Measuring Marine Biotoxins in a Hypersaline Coastal Lagoon
Abstract
:1. Introduction
2. Results and Discussion
2.1. Enhancements in the LC-MS Analytical Procedure
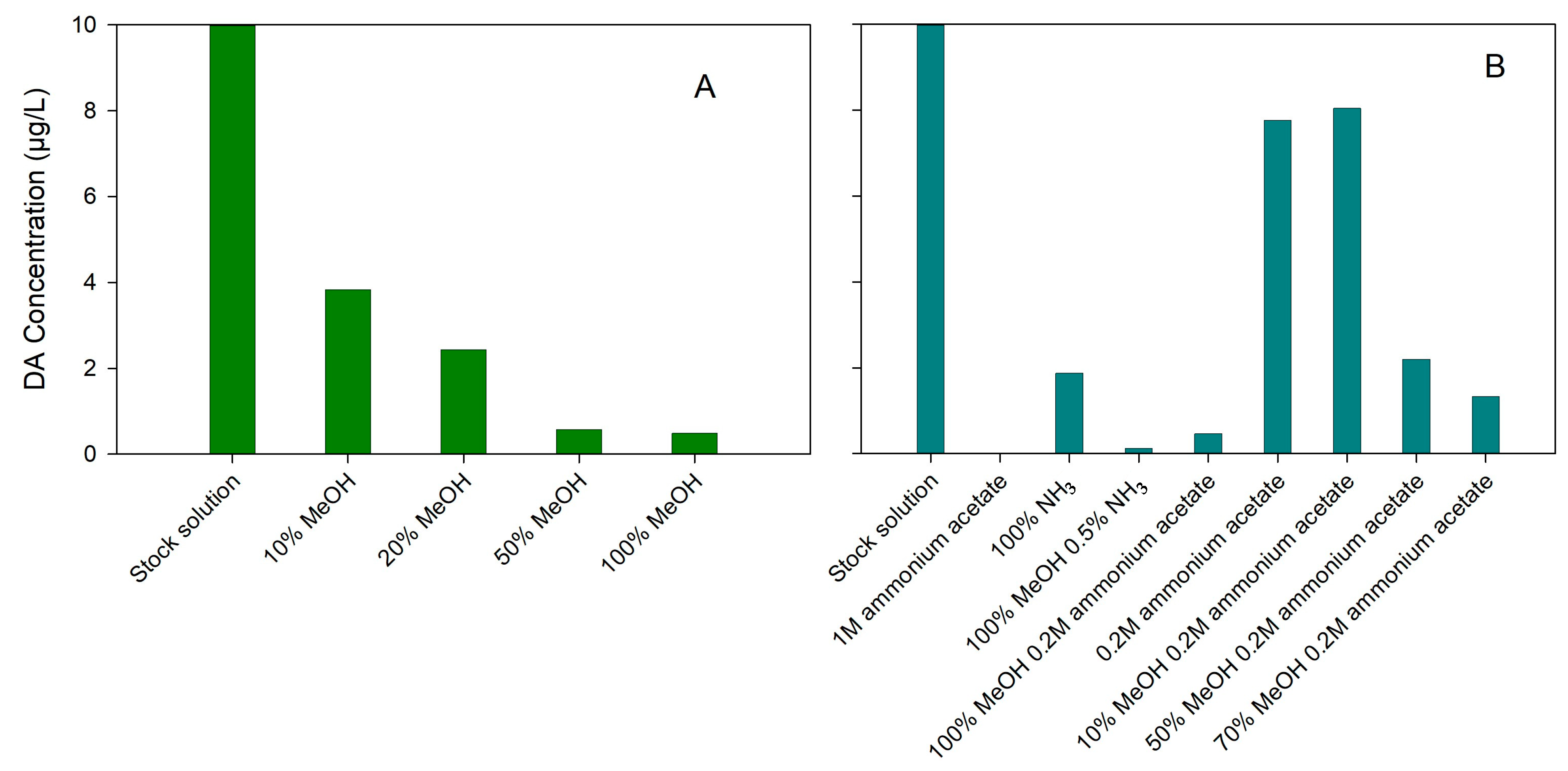
2.2. Enhancements in the SPE Procedure
2.3. Validation of the Measurements
2.4. Domoic Acid and Environmental Variables
3. Conclusions
4. Material and Methods
4.1. Study Area
4.2. Environmental Variables
4.3. LC-MS Analytical Method
4.4. Chemicals and Materials
4.5. Sampling and Pre-Treatment
4.6. Instrumentation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orellana, G.; Van Meulebroek, L.; De Rijcke, M.; Janssen, C.R.; Vanhaecke, L. High resolution mass spectrometry-based screening reveals lipophilic toxins in multiple trophic levels from the North Sea. Harmful Algae 2017, 64, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.T.; Tester, P.A. Toxic marine phytoplankton, zooplankton grazers, and pelagic food webs. Limnol. Oceanogr. 1997, 42, 1203–1213. [Google Scholar] [CrossRef]
- Doucette, G.; Maneiro, I.; Riveiro, I.; Svensen, C. Phycotoxin Pathways in Aquatic Food Webs: Transfer, Accumulation and Degradation. In Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2006; Volume 189, pp. 283–295. [Google Scholar]
- Li, J.; Ruan, Y.; Mak, Y.L.; Zhang, X.; Lam, J.C.W.; Leung, K.M.Y.; Lam, P.K.S. Occurrence and trophodynamics of marine lipophilic phycotoxins in a subtropical marine food web. Environ. Sci. Technol. 2021, 55, 8829–8838. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Fachon, E.; Bowers, E.K.; Kimmel, D.G.; Snyder, J.A.; Stimmelmayr, R.; Grebmeier, J.M.; Kibler, S.; Ransom Hardison, D.; Anderson, D.M.; et al. Paralytic shellfish toxins in Alaskan Arctic food webs during the anomalously warm ocean conditions of 2019 and estimated toxin doses to Pacific walruses and bowhead whales. Harmful Algae 2022, 114, 102205. [Google Scholar] [CrossRef]
- Fire, S.E.; Wang, Z.; Berman, M.; Langlois, G.W.; Morton, S.L.; Sekula-Wood, E.; Benitez-Nelson, C.R. Trophic transfer of the harmful algal toxin domoic acid as a cause of death in a minke whale (Balaenoptera acutorostrata) stranding in southern California. Aquat. Mamm. 2010, 36, 342–350. [Google Scholar] [CrossRef]
- Costa, P.R.; Garrido, S. Domoic acid accumulation in the sardine Sardina pilchardusand its relationship to Pseudo-nitzschia diatom ingestion. Mar. Ecol. Prog. Ser. 2004, 284, 261–268. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin foodstuffs. Off. J. Eur. Union 2004, 139, 55–64. [Google Scholar]
- Vilariño, N.; Louzao, M.C.; Fraga, M.; Botana, L.M. From Science to Policy: Dynamic Adaptation of Legal Regulations on Aquatic Biotoxins. In Climate Change and Marine and Freshwater Toxins; Botana, L.M., Louzao, M.C., Vilarino, N., Eds.; De Gruyter Mouton: Berlin, Germany, 2020; pp. 607–654. ISBN 9783110625738. [Google Scholar]
- FAO. Marine Biotoxins. In Food and Nutrition Paper 80; FAO: Rome, Italy, 2004. [Google Scholar]
