Polyvalent Snake Antivenoms: Production Strategy and Their Therapeutic Benefits
Abstract
:1. Introduction
2. Various Parameters Involved in the Production of Polyvalent AVs and the pAV Characteristics
2.1. The Number of Venoms to Be Included in the Production of a pAV
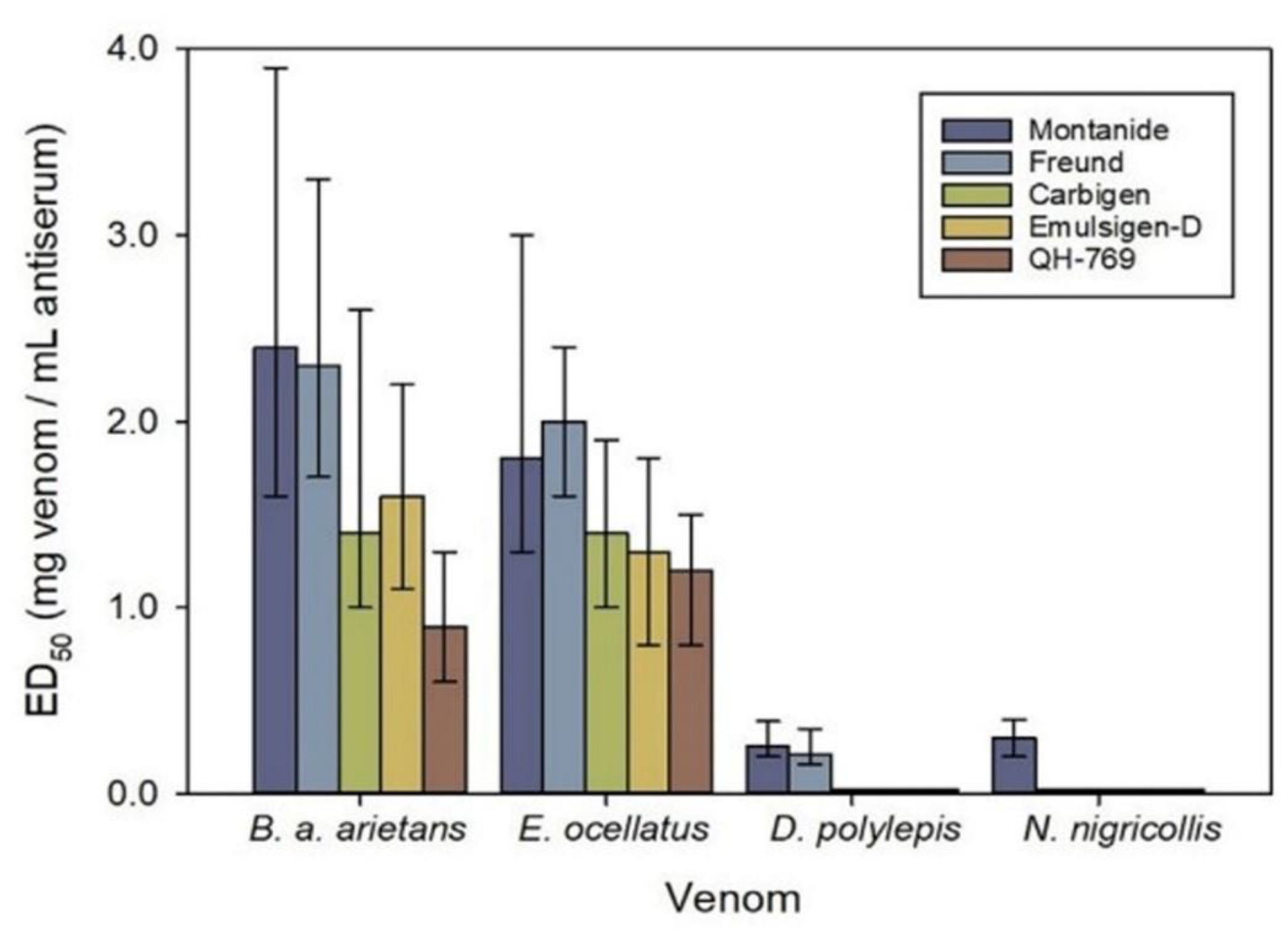
2.2. Use of Crude Venoms or Toxin Fractions
2.3. Antagonism and Synergism between the Constituent Venoms Used in pAV Production
2.4. Production of ‘Mixed pAVs’
2.5. pAVs with Combined Anti-Elapid and Anti-Viperid Activities
2.6. Paraspecificity of pAVs
2.7. The Benefits and Advantages of pAVs
3. Syndromic Polyvalent Antivenoms (s-pAVs)
3.1. The Paraspecificity of s-pAVs
3.2. Possibility of Wider Paraspecificity in the Syndromic pAVs
3.3. Future Prospects for Producing ‘Universal s-pAVs’
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Chippaux, J.P. Snake-bites: Appraisal of the global situation. Bull. World Health Organ. 1998, 76, 515–524. [Google Scholar] [PubMed]
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef]
- Ganthavorn, S. Toxicities of Thailand snake venoms and neutralization capacity of antivenin. Toxicon 1969, 7, 239–241. [Google Scholar] [CrossRef]
- Ake-umpan, S. Snake bite envenoming. In Annual Epidemiological Surveillance Report; Department of Disease Control, Ministry of Public Health: Bangkok, Thailand, 2018; pp. 26–218. ISBN 978-616-11-4161-5. [Google Scholar]
- Hawgood, B.J. Pioneers of anti-venomous serotherapy: Dr Vital Brazil (1865–1950). Toxicon 1992, 30, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Chotwiwatthanakun, C.; Pratanaphon, R.; Akesowan, S.; Sriprapat, S.; Ratanabanangkoon, K. Production of potent polyvalent antivenom against three elapid venoms using a low dose, low volume, multi-site immunization protocol. Toxicon 2001, 39, 1487–1494. [Google Scholar] [CrossRef]
- Laing, G.D.; Renjifo, J.M.; Ruiz, F.; Harrison, R.A.; Nasidi, A.; Gutierrez, J.M.; Rowley, P.D.; Warrell, D.A.; Theakston, R.D. A new Pan African polyspecific antivenom developed in response to the antivenom crisis in Africa. Toxicon 2003, 42, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Dos-Santos, M.C.; Arroyo, C.; Solano, S.; Herrera, M.; Villalta, M.; Segura, A.; Estrada, R.; Gutierrez, J.M.; Leon, G. Comparison of the effect of Crotalus simus and Crotalus durissus ruruima venoms on the equine antibody response towards Bothrops asper venom: Implications for the production of polyspecific snake antivenoms. Toxicon 2011, 57, 237–243. [Google Scholar] [CrossRef]
- Ramos-Cerrillo, B.; de Roodt, A.R.; Chippaux, J.P.; Olguin, L.; Casasola, A.; Guzman, G.; Paniagua-Solis, J.; Alagon, A.; Stock, R.P. Characterization of a new polyvalent antivenom (Antivipmyn Africa) against African vipers and elapids. Toxicon 2008, 52, 881–888. [Google Scholar] [CrossRef]
