221980. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Garcia, A.; Foote, C.S. Some Chemical Constituents of the Secretion from the Temporal Gland of the African Elephant (Loxodonta africana). J. Chem. Ecol. 1978, 4, 17–25. [Google Scholar] [CrossRef]
- Rajagopal, T.; Mahalakshmi, S.; Gayathri, T.R.; Muruganantham, N.; Muthukatturaja, M.; Rajesh, D.; Rameshkumar, K.; Ponmanickam, P.; Akbarsha, M.A.; Archunan, G. Histomorphology and Chemical Constituents of Interdigital Gland of Vembur Sheep, Ovis aries. Vet. Sci. 2022, 9, 647. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Krane, S.; Itagaki, Y.; Nakanishi, K.; Weldon, P.J. “Venom” of the Slow Loris: Sequence Similarity of Prosimian Skin Gland Protein and Fel d 1 Cat Allergen. Naturwissenschaften 2003, 90, 60–62. [Google Scholar] [CrossRef]
- Ligabue-Braun, R. Hello, Kitty: Could Cat Allergy Be a Form of Intoxication? J. Venom. Anim. Toxins Incl. Trop. Dis 2020, 26, e20200051. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Jackson, B.C.; Thompson, D.C.; Wright, M.W.; McAndrews, M.; Bernard, A.; Nebert, D.W.; Vasiliou, V. Update of the Human Secretoglobin (SCGB) Gene Superfamily and an Example of “evolutionary Bloom” of Androgen-Binding Protein Genes within the Mouse Scgb Gene Superfamily. Hum. Genom. 2011, 5, 691. [Google Scholar] [CrossRef]
- Mootz, M.; Jakwerth, C.A.; Schmidt-Weber, C.B.; Zissler, U.M. Secretoglobins in the Big Picture of Immunoregulation in Airway Diseases. Allergy 2022, 77, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Janssen-Weets, B.; Kerff, F.; Swiontek, K.; Kler, S.; Czolk, R.; Revets, D.; Kuehn, A.; Bindslev-Jensen, C.; Ollert, M.; Hilger, C. Mammalian Derived Lipocalin and Secretoglobin Respiratory Allergens Strongly Bind Ligands with Potentially Immune Modulating Properties. Front. Allergy 2022, 3, 958711. [Google Scholar] [CrossRef] [PubMed]
- Grönlund, H.; Saarne, T.; Gafvelin, G.; van Hage, M. The Major Cat Allergen, Fel d 1, in Diagnosis and Therapy. Int. Arch. Allergy Immunol. 2009, 151, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Ligabue-Braun, R.; Sachett, L.G.; Pol-Fachin, L.; Verli, H. The Calcium Goes Meow: Effects of Ions and Glycosylation on Fel d 1, the Major Cat Allergen. PLoS ONE 2015, 10, e0132311. [Google Scholar] [CrossRef][Green Version]
- Madani, G.; Nekaris, K.A.-I. Anaphylactic Shock Following the Bite of a Wild Kayan Slow Loris (Nycticebus kayan): Implications for Slow Loris Conservation. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 43. [Google Scholar] [CrossRef]
- Aprilia, W. Proteomics Characterisation of Bioactive Components from Javan Slow Loris (Nycticebus javanicus) Saliva. Master’s Thesis, University of Queensland, Brisbane, Australia, 2015. [Google Scholar]
- Tandler, B.; Pinkstaff, C.A.; Nagato, T.; Phillips, C.J. Giant Secretory Granules in the Ducts of the Parotid and Submandibular Glands of the Slow Loris. Tissue Cell 1996, 28, 321–329. [Google Scholar] [CrossRef]
- Thamadilok, S.; Choi, K.-S.; Ruhl, L.; Schulte, F.; Kazim, A.L.; Hardt, M.; Gokcumen, O.; Ruhl, S. Human and Nonhuman Primate Lineage-Specific Footprints in the Salivary Proteome. Mol. Biol. Evol. 2020, 37, 395–405. [Google Scholar] [CrossRef]
- Behringer, V.; Borchers, C.; Deschner, T.; Möstl, E.; Selzer, D.; Hohmann, G. Measurements of Salivary Alpha Amylase and Salivary Cortisol in Hominoid Primates Reveal within-Species Consistency and Between-Species Differences. PLoS ONE 2013, 8, e60773. [Google Scholar] [CrossRef]
- Cabana, F.; Dierenfeld, E.; Wirdateti, W.; Donati, G.; Nekaris, K.A.I. Slow Lorises (Nycticebus spp.) Really Are Slow: A Study of Food Passage Rates. Int. J. Primatol. 2017, 38, 900–913. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Kucharska, E.; Kowalczyk, T.; Zajdel, K.; Cegliński, T.; Zajdel, R. Antioxidant Properties of Plant-Derived Phenolic Compounds and Their Effect on Skin Fibroblast Cells. Antioxidants 2021, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Nekaris, K.A.I.; Bhattacharjee, P.C. Medicinal Plant Exudativory by the Bengal Slow Loris Nycticebus bengalensis. Endanger. Species Res. 2014, 23, 149–157. [Google Scholar] [CrossRef]
- Fuller, G.; Lukas, K.; Kuhar, C.; Dennis, P. A Retrospective Review of Mortality in Lorises and Pottos in North American Zoos, 1980–2010. Endanger. Species Res. 2014, 23, 205–217. [Google Scholar] [CrossRef]
- Fuller, G.; Eggen, W.F.; Wirdateti, W.; Nekaris, K.A.I. Welfare Impacts of the Illegal Wildlife Trade in a Cohort of Confiscated Greater Slow Lorises, Nycticebus coucang. J. Appl. Anim. Welf. Sci. 2018, 21, 224–238. [Google Scholar] [CrossRef]