- Brown, A.R.; Lilley, M.; Shutler, J.; Lowe, C.; Artioli, Y.; Torres, R.; Berdalet, E.; Tyler, C.R. Assessing risks and mitigating impacts of harmful algal blooms on mariculture and marine fisheries. Rev. Aquac. 2020, 12, 1663–1688. [Google Scholar] [CrossRef]
- Sanseverino, I.; Conduto, D.; Pozzoli, L.; Dobricic, S.; Lettieri, T. Algal Bloom and Its Economic Impact; European Commission, Joint Research Centre Institute for Environment and Sustainability: Ispra, Italy, 2016. [Google Scholar]
- Díaz, P.A.; Álvarez, G.; Varela, D.; Pérez-Santos, I.; Díaz, M.; Molinet, C.; Seguel, M.; Aguilera-Belmonte, A.; Guzmán, L.; Uribe, E.; et al. Impacts of harmful algal blooms on the aquaculture industry: Chile as a case study. Perspect. Phycol. 2019, 6, 39–50. [Google Scholar] [CrossRef]
- Brescianini, C.; Grillo, C.; Melchiorre, N.; Bertolotto, R.; Ferrari, A.; Vivaldi, B.; Icardi, G.; Gramaccioni, L.; Funari, E.; Scardala, S. Ostreopsis ovata algal blooms affecting human health in Genova, Italy, 2005 and 2006. Eurosurveillance 2006, 11, 3040. [Google Scholar] [CrossRef]
- Durando, P.; Ansaldi, F.; Oreste, P.; Moscatelli, P.; Marensi, L.; Grillo, C.; Gasparini, R.; Icardi, G. Ostreopsis ovata and human health: Epidemiological and clinical features of respiratory syndrome outbreaks from a two-year syndromic surveillance, 2005–2006, in north-west Italy. Eurosurveillance 2007, 12, 2005. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Grillo, C.; Melchiorre, N. Putative palytoxin and its new analogue, ovatoxin-a, in Ostreopsis ovata collected along the ligurian coasts during the 2006 toxic outbreak. J. Am. Soc. Mass Spectrom. 2008, 19, 111–120. [Google Scholar] [CrossRef]
- Kermarec, F.; Dor, F.; Armengaud, A.; Charlet, F.; Kantin, R.; Sauzade, D.; De Haro, L. Les risques sanitaires liés à la présence d’Ostreopsis ovata dans les eaux de baignade ou d’activités nautiques. Environ. Risques Et. Sante 2008, 7, 357–363. [Google Scholar]
- Tichadou, L.; Glaizal, M.; Armengaud, A.; Grossel, H.; Lemée, R.; Kantin, R.; Lasalle, J.L.; Drouet, G.; Rambaud, L.; Malfait, P.; et al. Health impact of unicellular algae of the Ostreopsis genus blooms in the Mediterranean Sea: Experience of the French Mediterranean coast surveillance network from 2006 to 2009. Clin. Toxicol. 2010, 48, 839–844. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament and the Council of the European Union. European Parliament Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 concerning the management of bathing water quality and repealing Directive 76/160/EEC. Off. J. Eur. Union 2006, 64, 37–51. [Google Scholar]
- USEPA. Water quality criteria. In Water Quality Standards Handbook; Office of Water: Washington, DC, USA, 2017; pp. 1–26. ISBN EPA-823-B-17-001. [Google Scholar]
- EFSA. Panel on contaminants in the food chain (CONTAM) Marine biotoxins in shellfish: Summary on regulated marine biotoxins. EFSA J. 2009, 1306, 1–23. [Google Scholar]
