The Presence of Pseudo-nitzschia australis in North Atlantic Aquaculture Sites, Implications for Monitoring Amnesic Shellfish Toxins
Abstract
:1. Introduction
2. Results
2.1. Observed Cell Densities of Pseudo-nitzschia spp. and AST Concentrations during 2020
2.2. Observed Concentrations of DST and AST during 2020
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Microplankton Sample Collection
5.2. Shellfish Sample Collection
5.3. Microplankton analysis
5.4. Shellfish Analysis
5.5. Scanning Electron Microscopy (SEM) Analysis
5.5.1. Bleaching of Diatom Frustules
5.5.2. Sputter Coating for SEM
5.5.3. SEM Imaging
5.6. Sample Preparation and Sequencing of Pseudo-nitzschia Samples
5.6.1. DNA Sample Preparation
5.6.2. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scotlands Marine Economic Statistics 2020, Marine Scotland. 2023. Available online: https://www.gov.scot/publications/scotlands-marine-economic-statistics-2020/pages/5/#:~:text=Aquaculture%20employed%202%2C391%20people%2C%200.09,3%25%20of%20marine%20economy%20employment (accessed on 5 July 2022).
- Munro, L.A. Scottish Shellfish Production Survey 2021; Marine Scotland Science: Aberdeen, UK, 2022; ISBN 978-1-80201-102-9. Available online: https://www.gov.scot/publications/scottish-shellfish-farm-production-survey-2021/documents/ (accessed on 7 July 2022).
- Scotland Food and Drink. Aquaculture Growth to 2030: A Strategic Plan for Farming Scotland’s Seas. 2016. Available online: https://foodanddrink.scot/helping-business/other-resources/publications/aquaculture-growth-to-2030-a-strategic-plan-for-farming-scotland-s-seas/ (accessed on 7 July 2022).
- Gianella, F.; Burrows, M.T.; Swan, S.C.; Turner, A.D.; Davidson, K. Temporal and spatial patterns of harmful algae affecting Scottish shellfish aquaculture. Front. Mar. Sci. 2021, 8, 785174. [Google Scholar] [CrossRef]
- Nielsen, P.; Vismann, B. Clearance Rate of Mytilus Edulis (L.) as a Function of Current Velocity and Mussel Aggregation. J. Shellfish. Res. 2014, 33, 457–463. [Google Scholar] [CrossRef]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.; John, U.; Savela, H.; Kremp, A. Alexandrium spp.: Genetic and ecological factors influencing saxitoxin production and proliferation. In Climate Change and Marine and Freshwater Toxins; De Gruyter: Berlin, Germany, 2015; pp. 123–139. [Google Scholar]
- Reguera, B.; Riobó, P.; Rodríguez, F.; Díaz, P.A.; Pizarro, G.; Paz, B.; Franco, J.M.; Blanco, J. Dinophysis toxins: Causative organisms, distribution and fate in shellfish. Mar. Drugs 2014, 12, 394–461. [Google Scholar] [CrossRef]
- Lundholm, N.; Skov, J.; Pocklington, R.; Moestrup, Ø. Domoic acid, the toxic amino acid responsible for amnesic shellfish poisoning, now in Pseudonitzschia seriata (Bacillariophyceae) in Europe. Phycologia 1994, 33, 475–478. [Google Scholar] [CrossRef]
- Hasle, G.R. Are most of the domoic acid-producing species of the diatom genus Pseudo-nitzschia cosmopolites? Harmful Algae 2002, 1, 137–146. [Google Scholar] [CrossRef]
- Smayda, T.J. Harmful Algal Bloom Communities in Scottish Coastal Waters: Relationship to Fish Farming and Regional Comparisons—A Review Paper 2006/3. 2006. Available online: https://webarchive.nrscotland.gov.uk/20190128102835/https://www2.gov.scot/Publications/2006/02/03095327/0 (accessed on 1 July 2023).