- Burrows, A.M.; Hartstone-Rose, A.; Nash, L.T. Exudativory in the Asian Loris, Nycticebus: Evolutionary Divergence in the Toothcomb and M3. Am. J. Phys. Anthropol. 2015, 158, 663–672. [Google Scholar] [CrossRef]
- Gillespie, T.R.; Chapman, C.A. Prediction of Parasite Infection Dynamics in Primate Metapopulations Based on Attributes of Forest Fragmentation. Conserv. Biol. 2006, 20, 441–448. [Google Scholar] [CrossRef]
- Solórzano-García, B.; Pérez-Ponce de León, G. Parasites of Neotropical Primates: A Review. Int. J. Primatol. 2018, 39, 155–182. [Google Scholar] [CrossRef]
- Ni, Q.; Dong, S.; Fan, Y.; Wan, W.; Teng, P.; Zhu, S.; Liang, X.; Xu, H.; Yao, Y.; Zhang, M.; et al. Molecular Epidemiology of Blastocystis in Confined Slow Lorises, Macaques, and Gibbons. Animals 2022, 12, 2992. [Google Scholar] [CrossRef]
- Frias, L.; Stark, D.J.; Lynn, M.S.; Nathan, S.K.; Goossens, B.; Okamoto, M.; MacIntosh, A.J.J. Lurking in the Dark: Cryptic Strongyloides in a Bornean Slow Loris. Int. J. Parasitol. Parasites Wildl. 2018, 7, 141–146. [Google Scholar] [CrossRef]
- Ikeda, Y.; Fujisaki, A.; Murata, K.; Hasegawa, H. Redescription of Pterygodermatites (Mesopectines) nycticebi (Mönnig, 1920) (Nematoda: Rictulariidae), a Parasite of Slow Loris Nycticebus coucang (Mammalia: Primates). Folia Parasitol. 2003, 50, 115–120. [Google Scholar] [CrossRef]
- Rode-Margono, E.J.; Albers, M.; Wirdateti, W.; Abinawanto, A.; Nekaris, K.A.I. Gastrointestinal Parasites and Ectoparasites in Wild Javan Slow Loris (Nycticebus javanicus), and Implications for Captivity and Animal Rescue. J. Zoo Aquar. Res. 2015, 3, 80–86. [Google Scholar] [CrossRef]
- Nixon, S.A.; Robinson, S.D.; Agwa, A.J.; Walker, A.A.; Choudhary, S.; Touchard, A.; Undheim, E.A.B.; Robertson, A.; Vetter, I.; Schroeder, C.I.; et al. Multipurpose Peptides: The Venoms of Amazonian Stinging Ants Contain Anthelmintic Ponericins with Diverse Predatory and Defensive Activities. Biochem. Pharmacol. 2021, 192, 114693. [Google Scholar] [CrossRef] [PubMed]
- Broeckhoven, C. Intraspecific Competition: A Missing Link in Dermal Armour Evolution? J. Anim. Ecol. 2022, 91, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Itescu, Y.; Schwarz, R.; Meiri, S.; Pafilis, P. Intraspecific Competition, Not Predation, Drives Lizard Tail Loss on Islands. J. Anim. Ecol. 2016, 86, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.C.; Hughes, C.; Rogers, T.L. Effects of Intraspecific Competition and Body Mass on Diet Specialization in a Mammalian Scavenger. Ecol. Evol. 2022, 12, e8338. [Google Scholar] [CrossRef]
- Pafilis, P.; Meiri, S.; Foufopoulos, J.; Valakos, E. Intraspecific Competition and High Food Availability Are Associated with Insular Gigantism in a Lizard. Naturwissenschaften 2009, 96, 1107–1113. [Google Scholar] [CrossRef]
- Ashwood, L.M.; Norton, R.S.; Undheim, E.A.B.; Hurwood, D.A.; Prentis, P.J. Characterising Functional Venom Profiles of Anthozoans and Medusozoans within Their Ecological Context. Mar. Drugs 2020, 18, 202. [Google Scholar] [CrossRef]
- Rasmussen, D. A Review of Bite Wounds in Lorises. Duke Lemur Center: Durham, NC, USA, 1984; Unpublished Document. [Google Scholar]
- Barrett, M.; Campera, M.; Morcatty, T.Q.; Weldon, A.V.; Hedger, K.; Maynard, K.Q.; Imron, M.A.; Nekaris, K.A.I. Risky Business: The Function of Play in a Venomous Mammal—The Javan Slow Loris (Nycticebus javanicus). Toxins 2021, 13, 318. [Google Scholar] [CrossRef]
- Wiens, F. Behavior and Ecology of Wild Slow Lorises (Nycticebus coucang): Social Organization, Infant Care System, and Diet. Ph.D. Thesis, University of Bayreuth, Bayreuth, Germany, 2002. [Google Scholar]
- Fuller, G.; Nijman, V.; Wirdateti, W.; Nekaris, K.A.I. Do Chemical Cues in the Venom of Slow Lorises Repel Avian Predators? EMU 2016, 116, 435–439. [Google Scholar] [CrossRef]
- Hardus, M.E.; Lameira, A.R.; Zulfa, A.; Atmoko, S.S.U.; de Vries, H.; Wich, S.A. Behavioral, Ecological, and Evolutionary Aspects of Meat-Eating by Sumatran Orangutans (Pongo abelii). Int. J. Primatol. 2012, 33, 287–304. [Google Scholar] [CrossRef]
- Utami, S.S.; van Hooff, J.A.R.A.M. Meat-Eating by Adult Female Sumatran Orangutans (Pongo pygmæus abelii). Am. J. Primatol. 1997, 43, 159–165. [Google Scholar] [CrossRef]