- EFSA. Panel on Contaminants in the Food Chain Marine biotoxins in shellfish—Domoic acid. EFSA J. 2009, 1181, 1–61. [Google Scholar]
- Work, T.M.; Barr, B.; Beale, A.M.; Fritz, L.; Michael, A.; Wright, J.L.C.; Url, S. Epidemiology of domoic acid poisoning in brown pelicans (Pelecanus occidentalis) and Brandt’s Cormorants (Phalacrocorax penicillatus) in California. J. Zoo Wildl. Med. 1993, 24, 54–62. [Google Scholar]
- Beltrán, A.S.; Palafox-Uribe, M.; Grajales-Montiel, J.; Cruz-Villacorta, A.; Ochoa, J.L. Sea bird mortality at Cabo San Lucas, Mexico: Evidence that toxic diatom blooms are spreading. Toxicon 1997, 35, 447–453. [Google Scholar] [CrossRef]
- Scholin, C.A.; Gulland, F.; Doucette, G.J.; Benson, S.; Busman, M.; Chavez, F.P.; Cordaro, J.; DeLong, R.; Lefebvre, K.A.; Lipscomb, T.; et al. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Lett. Nat. 2000, 403, 80–84. [Google Scholar] [CrossRef]
- Cook, K.B.; Lacaze, J.P.; MacHairopoulou, M.; Bresnan, E. Investigations into the relationship between domoic acid and copepods in Scottish waters. ICES J. Mar. Sci. 2022, 79, 963–973. [Google Scholar] [CrossRef]
- Oller-Ruiz, A.; Campillo, N.; Hernández-Córdoba, M.; Gilabert, J.; Viñas, P. Monitoring lipophilic toxins in seawater using dispersive liquid—Liquid microextraction and liquid chromatography with triple quadrupole mass spectrometry. Toxins 2021, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Sahraoui, I.; Grami, B.; Bates, S.S.; Bouchouicha, D.; Chikhaoui, M.A.; Mabrouk, H.H.; Hlaili, A.S. Response of potentially toxic Pseudo-nitzschia (Bacillariophyceae) populations and domoic acid to environmental conditions in a eutrophied, SW Mediterranean coastal lagoon (Tunisia). Estuar. Coast. Shelf Sci. 2012, 102–103, 95–104. [Google Scholar] [CrossRef]
- Barbaro, E.; Zangrando, R.; Rossi, S.; Cairns, W.R.L.; Piazza, R.; Corami, F.; Barbante, C.; Gambaro, A. Domoic acid at trace levels in lagoon waters: Assessment of a method using internal standard quantification. Anal. Bioanal. Chem. 2013, 405, 9113–9123. [Google Scholar] [CrossRef]
- Bazzoni, A.M.; Mudadu, A.G.; Esposito, G.; Urru, R.; Ortu, S.; Mara, L.; Uda, M.T.; Arras, I.; Lorenzoni, G.; Sanna, G.; et al. Bacterial and viral investigations combined with determination of phytoplankton and algal biotoxins in mussels and water from a Mediterranean coastal lagoon (Sardinia, Italy). J. Food Prot. 2019, 82, 1501–1511. [Google Scholar] [CrossRef]
- Marrouchi, R.; Abdennadher, M.; Feki-Sahnoun, W.; Marzougui, Z.; Hamza, I.S.; Hamza, A.; Kharrat, R. Marine biotoxin profile and Karenia selliformis and Alexandrium minitum occurrence in Boughrara lagoon during the last decade. Ecol. Chem. Eng. Sci. 2022, 29, 501–510. [Google Scholar] [CrossRef]
- Bosch-Orea, C.; Sanchís, J.; Farré, M.; Barceló, D. Analysis of lipophilic marine biotoxins by liquid chromatography coupled with high-resolution mass spectrometry in seawater from the Catalan Coast. Anal. Bioanal. Chem. 2017, 409, 5451–5462. [Google Scholar] [CrossRef]