- Fox, J.W.; Serrano, S.M. Exploring snake venom proteomes: Multifaceted analyses for complex toxin mixtures. Proteomics 2008, 8, 909–920. [Google Scholar] [CrossRef]
- Hunt, J.D.; Jackson, D.C.; Wood, P.R.; Stewart, D.J.; Brown, L.E. Immunological parameters associated with antigenic competition in a multivalent footrot vaccine. Vaccine 1995, 13, 1649–1657. [Google Scholar] [CrossRef]
- Ratanabanangkoon, K.; Tan, K.Y.; Eursakun, S.; Tan, C.H.; Simsiriwong, P.; Pamornsakda, T.; Wiriyarat, W.; Klinpayom, C.; Tan, N.H. A Simple and Novel Strategy for the Production of a Pan-specific Antiserum against Elapid Snakes of Asia. PLoS Negl. Trop. Dis. 2016, 10, e0004565. [Google Scholar] [CrossRef]
- Ratanabanangkoon, K. A Quest for a Universal Plasma-Derived Antivenom Against All Elapid Neurotoxic Snake Venoms. Front. Immunol. 2021, 12, 668328. [Google Scholar] [CrossRef]
- Li, Q.; Ownby, C.L. Evaluation of four different immunogens for the production of snake antivenoms. Toxicon 1992, 30, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Ratanabanangkoon, K.; Tan, K.Y.; Pruksaphon, K.; Klinpayom, C.; Gutierrez, J.M.; Quraishi, N.H.; Tan, C.H. A pan-specific antiserum produced by a novel immunization strategy shows a high spectrum of neutralization against neurotoxic snake venoms. Sci. Rep. 2020, 10, 11261. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Beltran, M.C.; Hurtado-Gomez, J.P.; Corredor-Espinel, V.; Ruiz-Gomez, F.J. A polyvalent coral snake antivenom with broad neutralization capacity. PLoS Negl. Trop. Dis. 2019, 13, e0007250. [Google Scholar] [CrossRef]
- Patra, A.; Kalita, B.; Khadilkar, M.V.; Salvi, N.C.; Shelke, P.V.; Mukherjee, A.K. Assessment of quality and pre-clinical efficacy of a newly developed polyvalent antivenom against the medically important snakes of Sri Lanka. Sci. Rep. 2021, 11, 18238. [Google Scholar] [CrossRef]
- Villalta, M.; Sanchez, A.; Herrera, M.; Vargas, M.; Segura, A.; Cerdas, M.; Estrada, R.; Gawarammana, I.; Keyler, D.E.; McWhorter, K.; et al. Development of a new polyspecific antivenom for snakebite envenoming in Sri Lanka: Analysis of its preclinical efficacy as compared to a currently available antivenom. Toxicon 2016, 122, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Lomonte, B.; Sanz, L.; Calvete, J.J.; Pla, D. Immunological profile of antivenoms: Preclinical analysis of the efficacy of a polyspecific antivenom through antivenomics and neutralization assays. J. Proteom. 2014, 105, 340–350. [Google Scholar] [CrossRef]
- Barber, C.M.; Isbister, G.K.; Hodgson, W.C. Alpha neurotoxins. Toxicon 2013, 66, 47–58. [Google Scholar] [CrossRef]
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon 2010, 56, 855–867. [Google Scholar] [CrossRef]