- Makur, K.P.; Utami-Atmoko, S.S.; Setia, T.M.; van Noordwijk, M.A.; Vogel, E.R. Slow Loris (Nycticebus borneanus) Consumption by a Wild Bornean Orangutan (Pongo pygmaeus wurmbii). Primates 2022, 63, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Angulo, Y.; Lomonte, B. Biochemistry and Toxicology of Toxins Purified from the Venom of the Snake Bothrops asper. Toxicon 2009, 54, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Simpson, G.M.; Fuller, G.; Lukas, K.E.; Kuhar, C.W.; Fitch-Snyder, H.; Taylor, J.; Dennis, P.M. Sources of Morbidity in Lorises and Pottos in North American Zoos: A Retrospective Review, 1980–2010. Zoo Biol. 2018, 37, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, M. The Cytotoxic Effect of Slow Loris (Nycticebus) Venom on Human Epidermal Carcinoma Cells; Oxford Brookes University: Oxford, UK, 2017. [Google Scholar]
- Bal, A.K.; Giordano, A.J.; Gouda, S. Effects of a Bengal Slow Loris Nycticebus bengalensis (Primates: Lorisidae) Bite: A Case Study from Murlen National Park, Mizoram, India. J. Threat. Taxa 2022, 14, 21449–21452. [Google Scholar] [CrossRef]
- Inoue, F.; Inoue, A.; Tsuboi, T.; Ichikawa, T.; Suga, M.; Ishihara, S.; Nakayama, S. Severe Anaphylactic Shock Following a Slow Loris Bite in a Patient with Cat Allergy. Intern. Med. 2021, 60, 3037–3039. [Google Scholar] [CrossRef]
- Utap, M.S.; Jamal, M.S.B.M. Anaphylactic Shock Following a Bite of a Wild Kayan Slow Loris (Nycticebus kayan) in Rural Sarawak, Malaysian Borneo. Rural Remote Health 2019, 19, 3. [Google Scholar] [CrossRef]
- Fung, H.; Wong, O. Clinical Quiz: A Potentially Toxic Primate Bite. Hong Kong J. Emerg. Med. 2016, 23, 301–303. [Google Scholar] [CrossRef]
- Wilde, H. Anaphylactic Shock Following Bite by a ‘Slow Loris’, Nycticebus coucang. Am. J. Trop. Med. Hyg. 1972, 21, 592–594. [Google Scholar] [CrossRef]
- Raab, W.P. Renal Enzyme Excretion Following Anaphylactic Shock. Nature 1966, 212, 953. [Google Scholar] [CrossRef]
- Licá, I.C.L.; Soares, A.M.d.S.; de Mesquita, L.S.S.; Malik, S. Biological Properties and Pharmacological Potential of Plant Exudates. Food Res. Int. 2018, 105, 1039–1053. [Google Scholar] [CrossRef] [PubMed]
- Nussinovitch, A. Plant Gum Exudates of the World: Sources, Distribution, Properties, and Applications; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-1-4200-5224-4. [Google Scholar]
- Power, M.L.; Myers, E.W. Digestion in the Common Marmoset (Callithrix Jacchus), a Gummivore-Frugivore. Am. J. Primatol. 2009, 71, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Dewi, T.; Imron, M.A.; Lukmandaru, G.; Hedger, K.; Campera, M.; Nekaris, K.A.I. The Sticky Tasty: The Nutritional Content of the Exudativorous Diet of the Javan Slow Loris in a Lowland Forest. Primates 2022, 63, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Swapna, N.; Radhakrishna, S.; Gupta, A.; Kumar, A. Exudativory in the Bengal Slow Loris (Nycticebus bengalensis) in Trishna Wildlife Sanctuary, Tripura, Northeast India. Am. J. Primatol. 2009, 72, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W. Final Report of the Safety Assessment of Acacia catechu Gum, Acacia concinna Fruit Extract, Acacia dealbata Leaf Extract, Acacia dealbata Leaf Wax, Acacia decurrens Extract, Acacia farnesiana Extract, Acacia farnesiana Flower Wax, Acacia farnesiana Gum, Acacia senegal Extract, Acacia senegal Gum, and Acacia senegal Gum Extract. Int. J. Toxicol. 2005, 24, 75–118. [Google Scholar] [CrossRef]
- Majee, S.K.; Bera, K.; Raja, W.; Ghosh, K.; Ray, S.; Ray, B. Structural Highlights of an Antioxidative Arabinogalactan Protein of Lannea grandis Gum That Stabilizes β-Lactoglobulin. Food Hydrocoll. 2016, 61, 720–729. [Google Scholar] [CrossRef]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary PH: A Diagnostic Biomarker. J. Indian Soc. Periodontol. 2013, 17, 461–465. [Google Scholar] [CrossRef]
- Sánchez, G.A.; Fernandez De Preliasco, M.V. Salivary PH Changes during Soft Drinks Consumption in Children. Int. J. Paediatr. Dent. 2003, 13, 251–257. [Google Scholar] [CrossRef]
- Ramírez-Torres, C.E.; Espinosa-Gómez, F.C.; Morales-Mávil, J.E.; Reynoso-Cruz, J.E.; Laska, M.; Hernández-Salazar, L.T. Influence of Tannic Acid Concentration on the Physicochemical Characteristics of Saliva of Spider Monkeys (Ateles Geoffroyi). PeerJ 2022, 10, e14402. [Google Scholar] [CrossRef]
- Saporito, R.A.; Donnelly, M.A.; Spande, T.F.; Garraffo, H.M. A Review of Chemical Ecology in Poison Frogs. Chemoecology 2012, 22, 159–168. [Google Scholar] [CrossRef]
- Savitzky, A.H.; Mori, A.; Hutchinson, D.A.; Saporito, R.A.; Burghardt, G.M.; Lillywhite, H.B.; Meinwald, J. Sequestered Defensive Toxins in Tetrapod Vertebrates: Principles, Patterns, and Prospects for Future Studies. Chemoecology 2012, 22, 141–158. [Google Scholar] [CrossRef]