- Doucette, G.J.; King, K.L.; Thessen, A.E.; Dortch, Q. The effect of salinity on domoic acid production by the diatom Pseudo-nitzschia multiseries. Nov. Hedwig. 2008, 133, 31–46. [Google Scholar]
- Estevez, P.; Gago-Martinez, A. Contribution of mass spectrometry to the advances in risk characterization of marine biotoxins: Towards the characterization of metabolites implied in human intoxications. Toxins 2023, 15, 103. [Google Scholar] [CrossRef] [PubMed]
- Moreiras, G.; Leão, J.M.; Gago-Martínez, A. Analysis of cyclic imines in mussels (Mytilus galloprovincialis) from Galicia (NW Spain) by LC-MS/MS. Int. J. Environ. Res. Public Health 2020, 17, 281. [Google Scholar] [CrossRef]
- Blanco, J.; Arévalo, F.; Moroño, Á.; Correa, J.; Muñíz, S.; Mariño, C.; Martín, H. Presence of azaspiracids in bivalve molluscs from Northern Spain. Toxicon 2017, 137, 135–143. [Google Scholar] [CrossRef]
- Chan, I.O.M.; Tsang, V.W.H.; Chu, K.K.; Leung, S.K.; Lam, M.H.W.; Lau, T.C.; Lam, P.K.S.; Wu, R.S.S. Solid-phase extraction-fluorimetric high performance liquid chromatographic determination of domoic acid in natural seawater mediated by an amorphous titania sorbent. Anal. Chim. Acta 2007, 583, 111–117. [Google Scholar] [CrossRef] [PubMed]
- De la Iglesia, P.; Giménez, G.; Diogène, J. Determination of dissolved domoic acid in seawater with reversed-phase extraction disks and rapid resolution liquid chromatography tandem mass spectrometry with head-column trapping. J. Chromatogr. A 2008, 1215, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Ao, J.; Gu, J.; Yuan, T.; Li, D.; Ma, Y.; Shen, Z. Applying molecular modelling and experimental studies to develop molecularly imprinted polymer for domoic acid enrichment from both seawater and shellfish. Chemosphere 2018, 199, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.M.; Liu, S.; Shi, X.; Liu, M.M.; Zhang, Y.D.L.; Wang, Q. Quality evaluation of a herbal prescription through quantification of 40 components by HPLC-ESI-MS/MS. Phytochem. Anal. 2012, 23, 365–372. [Google Scholar] [CrossRef]
- D’amore, T.; Lo Magro, S.; Vita, V.; Di Taranto, A. Optimization and validation of a high throughput UHPLC-MS/MS method for determination of the EU regulated lipophilic marine toxins and occurrence in fresh and processed shellfish. Mar. Drugs 2022, 20, 173. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; He, X.; Hao, S.; Wang, Y.; Zheng, X.; Wang, B. Simple determination of six groups of lipophilic marine algal toxins in seawater by automated on-line solid phase extraction coupled to liquid chromatography-tandem mass spectrometry. Chemosphere 2021, 262, 128374. [Google Scholar] [CrossRef] [PubMed]
- ISO 5725-1:1994(E); Accuracy (Trueness and Precision) of Measurements Methods and Results—Part 1: General Principles and Definitions. International Organization for Standarization (ISO): Geneva, Switzerland, 1994.