- Mendez, I.; Gutierrez, J.M.; Angulo, Y.; Calvete, J.J.; Lomonte, B. Comparative study of the cytolytic activity of snake venoms from African spitting cobras (Naja spp., Elapidae) and its neutralization by a polyspecific antivenom. Toxicon 2011, 58, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Arguedas, M.; Umana, D.; Moscoso, E.; Garcia, A.; Pereira, C.; Sanchez, A.; Duran, G.; Cordero, D.; Sanchez, A.; Segura, A.; et al. Comparison of adjuvant emulsions for their safety and ability to enhance the antibody response in horses immunized with African snake venoms. Vaccine X 2022, 12, 100233. [Google Scholar] [CrossRef]
- Patra, A.; Kalita, B.; Mukherjee, A.K. Assessment of quality, safety, and pre-clinical toxicity of an equine polyvalent anti-snake venom (Pan Africa): Determination of immunological cross-reactivity of antivenom against venom samples of Elapidae and Viperidae snakes of Africa. Toxicon 2018, 153, 120–127. [Google Scholar] [CrossRef]
- Senji Laxme, R.R.; Khochare, S.; de Souza, H.F.; Ahuja, B.; Suranse, V.; Martin, G.; Whitaker, R.; Sunagar, K. Beyond the ‘big four’: Venom profiling of the medically important yet neglected Indian snakes reveals disturbing antivenom deficiencies. PLoS Negl. Trop. Dis. 2019, 13, e0007899. [Google Scholar] [CrossRef] [PubMed]
- Waghmare, A.B.; Salvi, N.C.; Deopurkar, R.L.; Shenoy, P.A.; Sonpetkar, J.M. Evaluation of health status of horses immunized with snake venom and montanide adjuvants, IMS 3012 (nanoparticle), ISA 206 and ISA 35 (emulsion based) during polyvalent snake antivenom production: Hematological and biochemical assessment. Toxicon 2014, 82, 83–92. [Google Scholar] [CrossRef]
- Tan, C.H.; Tan, K.Y.; Lim, S.E.; Tan, N.H. Venomics of the beaked sea snake, Hydrophis schistosus: A minimalist toxin arsenal and its cross-neutralization by heterologous antivenoms. J. Proteom. 2015, 126, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Leong, P.K.; Tan, N.H.; Fung, S.Y.; Sim, S.M. Cross neutralisation of Southeast Asian cobra and krait venoms by Indian polyvalent antivenoms. Trans. R Soc. Trop. Med. Hyg. 2012, 106, 731–737. [Google Scholar] [CrossRef]
- Garcia-Arredondo, A.; Martinez, M.; Calderon, A.; Saldivar, A.; Soria, R. Preclinical Assessment of a New Polyvalent Antivenom (Inoserp Europe) against Several Species of the Subfamily Viperinae. Toxins 2019, 11, 149. [Google Scholar] [CrossRef]
- Leong, P.K.; Tan, C.H.; Sim, S.M.; Fung, S.Y.; Sumana, K.; Sitprija, V.; Tan, N.H. Cross neutralization of common Southeast Asian viperid venoms by a Thai polyvalent snake antivenom (Hemato Polyvalent Snake Antivenom). Acta Trop. 2014, 132, 7–14. [Google Scholar] [CrossRef]
- Tan, C.H.; Tan, N.H.; Tan, K.Y.; Kwong, K.O. Antivenom cross-neutralization of the venoms of Hydrophis schistosus and Hydrophis curtus, two common sea snakes in Malaysian waters. Toxins 2015, 7, 572–581. [Google Scholar] [CrossRef]