- Brown, L.; Bresnan, E.; Graham, J.; Lacaze, J.P.; Turrell, E.; Collins, C. Distribution, diversity and toxin composition of the genus Alexandrium (Dinophyceae) in Scottish waters. Eur. J. Phycol. 2010, 45, 375–393. [Google Scholar] [CrossRef]
- Bresnan, E.; Fryer, R.J.; Fraser, S.; Smith, N.; Stobo, L.; Brown, N.; Turrell, E. The relationship between Pseudo-nitzschia (Peragallo) and domoic acid in Scottish shellfish. Harmful Algae 2017, 63, 193–202. [Google Scholar] [CrossRef]
- Gallacher, S.; Howard, G.; Hess, P.; MacDonald, E.; Kelly, M.C.; Bates, L.A.; Brown, N.; MacKenzie, M.; Gillibrand, P.; Turrell, W.R. The occurrence of amnesic shellfish poisons in Scottish waters. Harmful Algal Bloom. 2000 2001, 30–33. [Google Scholar]
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef]
- Hinder, S.L.; Hays, G.C.; Brooks, C.J.; Davies, A.P.; Edwards, E.; Walne, A.W.; Gravenor, M.B. Toxic marine microalgae and shellfish poisoning in the British Isles: History, review of epidemiology, and future implications. Environ. Health 2011, 10, 54. [Google Scholar] [CrossRef]
- Brown, J.; Fernand, L.; Horsburgh, K.J.; Hill, A.E.; Read, J.W. Paralytic Shellfish Poisoning on the East Coast of the UK in Relation to Seasonal Density-driven Circulation. J. Plankton Res. 2001, 23, 105–116. [Google Scholar] [CrossRef]
- Whyte, C.; Swan, S.; Davidson, K. Changing wind patterns linked to unusually high Dinophysis blooms around the Shetland Islands, Scotland. Harmful Algae 2014, 39, 365–373. [Google Scholar] [CrossRef]
- Davidson, K.; Tett, P.; Gowen, R.J. Harmful Algal Blooms. In Marine Pollution and Human Health; Hester, R.E., Harrison, R.M., Eds.; Issues in Environmental Science and Technology; RSC Publishing: Cambridge, UK, 2011; pp. 95–127. ISBN 978-1-84973-240-6. [Google Scholar]
- Berdalet, E.; Fleming, L.E.; Gowen, R.; Davidson, K.; Hess, P.; Backer, L.C.; Moore, S.K.; Hoagland, P.; Enevoldsen, H. Marine harmful algal blooms, human health and wellbeing: Challenges and opportunities in the 21st century. J. Mar. Biol. Assoc. UK 2016, 96, 61–91. [Google Scholar] [CrossRef] [PubMed]
- Food Standard Scotland, Biotoxin and Phytoplankton Monitoring. 2023. Available online: https://www.foodstandards.gov.scot/business-and-industry/industry-specific-advice/shellfish (accessed on 16 January 2023).
- Regulation (EU) 2017/625 of the European Parliament and the Council. Available online: https://www.legislation.gov.uk/eur/2017/625/contents (accessed on 1 July 2023).
- Algoet, M.; Coates, L.; Swan, S.; Bickerstaff, L.; Ford, C.; Panton, S. The Shellfish Official Control Monitoring Programmes for Scotland. Summary Report for 2020. 2021. Available online: https://www.cefas.co.uk/media/4nxk5h1u/c7710-fss-2020-summary-report-final-v1-150721-to-fss.pdf (accessed on 28 August 2023).
- Holtrop, G.; Horgan, G.W. Risk Assessment of the FSA Scotland Monitoring Programme for Biotoxins in Shellfish Harvested from Classified Inshore Areas in Scotland: Evaluation of the Current Scheme and Development of Improved Alternatives Based on Historical Data; Report to Food Standards Agency Scotland, Project code S01026; Food Standards Scotland: Scotland, UK, 2004.
- Holtrop, G. Risk Assessment of the FSA Scotland Inshore Shellfish Monitoring Programme Based on Historical Toxin Data from 2004–2006; Report to Food Standards Agency Scotland, Project code S14036; Food Standards Scotland: Scotland, UK, 2008.
- Holtrop, G.; Swan, S.; Duff, B.; Wilding, T.; Narayanaswamy, B.; Davidson, K. Risk Assessment of the Scottish Monitoring Programme for Marine Biotoxins in Shellfish Harvested from Classified Production Areas: Review of the current Sampling Scheme to Develop an Improved Programme Based on Evidence of Risk; Report to Food Standards Scotland, FSS/2015/021; Food Standards Scotland: Scotland, UK, 2016.