- European Commission. Commission Decision (2002/657/EC) of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Communities 2004, 180, 84–109. [Google Scholar] [CrossRef]
- Lelong, A.; Hégaret, H.; Soudant, P.; Bates, S.S. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: Revisiting previous paradigms. Phycologia 2012, 51, 168–216. [Google Scholar] [CrossRef]
- Brunson, J.K.; McKinnie, S.M.K.; Chekan, J.R.; McCrow, J.P.; Miles, Z.D.; Bertrand, E.M.; Bielinski, V.A.; Luhavaya, H.; Oborník, M.; Smith, G.J.; et al. Biosynthesis of the neurotoxin domoic acid in a bloom-forming diatom. Science 2018, 361, 1356–1358. [Google Scholar] [CrossRef]
- Wells, M.L.; Trick, C.G.; Cochlan, W.P.; Hughes, M.P.; Trainer, V.L. Domoic acid: The synergy of iron, copper, and the toxicity of diatoms. Limnol. Oceanogr. 2005, 50, 1908–1917. [Google Scholar] [CrossRef]
- Silver, M.W.; Bargu, S.; Coale, S.L.; Benitez-Nelson, C.R.; Garcia, A.C.; Roberts, K.J.; Sekula-Wood, E.; Bruland, K.W.; Coale, K.H. Toxic diatoms and domoic acid in natural and iron enriched waters of the oceanic Pacific. Proc. Natl. Acad. Sci. USA 2010, 107, 20762–20767. [Google Scholar] [CrossRef] [PubMed]
- Alorda-Montiel, I.; Rodríguez-Puig, J.; Alorda-Kleinglass, A.; Diego-Feliu, M.; Rodellas, V.; Garcia-Orellana, J. Historical record and sources of contaminants in sediments from Mar Menor coastal lagoon. In Proceedings of the EGU General Assembly 2021, Online, 19–30 April 2021; p. EGU21-15883. [Google Scholar] [CrossRef]
- René, C.; Ruiz, G.; Rodellas, V.; Rodriguez-puig, J.; Santos-echeandía, J. Potentially toxic element input into Mar Menor coastal lagoon (Spain) through submarine groundwater discharge. In Proceedings of the EGU General Assembly 2023, Vienna, Austria, 24–28 April 2023; pp. 24–28. [Google Scholar]
- Liefer, J.D.; MacIntyre, H.L.; Novoveská, L.; Smith, W.L.; Dorsey, C.P. Temporal and spatial variability in Pseudo-nitzschia spp. in Alabama coastal waters: A “hot spot” linked to submarine groundwater discharge? Harmful Algae 2009, 8, 706–714. [Google Scholar] [CrossRef]
- Bates, S.S.; Garrison, D.L.; Horner, R.A. Bloom Dynamics and Physiology Producing Pseudo-nitzschia Species. In Physiological Ecology of Harmful Algal Blooms; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 267–292. [Google Scholar]
- Kudela, R.; Roberts, A.; Armstrong, M. Laboratory Analyses of Nutrient Stress and Toxin Production in Pseudo-nitzschia spp. from Monterey Bay, California. In Harmful Algal Blooms 2002; Steidinger, K.A., Landsberg, J.H., Tomas, C.R., Vargo, G.A., Eds.; Florida and Wildlife Conservation Commission: Fort Myers, FL, USA; Institute of Oceanography: St. Petersburg, FL, USA; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2003. [Google Scholar]
- Sun, J.; Hutchins, D.A.; Feng, Y.; Seubert, E.L.; Caron, D.A.; Fu, F.X. Effects of changing pCO2 and phosphate availability on domoic acid production and physiology of the marine harmful bloom diatom Pseudo-nitzschia multiseries. Limnol. Oceanogr. 2011, 56, 829–840. [Google Scholar] [CrossRef]
- Lema, K.A.; Latimier, M.; Nézan, É.; Fauchot, J.; Le Gac, M. Inter and intra-specific growth and domoic acid production in relation to nutrient ratios and concentrations in Pseudo-nitzschia: Phosphate an important factor. Harmful Algae 2017, 64, 11–19. [Google Scholar] [CrossRef]