- Arce, V.; Rojas, E.; Ownby, C.L.; Rojas, G.; Gutierrez, J.M. Preclinical assessment of the ability of polyvalent (Crotalinae) and anticoral (Elapidae) antivenoms produced in Costa Rica to neutralize the venoms of North American snakes. Toxicon 2003, 41, 851–860. [Google Scholar] [CrossRef]
- Tan, C.H.; Tan, N.H.; Sim, S.M.; Fung, S.Y.; Jayalakshmi, P.; Gnanathasan, C.A. Nephrotoxicity of hump-nosed pit viper (Hypnale hypnale) venom in mice is preventable by the paraspecific Hemato polyvalent antivenom (HPA). Toxicon 2012, 60, 1259–1262. [Google Scholar] [CrossRef]
- Tan, C.H.; Leong, P.K.; Fung, S.Y.; Sim, S.M.; Ponnudurai, G.; Ariaratnam, C.; Khomvilai, S.; Sitprija, V.; Tan, N.H. Cross neutralization of Hypnale hypnale (hump-nosed pit viper) venom by polyvalent and monovalent Malayan pit viper antivenoms in vitro and in a rodent model. Acta Trop. 2011, 117, 119–124. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Tsai, W.C.; Pla, D.; Solano, G.; Lomonte, B.; Sanz, L.; Angulo, Y.; Calvete, J.J. Preclinical assessment of a polyspecific antivenom against the venoms of Cerrophidion sasai, Porthidium nasutum and Porthidium ophryomegas: Insights from combined antivenomics and neutralization assays. Toxicon 2013, 64, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Solano, G.; Gomez, A.; Corrales, G.; Chacon, D.; Estrada, R.; Leon, G. Contributions of the snake venoms of Bothrops asper, Crotalus simus and Lachesis stenophrys to the paraspecificity of the Central American polyspecific antivenom (PoliVal-ICP). Toxicon 2018, 144, 1–6. [Google Scholar] [CrossRef]
- Chaisakul, J.; Rusmili, M.R.A.; Alsolaiss, J.; Albulescu, L.O.; Harrison, R.A.; Othman, I.; Casewell, N.R. In Vitro Immunological Cross-Reactivity of Thai Polyvalent and Monovalent Antivenoms with Asian Viper Venoms. Toxins 2020, 12, 766. [Google Scholar] [CrossRef]
- Segura, A.; Castillo, M.C.; Nunez, V.; Yarleque, A.; Goncalves, L.R.; Villalta, M.; Bonilla, C.; Herrera, M.; Vargas, M.; Fernandez, M.; et al. Preclinical assessment of the neutralizing capacity of antivenoms produced in six Latin American countries against medically-relevant Bothrops snake venoms. Toxicon 2010, 56, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Theakston, R.D.; Warrell, D.A. Confronting the neglected problem of snake bite envenoming: The need for a global partnership. PLoS Med. 2006, 3, e150. [Google Scholar] [CrossRef]
- Warrell, D.A.; Williams, D.J. Clinical aspects of snakebite envenoming and its treatment in low-resource settings. Lancet 2023, 401, 1382–1398. [Google Scholar] [CrossRef]