- Li, M.-L.; Wang, S.; Xu, P.; Tian, H.-Y.; Bai, M.; Zhang, Y.-P.; Shao, Y.; Xiong, Z.-J.; Qi, X.-G.; Cooper, D.N.; et al. Functional Genomics Analysis Reveals the Evolutionary Adaptation and Demographic History of Pygmy Lorises. Proc. Natl. Acad. Sci. USA 2022, 119, e2123030119. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Shafaghat, F.; Kovanen, P.T.; Meri, S. Mast Cells and Complement System: Ancient Interactions between Components of Innate Immunity. Allergy 2020, 75, 2818–2828. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, H. An Overview of Complement Systems in Teleosts. Dev. Comp. Immunol. 2022, 137, 104520. [Google Scholar] [CrossRef] [PubMed]
- Sim, R.; Tsiftsoglou, S. Proteases of the Complement System. Biochem. Soc. Trans. 2004, 32, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Rother, K.; Till, G.O. The Complement System; Springer Science & Business Media: Berlin, Germany, 2012; ISBN 978-3-642-97038-2. [Google Scholar]
- Vogel, C.-W.; Fritzinger, D.C. Cobra Venom Factor: Structure, Function, and Humanization for Therapeutic Complement Depletion. Toxicon 2010, 56, 1198–1222. [Google Scholar] [CrossRef]
- Cochrane, C.G.; Müller-Eberhard, H.J.; Aikin, B.S. Depletion of Plasma Complement in Vivo by a Protein of Cobra Venom: Its Effect on Various Immunologic Reactions1. J. Immunol. 1970, 105, 55–69. [Google Scholar] [CrossRef]
- Dutertre, S.; Jin, A.-H.; Vetter, I.; Hamilton, B.; Sunagar, K.; Lavergne, V.; Dutertre, V.; Fry, B.G.; Antunes, A.; Venter, D.J.; et al. Evolution of Separate Predation- and Defence-Evoked Venoms in Carnivorous Cone Snails. Nat. Commun. 2014, 5, 3521. [Google Scholar] [CrossRef]
- Fischer, M.L.; Wielsch, N.; Heckel, D.G.; Vilcinskas, A.; Vogel, H. Context-Dependent Venom Deployment and Protein Composition in Two Assassin Bugs. Ecol. Evol. 2020, 10, 9932–9947. [Google Scholar] [CrossRef]
- Walker, A.A.; Mayhew, M.L.; Jin, J.; Herzig, V.; Undheim, E.A.B.; Sombke, A.; Fry, B.G.; Meritt, D.J.; King, G.F. The Assassin Bug Pristhesancus Plagipennis Produces Two Distinct Venoms in Separate Gland Lumens. Nat. Commun. 2018, 9, 755. [Google Scholar] [CrossRef]
- Kazandjian, T.D.; Hamilton, B.R.; Robinson, S.D.; Hall, S.R.; Bartlett, K.E.; Rowley, P.; Wilkinson, M.C.; Casewell, N.R.; Undheim, E.A.B. Physiological Constraints Dictate Toxin Spatial Heterogeneity in Snake Venom Glands. BMC Biol. 2022, 20, 148. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, G.A. The Role of Antlers in the Behaviour of Red Deer. J. Exp. Zool. 1972, 182, 233–249. [Google Scholar] [CrossRef]
- Mayor, P.; Mamani, J.; Montes, D.; González-Crespo, C.; Sebastián, M.A.; Bowler, M. Proximate Causes of the Red Face of the Bald Uakari Monkey (Cacajao calvus). R. Soc. Open Sci. 2015, 2, 150145. [Google Scholar] [CrossRef] [PubMed]
- Setchell, J.M.; Smith, T.; Wickings, E.J.; Knapp, L.A. Social Correlates of Testosterone and Ornamentation in Male Mandrills. Horm. Behav. 2008, 54, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Candolin, U. Male-Male Competition Ensures Honest Signaling of Male Parental Ability in the Three-Spined Stickleback (Gasterosteus aculeatus). Behav. Ecol. Sociobiol. 2000, 49, 57–61. [Google Scholar] [CrossRef]
- Palaoro, A.V.; Peixoto, P.E.C. The Hidden Links between Animal Weapons, Fighting Style, and Their Effect on Contest Success: A Meta-Analysis. Biol. Rev. 2022, 97, 1948–1966. [Google Scholar] [CrossRef]
- Soares, S.C.; Lindström, B.; Esteves, F.; Öhman, A. The Hidden Snake in the Grass: Superior Detection of Snakes in Challenging Attentional Conditions. PLoS ONE 2014, 9, e114724. [Google Scholar] [CrossRef]
- Meno, W.; Coss, R.G.; Perry, S. Development of Snake-Directed Antipredator Behavior by Wild White-Faced Capuchin Monkeys: I. Snake-Species Discrimination: Development of Snake Discrimination. Am. J. Primatol. 2013, 75, 281–291. [Google Scholar] [CrossRef]
- Harris, R.J.; Nekaris, K.A.-I.; Fry, B.G. Monkeying around with Venom: An Increased Resistance to α-Neurotoxins Supports an Evolutionary Arms Race between Afro-Asian Primates and Sympatric Cobras. BMC Biol. 2021, 19, 253. [Google Scholar] [CrossRef]
- Isbell, L.A. Snakes as Agents of Evolutionary Change in Primate Brains. J. Hum. Evol. 2006, 51, 1–35. [Google Scholar] [CrossRef]
- Ashby, J. Dismissing Australian Mammals as Weird Hurts Efforts to Conserve Them. Available online: https://www.newscientist.com/article/mg25433860-500-dismissing-australian-mammals-as-weird-hurts-efforts-to-conserve-them/ (accessed on 7 July 2023).