- Lundholm, N.; Hansen, P.J.; Kotaki, Y. Effect of pH on growth and domoic acid production by potentially toxic diatoms of the genera Pseudo-nitzschia and Nitzschia. Mar. Ecol. Prog. Ser. 2004, 273, 1–15. [Google Scholar] [CrossRef]
- Hasle, G.R.; Syvertsen, E.E. Marine Diatoms. In The Diatoms: Biology and Morphology of the Genera; Round, F.E., Crawford, R.M., Eds.; University Press: Cambridge, UK, 1997; pp. 563–584. [Google Scholar]
- Bates, S.S.; Trainer, V.L. The ecology, evolution, and oceanography of harmful algal blooms. Limnol. Oceanogr. 2014, 59, 751–766. [Google Scholar]
- Turner, J.T.; Tester, P.A.; Gelsleichter, J. Persistence of domoic acid in seawater under laboratory and field conditions. Nat. Toxins 2000, 8, 271–279. [Google Scholar]
- Conesa, H.M.; Jiménez-Cárceles, F.J. The Mar Menor lagoon (SE Spain): A singular natural ecosystem threatened by human activities. Mar. Pollut. Bull. 2007, 54, 839–849. [Google Scholar] [CrossRef]
- Bernardeau-Esteller, J.; Sandoval-Gil, J.M.; Belando, M.D.; Ramos-Segura, A.; García-Muñoz, R.; Marín-Guirao, L.; Ruiz, J.M. The role of Cymodocea nodosa and Caulerpa prolifera meadows as nitrogen sinks in temperate coastal lagoons. Diversity 2023, 15, 172. [Google Scholar] [CrossRef]
- Burns, J.M.; Ferry, J.L. Adsorption of domoic acid to marine sediments and clays. J. Environ. Monit. 2007, 9, 1373–1377. [Google Scholar] [CrossRef]
- Smith, J.; Shultz, D.; Howard, M.D.A.; Robertson, G.; Phonsiri, V.; Renick, V.; Caron, D.A.; Kudela, R.M.; McLaughlin, K. Persistent domoic acid in marine sediments and benthic infauna along the coast of Southern California. Harmful Algae 2021, 108, 102103. [Google Scholar] [CrossRef]
- Gilabert, J. Seasonal plankton dynamics in a Mediterranean hypersaline coastal lagoon: The Mar Menor. J. Plankton Res. 2001, 23, 207–218. [Google Scholar] [CrossRef]
- López Castejón, F. Caracterización de la Hidrodinámica del Mar Menor y los Flujos de Intercambio con el Mediterráneo mediante Datos in situ y Modelado Numérico. Ph.D. Thesis, Universidad Politécnica de Cartagena, Cartagena, Spain, 2017; 208p. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; Campillo, S.; Fernández-Palacios, J.M.; García-Lacunza, A.; García-Oliva, M.; Ibañez, H.; Navarro-Martínez, P.C.; Pérez-Marcos, M.; Pérez-Ruzafa, I.M.; Quispe-Becerra, J.I.; et al. Long-term dynamic in nutrients, chlorophyll a, and water quality parameters in a coastal lagoon during a process of eutrophication for decades, a sudden break and a relatively rapid recovery. Front. Mar. Sci. 2019, 6, 26. [Google Scholar] [CrossRef]
- Mercado, J.M.; Cortés, D.; Gómez-Jakobsen, F.; García-Gómez, C.; Ouaissa, S.; Yebra, L.; Ferrera, I.; Valcárcel-Pérez, N.; López, M.; García-Muñoz, R.; et al. Role of small-sized phytoplankton in triggering an ecosystem disruptive algal bloom in a Mediterranean hypersaline coastal lagoon. Mar. Pollut. Bull. 2021, 164, 111989–111999. [Google Scholar] [CrossRef] [PubMed]
- Reguera, B.; Alonso, R.; Moreira, Á.; Méndez, S.; Bottein, M.-Y.D. Guide for Designing and Implementing a Plan to Monitor Toxin-Producing Microalgae. In IOC Manuals and Guides, 77; UNESCO: Paris, France; IAEA: Vienna, Austria, 2016; p. 66. [Google Scholar]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis; John Wiley & Sons: Hoboken, NJ, USA, 1999; ISBN 9783527613984. [Google Scholar]