- Williams, D.J.; Gutierrez, J.M.; Calvete, J.J.; Wuster, W.; Ratanabanangkoon, K.; Paiva, O.; Brown, N.I.; Casewell, N.R.; Harrison, R.A.; Rowley, P.D.; et al. Ending the drought: New strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J. Proteom. 2011, 74, 1735–1767. [Google Scholar] [CrossRef]
- Sapsutthipas, S.; Leong, P.K.; Akesowan, S.; Pratanaphon, R.; Tan, N.H.; Ratanabanangkoon, K. Effective equine immunization protocol for production of potent poly-specific antisera against Calloselasma rhodostoma, Cryptelytrops albolabris and Daboia siamensis. PLoS Negl. Trop. Dis. 2015, 9, e0003609. [Google Scholar] [CrossRef] [PubMed]
- Sapsutthipas, S. Production of Polyvalent Therapeutic Antivenom against Vipera russelli SIAMENSIS, Caloselama rhodostoma and Trimeresurus albolabris. Master’s Thesis, Mahidol University, Bangkok, Thailand, 1998. Available online: http://www.thaithesis.org/detail.php?id=42722 (accessed on 15 June 2023).
- Warrell, D. (Ed.) Guidelines for the Management of Snakebites, 2nd ed.; World Health Organization: Geneva, Switzerland, 2016; p. 144. [Google Scholar]
- Leong, P.K.; Sim, S.M.; Fung, S.Y.; Sumana, K.; Sitprija, V.; Tan, N.H. Cross neutralization of Afro-Asian cobra and Asian krait venoms by a Thai polyvalent snake antivenom (Neuro Polyvalent Snake Antivenom). PLoS Negl. Trop. Dis. 2012, 6, e1672. [Google Scholar] [CrossRef]
- Tan, K.Y.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Venomics, lethality and neutralization of Naja kaouthia (monocled cobra) venoms from three different geographical regions of Southeast Asia. J. Proteom. 2015, 120, 105–125. [Google Scholar] [CrossRef]
- Leong, P.K.; Fung, S.Y.; Tan, C.H.; Sim, S.M.; Tan, N.H. Immunological cross-reactivity and neutralization of the principal toxins of Naja sumatrana and related cobra venoms by a Thai polyvalent antivenom (Neuro Polyvalent Snake Antivenom). Acta Trop. 2015, 149, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Claus, I.; Mebs, D. Cross-neutralization of thrombin-like enzymes in snake venoms by polyvalent antivenoms. Toxicon 1989, 27, 1397–1399. [Google Scholar] [CrossRef]
- Beghini, D.G.; da Cruz-Hofling, M.A.; Randazzo-Moura, P.; Rodrigues-Simioni, L.; Novello, J.C.; Hyslop, S.; Marangoni, S. Cross-neutralization of the neurotoxicity of Crotalus durissus terrificus and Bothrops jararacussu venoms by antisera against crotoxin and phospholipase A2 from Crotalus durissus cascavella venom. Toxicon 2005, 46, 604–611. [Google Scholar] [CrossRef]
- Segura, A.; Herrera, M.; Vargas, M.; Villalta, M.; Uscanga-Reynell, A.; Leon, G.; Gutierrez, J.M. Preclinical efficacy against toxic activities of medically relevant Bothrops sp. (Serpentes: Viperidae) snake venoms by a polyspecific antivenom produced in Mexico. Rev. Biol. Trop. 2017, 65, 345–350. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins; WHO: Geneva, Switzerland, 2010.