- Nekaris, K.A.I.; Starr, C.R.; Collins, R.L.; Wilson, A. Comparative ecology of exudate feeding by lorises (Nycticebus, Loris) and pottos (Perodicticus, Arctocebus). In The Evolution of Exudativory in Primates; Springer: Berlin/Heidelberg, Germany, 2010; pp. 155–168. [Google Scholar]
- Pliosungnoen, M.; Savani, T. Spatial and Feeding Behavior of the Endangered Bengal Slow Loris, Nycticebus bengalensis in Khao Angrunai Wildlife Sanctuary, Thailand. Primate Society of Great Britain: Bristol, UK, 2008; Unpublish. [Google Scholar]
- Wiens, F.; Zitzmann, A.; Hussein, N.A. Fast food for slow lorises: Is low metabolism related to secondary compounds in high-energy plant diet? J. Mammal. 2006, 87, 790–798. [Google Scholar] [CrossRef]
- Rode-Margono, E.J.; Rademaker, M.; Wirdateti Strijkstra, A.; Nekaris, K.A.I. Noxious arthropods as potential prey of the venomous Javan slow loris (Nycticebus javanicus) in a West Javan volcanic agricultural system. J. Nat. Hist. 2015, 49, 1949–1959. [Google Scholar] [CrossRef]
- Anirudh, N.; Brown, E.; Sanchez, K.; Irpiandi, I. Sexual Differences in Feeding and Foraging of Released Philippine Slow Loris (Nycticebus menagensis). In Evolution, Ecology and Conservation of Lorises and Pottos; Nekaris, K., Burrows, A., Eds.; Cambridge University Press: Cambridge, UK, 2020; pp. 219–227. [Google Scholar] [CrossRef]
- Zofou, D.; Kuete, V.; Titanji, V.P. Antimalarial and other antiprotozoal products from African medicinal plants. Med. Plant Res. Afr. 2013, 661–709. [Google Scholar]
- Woo, H.; Kim, H.; Shin, S.; Shin, J.H.; Ryu, D.; Park, D.; Jung, E. Rhus semialata M. extract ameliorate para-phenylenediamine-induced toxicity in keratinocytes. Toxicol. Rep. 2021, 8, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Widiyarti, G.; Tiara, Y. Antioxidant Activity and Toxicity of Puspa (Schima wallichii) Leaves Extract from Indonesia. J. Trop. Life Sci. 2018, 8. [Google Scholar] [CrossRef]
- Attanayake, A.P. Toxicological investigation of Spondias pinnata (Linn. F.) Kurz.(Family: Anacardiaceae) bark extract in Wistar rats. Int. J. Green Pharm. (IJGP) 2015, 9, 26–31. [Google Scholar] [CrossRef]
- Sireeratawong, S.; Jaijoy, K.; Panunto, W.; Nanna, U.; Lertprasertsuke, N.; Soonthornchareonnon, N. Acute and chronic toxicity studies of the water extract from dried fruits of Terminalia bellerica (Gaertn.) Roxb. in Spargue-Dawley rats. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 223–231. [Google Scholar] [CrossRef]
- EL-Taher, E.M.; El-Sherei, M.M.; El Dine, R.S.; ElNaggar, D.M.; Khalil, W.K.; Kassem, S.M.; Elkhateeb, A.; Kassem, M.E. Acacia pennata L. leaves: Chemical profiling and impact on DNA damage, alteration of genotoxicity—Related genes expression and ROS generation in hepatic tissues of acetaminophen treated male rats. Adv. Tradit. Med. 2021, 22, 221–229. [Google Scholar] [CrossRef]
- Allahyari, S.; Delazar, A.; Najafi, M. Evaluation of general toxicity, anti-oxidant activity and effects of Ficus carica leaves extract on ischemia/reperfusion injuries in isolated heart of rat. Adv. Pharm. Bull. 2014, 4 (Suppl. 2), 577. [Google Scholar]
- Aneja, K.R.; Sharma, C.; Joshi, R. Antimicrobial activity of Terminalia arjuna Wight & Arn.: An ethnomedicinal plant against pathogens causing ear infection. Braz. J. Otorhinolaryngol. 2012, 78, 68–74. [Google Scholar]
- Anwar, R.; Hajardhini, P. Antibacterial Activity of Gallic Acid from the Leaves of Altingia excelsa Noronha to Enterococcus faecalis. Open Access Maced. J. Med. Sci. 2022, 10, 1–6. [Google Scholar] [CrossRef]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H.; Mahajan, R.T. Traditional uses, phytochemistry and pharmacology of Ficus carica: A review. Pharm. Biol. 2014, 52, 1487–1503. [Google Scholar] [CrossRef]
- Padgaonkar, A.V.; Suryavanshi, S.V.; Londhe, V.Y.; Kulkarni, Y.A. Acute toxicity study and anti-nociceptive activity of Bauhinia acuminata Linn. Leaf extracts in experimental animal models. Biomed. Pharmacother. 2018, 97, 60–66. [Google Scholar] [CrossRef]
- Mani, S.; Duraipandian, C.; Chidambaram, S.B. Analgesic, anti-inflammatory and acute oral toxicity profile of leaf and bark extracts of Albizia procera. BMC Complement. Med. Ther. 2022, 22, 1–9. [Google Scholar] [CrossRef]