- Utermöhl, H. Methods of collecting plankton for various purposes are discussed. SIL Commun. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- McNabb, P. Chemistry, Metabolism, and Chemical Analysis of Okadaic Acid Group Toxins. In Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection; Botana, L.M., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2008; p. 209. [Google Scholar]
- Gerssen, A.; Mulder, P.P.J.; de Boer, J. Screening of lipophilic marine toxins in shellfish and algae: Development of a library using liquid chromatography coupled to orbitrap mass spectrometry. Anal. Chim. Acta 2011, 685, 176–185. [Google Scholar] [CrossRef]
- Pocklington, R.; Milley, J.E.; Bates, S.S.; Bird, C.J.; De Freitas, A.S.W.; Quilliam, M.A. Trace determination of domoic acid in seawater and phytoplankton by high-performance liquid chromatography of the fluorenylmethoxycarbonyl (FMOC) derivative. Int. J. Environ. Anal. Chem. 1990, 38, 351–368. [Google Scholar] [CrossRef]
- Regueiro, J.; Martín-Morales, E.; Álvarez, G.; Blanco, J. Sensitive determination of domoic acid in shellfish by on-line coupling of weak anion exchange solid-phase extraction and liquid chromatography-diode array detection-tandem mass spectrometry. Food Chem. 2011, 129, 672–678. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, M.; Tong, P.; Lu, Q.; Zhang, L. Ferrite nanospheres-based magnetic solid-phase extraction for determination of domoic acid in seawater samples using high-performance liquid chromatography with tandem mass spectrometry. J. Chromatogr. A 2016, 1443, 54–61. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, J.; Wang, J.; Tan, L. Multipoint recognition of domoic acid from seawater by dummy template molecularly imprinted solid-phase extraction coupled with high-performance liquid chromatography. J. Chromatogr. A 2017, 1500, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, D.; Hong, Z.; Zhou, S.; Zhao, Y. Polymeric ion exchange material based dispersive micro solid-phase extraction of lipophilic marine toxins in seawater followed by the Q Exactive mass spectrometer analysis using a scheduled high resolution parallel reaction monitoring. Microchem. J. 2018, 138, 526–532. [Google Scholar] [CrossRef]



| Compound | MRM Transition (m/z) | CE (V) | Fragmentor Voltage (V) | Retention Time (min) |
|---|---|---|---|---|
| DA | 312 → 266 Q | 10 | 120 | 3.4 |
| 312 → 248 q | 10 | |||
| 312 → 161 q | 20 | |||
| YTX | 1141.5 → 1061.3 Q | 35 | 135 | 4.7 |
| 1141.5 → 925.5 q | 60 | |||
| hYTX | 1155.4 → 1075.5 Q | 35 | 135 | 4.7 |
| GYM | 508.3 → 490.6 Q | 40 | 200 | 6.9 |
| 13-desM | 692 → 164 Q | 50 | 200 | 7.7 |
| 692 → 444 q | 20 | |||
| 13,19-didesM | 678 → 164 Q | 50 | 200 | 7.3 |
| 678 → 430 q | 40 | |||
| SPX20G | 706 → 688 Q | 30 | 190 | 8.1 |
| 706 → 670 q | 35 | |||
| OA | 827 → 723 Q | 55 | 190 | 13.8 |
| 827 → 809 q | 45 | |||
| DTX2 | 827 → 723 Q | 55 | 190 | 14.7 |
| 827 → 809 q | 45 | |||
| AZA4 | 844 → 826 Q | 30 | 190 | 14.7 |
| 844 → 808 q | 45 | |||
| PTX2 | 881.5 → 837.5 Q | 60 | 230 | 14.8 |
| DTX1 | 841 → 823 Q | 45 | 190 | 17.5 |
| 841 → 737 q | 55 | |||
| AZA3 | 828 → 810 Q | 30 | 190 | 18.6 |
| 828 → 792 q | 45 | |||
| AZA5 | 844 → 826 Q | 30 | 180 | 19.0 |
| 844 → 808 q | 40 | |||
| AZA1 | 842 → 824 Q | 30 | 190 | 19.4 |
| 842 → 806 q | 45 | |||
| AZA2 | 856 → 838 Q | 30 | 190 | 19.9 |