- Raweerith, R.; Ratanabanangkoon, K. Immunochemical and biochemical comparisons of equine monovalent and polyvalent snake antivenoms. Toxicon 2005, 45, 369–375. [Google Scholar] [CrossRef]
- Vieira, D.F.; Watanabe, L.; Sant’ana, C.D.; Marcussi, S.; Sampaio, S.V.; Soares, A.M.; Arni, R.K. Purification and characterization of jararassin-I, A thrombin-like enzyme from Bothrops jararaca snake venom. Acta Biochim. Biophys. Sin. 2004, 36, 798–802. [Google Scholar] [CrossRef]
- Gaebert, A.K. Isolation of thrombin-like factors from Malayan pit viper venom by affinity chromatography. Toxicon 1977, 15, 217–224. [Google Scholar] [CrossRef]
- Rock, C.O.; Snyder, F. Rapid purification of phospholipase A2 from Crotalus adamanteus venom by affinity chromatography. J. Biol. Chem. 1975, 250, 6564–6566. [Google Scholar] [CrossRef] [PubMed]
- Aarsman, A.J.; Neys, F.; Van den Bosch, H. A simple and versatile affinity column for phospholipase A2. Biochim. Biophys. Acta 1984, 792, 363–366. [Google Scholar] [CrossRef]
- Stroka, A.; Donato, J.L.; Bon, C.; Hyslop, S.; de Araujo, A.L. Purification and characterization of a hemorrhagic metalloproteinase from Bothrops lanceolatus (Fer-de-lance) snake venom. Toxicon 2005, 45, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Samel, M.; Siigur, J. Isolation and characterization of hemorrhagic metalloproteinase from Vipera berus berus (common viper) venom. Comp. Biochem. Physiol. C Comp. Pharm. Toxicol. 1990, 97, 209–214. [Google Scholar] [CrossRef] [PubMed]
- WHO Target Product Profiles for Animal Plasma-Derived Antivenoms: Antivenoms for Treatment of Snakebite Envenoming in Sub-Saharan Africa. July 2023. Available online: https://www.who.int/publications/i/item/9789240074569 (accessed on 15 July 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratanabanangkoon, K. Polyvalent Snake Antivenoms: Production Strategy and Their Therapeutic Benefits. Toxins 2023, 15, 517. https://doi.org/10.3390/toxins15090517
Ratanabanangkoon K. Polyvalent Snake Antivenoms: Production Strategy and Their Therapeutic Benefits. Toxins. 2023; 15(9):517. https://doi.org/10.3390/toxins15090517
Chicago/Turabian StyleRatanabanangkoon, Kavi. 2023. "Polyvalent Snake Antivenoms: Production Strategy and Their Therapeutic Benefits" Toxins 15, no. 9: 517. https://doi.org/10.3390/toxins15090517
APA StyleRatanabanangkoon, K. (2023). Polyvalent Snake Antivenoms: Production Strategy and Their Therapeutic Benefits. Toxins, 15(9), 517. https://doi.org/10.3390/toxins15090517