- de Oliveira, A.M.; do Nascimento, M.F.; Ferreira, M.R.A.; de Moura, D.F.; dos Santos Souza, T.G.; da Silva, G.C.; da Silva Ramos, E.H.; Paiva, P.M.G.; de Medeiros, P.L.; da Silva, T.G.; et al. Evaluation of acute toxicity, genotoxicity and inhibitory effect on acute inflammation of an ethanol extract of Morus alba L.(Moraceae) in mice. J. Ethnopharmacol. 2016, 194, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Panda, N.; Kumar, S.; Banerjee, S.; Mandal, N.B.; Sahu, N.P. A triterpenoid saponin possessing antileishmanial activity from the leaves of Careya arborea. Phytochemistry 2006, 67, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Piletta, P.A.; Hausen, B.M.; Pasche-Koo, F.; French, L.E.; Saurat, J.H.; Hauser, C. Allergic contact dermatitis to east Indian rosewood (Dalbergia latifolia Roxb.). J. Am. Acad. Dermatol. 1996, 34, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.S.; Tiwari, A.K.; Reddy, S.V.; Aparna, P.; Rao, R.J.; Ali, A.Z.; Rao, J.M. Free-Radical-Scavenging and Xanthine Oxidase Inhibitory Constituents from Stereospermum personatum. J. Nat. Prod. 2005, 68, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Das, M.C.; Das, N.; Bhattacharjee, S. Evaluation of the antileishmanial potency, toxicity and phytochemical constituents of methanol bark extract of Sterculia villosa. Pharm. Biol. 2017, 55, 998–1009. [Google Scholar] [CrossRef]
- Costa, B.A.; de Oliveira, J.M.; Sales, P.A.; Lira, S.R.D.S.; Silva, S.M.D.S.; Costa, L.M.; Muratori, M.C.; Costa, A.P. Systemic and reproductive toxicity induced by Parkia platycephala ethanolic extract in female Wistar rats. Rev. Bras. Farmacogn. 2013, 23, 920–926. [Google Scholar] [CrossRef]
- Dwevedi, A.; Sharma, K.; Sharma, Y.K. Cadamba: A miraculous tree having enormous pharmacological implications. Pharmacogn. Rev. 2015, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.S.; da Silva, G.S.; Hilel, A.S.; Carvalho, A.C.; Remor, K.V.; Schlindwein, A.D.; Kanis, L.A.; Martins, D.F.; Kviecinski, M.R. Study of the potential adverse effects caused by the dermal application of Dillenia indica L. fruit extract standardized to betulinic acid in rodents. PLoS ONE 2019, 14, e0217718. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.; Divya, B.; Mary, R.A.; Viji, M.H.; Kalaichelvan, V.K.; Palanivel, V. Anti–ulcer activity of Ficus religiosa leaf ethanolic extract. Asian Pac. J. Trop. Biomed. 2013, 3, 554–556. [Google Scholar] [CrossRef]
- Gupta, S.; Singh, S.; Gupta, R.; Gurjeet Singh, T. Pharmacological and Phytochemical Updates on Pothos scandens L. Pharmacogn. Commun. 2018, 8. [Google Scholar] [CrossRef]
- Hafid, A.F.; Permanasari, A.A.; Tumewu, L.; Adianti, M.; Aoki, C.; Widyawaruyanti, A.; Lusida, M.I.; Hotta, H. Activities of Ficus fistulosa leave extract and fractions against Hepatitis C Virus. Procedia Chem. 2016, 18, 179–184. [Google Scholar] [CrossRef][Green Version]
- Saadullah, M.; Asif, M.; Arif, S.; Kanwal, B. A Comprehensive Review on Traditional Uses, Chemical Constituents, and Diverse Pharmacological Importance of the Genus Breynia. Rec. Nat. Prod. 2022, 6, 538–549. [Google Scholar]
- Kumar, K.A.; Rao, B.G.; Prabhakar, T. Evaluation of Acute Toxicity of Plant Gum of Lannea Coromandelica in Mice. Indian J. Res. Pharm. Biotechnol. (IJRPB) 2019, 7. [Google Scholar]
- Sultana, N.; Chung, H.J.; Emon, N.U.; Alam, S.; Taki, M.T.I.; Rudra, S.; Tahamina, A.; Alam, R.; Ahmed, F.; Mamun, A.A. Biological functions of Dillenia pentagyna Roxb. Against pain, inflammation, fever, diarrhea, and thrombosis: Evidenced from in vitro, in vivo, and molecular docking study. Front. Nutr. 2022, 9, 911274. [Google Scholar] [CrossRef]
- Lala, M. Characterization of Firmiana colorata (Roxb.) leaf extract and its silver nanoparticles reveal their antioxidative, anti-microbial, and anti-inflammatory properties. Int. Nano Lett. 2023, 1. Online ahead of print. [Google Scholar]
- Poivre, M.; Duez, P. Biological activity and toxicity of the Chinese herb Magnolia officinalis Rehder & E. Wilson (Houpo) and its constituents. J. Zhejiang Univ. Sci. B 2017, 18, 194. [Google Scholar]
- Jafari, M.; Naeini, K.M.; Lorigooini, Z.; Namjoo, R. Oral acute and sub-acute toxic effects of hydroalcoholic Terminalia chebula Retz and Achillea wilhelmsii extracts in BALB/c mice. BioMedicine 2019, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Jørs, E. The prevalence of skin and mucosal symptoms in gardeners handling Ficus benjamina (weeping fig) and Hedera helix (ivy). A cross-sectional study. Ugeskr. Laeger 2003, 165, 3526–3529. [Google Scholar] [PubMed]
- Khalid, M.; Alqarni, M.H.; Shoaib, A.; Arif, M.; Foudah, A.I.; Afzal, O.; Ali, A.; Ali, A.; Alqahtani, S.S.; Altamimi, A.S. Anti-arthritic and anti-inflammatory potential of Spondias mangifera extract fractions: An in silico, in vitro and in vivo approach. Plants 2021, 10, 825. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, V.; Pandey, K.; Kapil, A.; Singh, N.; Samant, M.; Dube, A. In vitro and in vivo leishmanicidal activity of Dysoxylum binectariferum and its fractions against Leishmania donovani. Phytomedicine 2007, 14, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, Y.; Zheng, J. Dioscorea bulbifera L.-induced hepatotoxicity and involvement of metabolic activation of furanoterpenoids. Drug Metab. Rev. 2020, 52, 568–584. [Google Scholar] [CrossRef] [PubMed]
- Lončarić, M.; Strelec, I.; Moslavac, T.; Šubarić, D.; Pavić, V.; Molnar, M. Lipoxygenase inhibition by plant extracts. Biomolecules 2021, 11, 152. [Google Scholar] [CrossRef]