| 856 → 820 q | 45 |
| Compound | Aqueous Standard Slope (L ng−1) | Natural Seawater Slope (L ng−1) | Synthetic Seawater Slope (L ng−1) | Lineal Range Studied (ng L−1) | LOD (ng L−1) | LOQ (ng L−1) | RSD (%) |
|---|---|---|---|---|---|---|---|
| DA | 5.41 | 6.29 | 5.74 | 45–900 | 14.8 | 44.6 | 6.19 |
| GYM | 25.4 | 20.2 | 22.8 | 16–100 | 35.0 | 105 | 2.31 |
| 13-desM | 469 | 444 | 479 | 20–200 | 0.06 | 0.17 | 10.2 |
| 13,19-didesM | 564 | 397 | 553 | 20–200 | 0.05 | 0.16 | 10.4 |
| SPX | 215 | 200 | 256 | 20–200 | 0.15 | 0.44 | 8.09 |
| OA | 0.64 | 0.69 | 0.75 | 200–2000 | 80.6 | 242 | 9.12 |
| PTX2 | 4.37 | 5.13 | 4.14 | 15–100 | 2.29 | 6.86 | 10.8 |
| AZA4 | 575 | 614 | 807 | 3–50 | 0.72 | 2.16 | 6.01 |
| DTX2 | 69.5 | 66.8 | 71.4 | 26–133 | 8.63 | 25.9 | 6.34 |
| AZA5 | 84.5 | 158 | 141 | 3–50 | 1.04 | 3.12 | 9.19 |
| DTX1 | 4.91 | 6.18 | 5.27 | 26–133 | 8.95 | 26.9 | 13.6 |
| hYTX | 2.31 | 2.50 | 2.22 | 100–1100 | 91.2 | 274 | 12.5 |
| YTX | 2.15 | 2.47 | 2.07 | 100–1100 | 51.7 | 155 | 6.34 |
| AZA3 | 235 | 247 | 353 | 3–50 | 1.71 | 5.13 | 14.9 |
| AZA1 | 427 | 595 | 722 | 3–50 | 0.15 | 0.45 | 0.44 |
| AZA2 | 315 | 408 | 481 | 3–50 | 0.20 | 0.61 | 3.36 |
| Compound | Level Concentration (ng L−1) | Recovery (%) |
|---|---|---|
| DA | 50 | 83 |
| 100 | 75 | |
| GYM | 500 | 118 |
| 1000 | 115 | |
| 13-desM | 20 | 80 |
| 50 | 79 | |
| 13,19-didesM | 20 | 82 |
| 50 | 74 | |
| SPX | 50 | 91 |
| 200 | 80 | |
| OA | 200 | 90 |
| 400 | 107 | |
| PTX2 | 50 | 119 |
| 200 | 90 | |
| AZA4 | 5 | 112 |
| 10 | 88 | |
| DTX2 | 25 | 106 |
| 50 | 107 | |
| AZA5 | 5 | 110 |
| 10 | 90 | |
| DTX1 | 50 | 120 |
| 200 | 122 | |
| hYTX | 50 | 81 |
| 200 | 77 | |
| YTX | 50 | 108 |
| 200 | 78 | |
| AZA3 | 5 | 87 |
| 10 | 105 | |
| AZA1 | 5 | 99 |
| 10 | 110 | |
| AZA2 | 5 | 114 |
| 10 | 120 |
| Max | Min | Mean | Std | |
|---|---|---|---|---|
| Temperature (°C) | 31 | 9.9 | 20.86 | 6.48 |
| Salinity (PSU) | 45.61 | 39.75 | 43.06 | 1.37 |
| Nitrate [NO3−] (μM) | 3.83 | 0.009 | 0.43 | 0.75 |
| Nitrite [NO2−] (μM) | 0.477 | 0.01 | 0.1 | 0.12 |
| Ammonia [NH4+] (μM) | 10.2 | 0.04 | 2.38 | 2.31 |
| Phosphates [PO4−] (μM) | 0.434 | 0.002 | 0.14 | 0.12 |
| Silicates [Si(OH)4] (μM) | 33.6 | 0.03 | 10.58 | 8.77 |
| DIN | 11.001 | 0.07 | 2.92 | 2.55 |
| DIN:P | 1872.5 | 0.389 | 92.72 | 267.18 |
| DIN:Si | 108.5 | 0.005 | 5.85 | 19.42 |
| Si:P | 1690 | 0.084 | 221.29 | 326.99 |
| Pseudo-nitzschia (cel/L) | 1.20 × 107 | 0 | 1.30 × 106 | 3.00 × 106 |
| DA (ng/L) | 224.06 | 14.84 | 80.09 | 56.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oller-Ruiz, A.; Alcaraz-Oliver, N.; Férez, G.; Gilabert, J. Measuring Marine Biotoxins in a Hypersaline Coastal Lagoon. Toxins 2023, 15, 526. https://doi.org/10.3390/toxins15090526
Oller-Ruiz A, Alcaraz-Oliver N, Férez G, Gilabert J. Measuring Marine Biotoxins in a Hypersaline Coastal Lagoon. Toxins. 2023; 15(9):526. https://doi.org/10.3390/toxins15090526
Chicago/Turabian StyleOller-Ruiz, Ainhoa, Nuria Alcaraz-Oliver, Gema Férez, and Javier Gilabert. 2023. "Measuring Marine Biotoxins in a Hypersaline Coastal Lagoon" Toxins 15, no. 9: 526. https://doi.org/10.3390/toxins15090526
APA StyleOller-Ruiz, A., Alcaraz-Oliver, N., Férez, G., & Gilabert, J. (2023). Measuring Marine Biotoxins in a Hypersaline Coastal Lagoon. Toxins, 15(9), 526. https://doi.org/10.3390/toxins15090526