- Malik, F.Z.A.; Allaudin, Z.N.; Loh, H.S.; Nee, T.K.; Hani, H.; Abdullah, R. Antiviral and virucidal activities of Duabanga grandiflora leaf extract against Pseudorabies virus in vitro. BMC Complement. Altern. Med. 2016, 16, 1–10. [Google Scholar] [CrossRef][Green Version]
- Mat, N.B.; Assim, Z.B.; Ahmad, F.B. Chemical Characterization and Biological Activities of Methanol Extract from Castanopsis megacarpa Seeds of Sarawak. Trans. Sci. Technol. 2017, 4, 330–335. [Google Scholar]
- Méndez, M.D.C.; Elias, F.; Riet-Correa, F.; Gimeno, E.J.; Portiansky, E.L. Intoxicação experimental com frutos de Melia azedarach (Meliaceae) em suínos. Pesqui. Veterinária Bras. 2006, 26, 26–30. [Google Scholar] [CrossRef][Green Version]
- Paritala, V.; Chiruvella, K.K.; Thammineni, C.; Ghanta, R.G.; Mohammed, A. Phytochemicals and antimicrobial potentials of mahogany family. Rev. Bras. Farmacogn. 2015, 25, 61–83. [Google Scholar] [CrossRef]
- Paul, S.; Ali, M.Y.; Rumpa, N.E.; Tanvir, E.M.; Hossen, M.S.; Saha, M.; Bhoumik, N.C.; Gan, S.H.; Khalil, M.I. Assessment of toxicity and beneficiary effects of Garcinia pedunculata on the hematological, biochemical, and histological homeostasis in rats. Evid.-Based Complement. Altern. Med. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Pelfrène, A.F. Rodenticides. In Hayes’ Handbook of Pesticide Toxicology; Academic Press: Cambridge, MA, USA, 2010; pp. 2153–2217. [Google Scholar]
- Pereira, V.D.J.; Kaplan, M.A.C. Artocarpus: Um gênero exótico de grande bioatividade. Floresta Ambiente 2013, 20, 1–15. [Google Scholar] [CrossRef]
- Puja, S.D.; Shahriar, K.R.; Hasan, C.M.; Ahsan, M. Flavonoids from the Leaves of Bridelia stipularis with In Vitro Antioxidant and Cytotoxicity Activity. Pharmacol. Pharm. 2020, 11, 137–146. [Google Scholar] [CrossRef]
- Rajput, D.; Patil, U.K.; Chauhan, D.N.; Shah, K.; Chauhan, N.S. Potentials of natural products in vector-borne diseases management: Current and future perspectives. In Natural Products in Vector-Borne Disease Management; Academic Press: Cambridge, MA, USA, 2023; pp. 1–25. [Google Scholar]
- Senapati, A.K.; Swain, S.R.; Satyanarayana, S. Toxicological Studies of the Hydroalcoholic Extract of Pterospermum Acerifolium Flowers. Pharmacologyonline 2009, 1221–1227. [Google Scholar]
- Shirsat, P.; AR, Z.; Kashikar, R.; Athavale, M.; Athavale, T.; Taware, P.; Saldanha, T.; Kolhe, S.; Tembhurne, S. Subacute toxicity study of the ethanolic extract of Mesua ferrea (L.) flowers in rats. Drug Chem. Toxicol. 2022, 45, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Siddhuraju, P.; Becker, K.; Makkar, H.P.S. Chemical composition, protein fractionation, essential amino acid potential and antimetabolic constituents of an unconventional legume, Gila bean (Entada phaseoloides Merrill) seed kernel. J. Sci. Food Agric. 2002, 82, 192–202. [Google Scholar] [CrossRef]
- Sireeratawong, S.; Thamaree, S.; Ingkaninan, K.; Piyabhan, P.; Vannasiri, S.; Khonsung, P.; Singhalak, T.; Jaijoy, K. Evaluation of acute and subacute oral toxicity of the ethanol extract from Antidesma Acidum Retz. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 465–469. [Google Scholar] [CrossRef][Green Version]
- Sobeh, M.; Mahmoud, M.F.; Hasan, R.A.; Abdelfattah, M.A.; Osman, S.; Rashid, H.O.; El-Shazly, A.M.; Wink, M. Chemical composition, antioxidant and hepatoprotective activities of methanol extracts from leaves of Terminalia bellirica and Terminalia sericea (Combretaceae). PeerJ 2019, 7, e6322. [Google Scholar] [CrossRef]
- Yorsin, S.; Sriwiriyajan, S.; Chongsa, W. Vasorelaxing effect of Garcinia cowa leaf extract in rat thoracic aorta and its underlying mechanisms. J. Tradit. Complement. Med. 2023, 13, 219–225. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, J.J.; Xu, J.; Feng, F.; Qu, W. Medicinal uses, phytochemistry and pharmacology of the genus Uncaria. J. Ethnopharmacol. 2015, 173, 48–80. [Google Scholar] [CrossRef]
- Nasrin, M.; Dash, P.R.; Ali, M.S. In Vitro antibacterial and in Vivo cytotoxic activities of Grewia paniculata. Avicenna J. Phytomedicine 2015, 5, 98. [Google Scholar]
- Jakobsson, H.E.; Jernberg, C.; Andersson, A.F.; Sjölund-Karlsson, M.; Jansson, J.K.; Engstrand, L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS ONE 2010, 5, e9836. [Google Scholar] [CrossRef]
- Marinova, G.; Batchvarov, V. Evaluation of the methods for determination of the free radical scavenging activity by DPPH. Bulg. J. Agric. Sci. 2011, 17, 11–24. [Google Scholar]
- Tedong, L.; Dzeufiet, P.D.D.; Dimo, T.; Asongalem, E.A.; Sokeng, S.D.; Flejou, J.F.; Callard, P.; Kamtchouing, P. Acute and subchronic toxicity of Anacardium occidentale Linn (Anacardiaceae) leaves hexane extract in mice. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 140–147. [Google Scholar] [CrossRef]
- Rohmawati, D.; Sutoyo, S. Steroid isolated from the dichlorometane extract of matoa’s stem bark (Pometia pinnata) and toxicity tests against Artemia salina Leach. In Seminar Nasional Kimia-National Seminar on Chemistry (SNK 2018); Atlantis Press: Amsterdam, The Netherlands, 2018; pp. 103–105. [Google Scholar]
- Siriyong, T.; Ontong, J.C.; Leejae, S.; Suwalak, S.; Coote, P.J.; Voravuthikunchai, S.P. In vivo safety assessment of rhodomyrtone, a potent compound, from Rhodomyrtus tomentosa leaf extract. Toxicol. Rep. 2020, 7, 919–924. [Google Scholar] [CrossRef]
- Chuah, L.O.; Yeap, S.K.; Ho, W.Y.; Beh, B.K.; Alitheen, N.B. In Vitro and In Vivo toxicity of Garcinia or hydroxycitric acid: A review. Evid.-Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Subarnas, A.; Diantini, A.; Abdulah, R.; Zuhrotun, A.; Yamazaki, C.; Nakazawa, M.; Koyama, H. Antiproliferative activity of primates-consumed plants against MCF-7 human breast cancer cell lines. E3 J. Med. Res. 2012, 1, 38–43. [Google Scholar]
- Stauffer, F.W.; Siegert, S.; Silberbauer-Gottsberger, I.; Gottsberger, G. Floral structure in the Asian palm Eugeissona tristis Griff.(Arecaceae: Calamoideae), and description of a new nectary type in the family. Plant Syst. Evol. 2016, 302, 629–639. [Google Scholar] [CrossRef]
- Ebbo, A.A.; Sani, D.; Suleiman, M.M.; Ahmad, A.; Hassan, A.Z. Acute and sub-chronic toxicity evaluation of the crude methanolic extract of Diospyros mespiliformis hochst ex a. Dc (ebenaceae) and its fractions. Toxicol. Rep. 2020, 7, 1138–1144. [Google Scholar] [CrossRef]
- Brenner, S.A.; Romeo, J.T. Fungitoxic effects of nonprotein imino acids on growth of saprophytic fungi isolated from the leaf surface of Calliandra haematocephala. Appl. Environ. Microbiol. 1986, 51, 690–693. [Google Scholar] [CrossRef]
- Saptarini, N.M.; Mustarichie, R. Acute toxicity of ethanolic extract of hantap (Sterculia urceolata JE Smith) leaves. Drug Invent. Today 2020, 14. [Google Scholar]
- Ragasa, C.Y.; Ng, V.A.S.; De Los Reyes, M.M.; Mandia, E.H.; Oyong, G.G.; Shen, C.C. Chemical constituents and cytotoxicity of the leaves of Dysoxylum gaudichaudianum (A. Juss.) Miq. Der Pharma Chem. 2014, 6, 182–187. [Google Scholar]
- Asuquo, E.G.; Udobi, C.E. Antibacterial and toxicity studies of the ethanol extract of Musa paradisiaca leaf. Cogent Biol. 2016, 2, 1219248. [Google Scholar] [CrossRef]
- Hasan, M.R.; Mondal, M.; Sweilam, S.H.; Hossain, M.M.; Hanif, M.A.; Islam, S.M.S.; Roy, A.K.; Hasan, M.M.; Sarkar, C.; Mondal, K.R.; et al. Acute and Sub-chronic Toxicity Evaluations of Mallotus repandus Stem Methanol Extract in Female Sprague-Dawley Rats. Res. Sq. 2022, preprint. [Google Scholar]
- Hsu, H.Y.; Lin, C.C.; Chen, J.Y.; Yang, J.J.; Zhang, R. Toxic effects of Erycibe obtusifolia, a Chinese medicinal herb, in mice. J. Ethnopharmacol. 1998, 62, 101–105. [Google Scholar] [CrossRef]
- Nair, R.R. Evaluation of acute and sub-acute oral toxicity of ethanolic root extract of Tetracera akara (Burm. f.) Merr., an ethnomedicinal plant used by the Kani tribe of Kerala. J. Tradit. Folk. Pract. 2018, 5. [Google Scholar] [CrossRef]
- Waleguele, C.C.; Mba’ning, B.M.; Awantu, A.F.; Bankeu, J.J.; Fongang, Y.S.; Ngouela, A.S.; Tsamo, E.; Sewald, N.; Lenta, B.N.; Krause, R.W. Antiparasitic constituents of Beilschmiedia louisii and Beilschmiedia obscura and some semisynthetic derivatives (Lauraceae). Molecules 2020, 25, 2862. [Google Scholar] [CrossRef]
- Jansakul, C.; Jusapalo, N.; Mahattanadul, S. Hypotensive effect of n-butanol extract from stem of Salacia chinensis in rats. Acta Hortic. 2005, 678, 107–114. [Google Scholar] [CrossRef]
- Du, M.; Huang, S.; Zhang, J.; Wang, J.; Hu, L.; Jiang, J. Toxicolological test of saponins from Sapindus mukorossi Gaerth. Open J. For. 2015, 5, 749. [Google Scholar]
- Lin, T.J.; Hsu, C.I.; Lee, K.H.; Shiu, L.L.; Deng, J.F. Two outbreaks of acute Tung Nut (Aleurites fordii) poisoning. J. Toxicol. Clin. Toxicol. 1996, 34, 87–92. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitzpatrick, L.L.J.; Ligabue-Braun, R.; Nekaris, K.A.-I. Slowly Making Sense: A Review of the Two-Step Venom System within Slow (Nycticebus spp.) and Pygmy Lorises (Xanthonycticebus spp.). Toxins 2023, 15, 514. https://doi.org/10.3390/toxins15090514
Fitzpatrick LLJ, Ligabue-Braun R, Nekaris KA-I. Slowly Making Sense: A Review of the Two-Step Venom System within Slow (Nycticebus spp.) and Pygmy Lorises (Xanthonycticebus spp.). Toxins. 2023; 15(9):514. https://doi.org/10.3390/toxins15090514
Chicago/Turabian StyleFitzpatrick, Leah Lucy Joscelyne, Rodrigo Ligabue-Braun, and K. Anne-Isola Nekaris. 2023. "Slowly Making Sense: A Review of the Two-Step Venom System within Slow (Nycticebus spp.) and Pygmy Lorises (Xanthonycticebus spp.)" Toxins 15, no. 9: 514. https://doi.org/10.3390/toxins15090514
APA StyleFitzpatrick, L. L. J., Ligabue-Braun, R., & Nekaris, K. A.-I. (2023). Slowly Making Sense: A Review of the Two-Step Venom System within Slow (Nycticebus spp.) and Pygmy Lorises (Xanthonycticebus spp.). Toxins, 15(9), 514. https://doi.org/10.3390/toxins15090514