- Davidson, K.; Whyte, C.; Aleynik, D.; Dale, A.; Kurekin, A.; McNeill, S.; Miller, P.; Porter, M.; Saxon, R. HABreports: Online early warning of harmful algal and biotoxin risk for the shellfish and finfish aquaculture industries. Front. Mar. Sci. 2021, 8, 631732. [Google Scholar] [CrossRef]
- Bresnan, E.; Kraberg, A.; Fraser, S.; Brown, L.; Hughes, S.; Wiltshire, K.H. Diversity and seasonality of Pseudo-nitzschia (Peragallo) at two North Sea time-series monitoring sites. Helgol. Mar. Res. 2015, 69, 193–204. [Google Scholar] [CrossRef]
- Fehling, J.; Davidson, K.; Bolch, C.; Tett, P. Seasonality of Pseudo-nitzschia spp. (Bacillariophyceae) in western Scottish waters. Mar. Ecol. Prog. Ser. 2006, 323, 91–105. [Google Scholar] [CrossRef]
- Fehling, J.; Green, D.H.; Davidson, K.; Bolch, C.J.; Bates, S.S. Domoic acid production by Pseudo-nitzschia seriata (bacillariophyceae) in Scottish waters. J. Phycol. 2004, 40, 622–630. [Google Scholar] [CrossRef]
- Todd, E.C.D. Domoic Acid and Amnesic Shellfish Poisoning—A Review. J. Food Prot. 1993, 56, 69–83. [Google Scholar] [CrossRef]
- Rowland-Pilgrim, S.; Swan, S.C.; O’Neill, A.; Johnson, S.; Coates, L.; Stubbs, P.; Dean, K.; Parks, R.; Harrison, K.; Alves, M.T. Variability of amnesic shellfish toxin and Pseudo-nitzschia occurrence in bivalve molluscs and water samples–analysis of ten years of the official control monitoring programme. Harmful Algae 2019, 87, 101623. [Google Scholar] [CrossRef]
- Skov, J.; Lundholm, N.; Moestrup, Ø.; Larsen, J. Potentially Toxic Phytoplankton, Genus Pseudo-nitzschia Bacillariophyceae/Diatomophyceae. ICES Identification Leaflets for Phytoplankton. Leaflet No. 185, 4. 1999. Available online: https://www.ices.dk/sites/pub/Publication%20Reports/Plankton%20leaflets/IDPlankton_185.PDF (accessed on 28 August 2023).
- Hallegraeff, G.M.; Anderson, D.M.; Cembella, A.D.; Enevoldsen, H.O. (Eds.) Manual on Harmful Marine Microalgae, 2nd revised ed.; UNESCO: Paris, France, 2004; (Monographs on Oceanographic Methodology, 11). [Google Scholar] [CrossRef]
- Lelong, A.; Hégaret, H.; Soudant, P.; Bates, S.S. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: Revisiting previous paradigms. Phycologia 2012, 51, 168–216. [Google Scholar] [CrossRef]
- Lim, H.C.; Tan, S.N.; Teng, S.T.; Lundholm, N.; Orive, E.; David, H.; Quijano-Scheggia, S.; Leong, S.C.Y.; Wolf, M.; Bates, S.S.; et al. Phylogeny and species delineation in the marine diatom Pseudo-nitzschia (Bacillariophyta) using cox1, LSU, and ITS2 rRNA genes: A perspective in character evolution. J. Phycol. 2018, 54, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Karlson, B.; Andersen, P.; Arneborg, L.; Cembella, A.; Eikrem, W.; John, U.; Joy West, J.; Klemm, K.; Kobos, J.; Lehtinen, S.; et al. Harmful algal blooms and their effects in coastal seas of Northern Europe. Harmful Algae 2021, 102, 101989. [Google Scholar] [CrossRef]
- Álvarez, G.; Uribe, E.; Quijano-Scheggia, S.I.; López-Rivera, A.; Mariño, C.; Blanco, J. Domoic acid production by Pseudo-nitzschia australis and Pseudo-nitzschia calliantha isolated from North Chile. Harmful Algae 2009, 8, 938–945. [Google Scholar] [CrossRef]
- Díaz, P.A.; Ruiz-Villarreal, M.; Velo-Suárez, L.; Ramilo, I.; Gentien, P.; Lunven, M.; Fernand, L.; Raine, R.; Reguera, B. Tidal and wind-event variability and the distribution of two groups of Pseudo-nitzschia species in an upwelling-influenced Ría. Deep Sea Res. II 2014, 101, 163–179. [Google Scholar] [CrossRef]
- Santiago-Morales, I.S.; García-Mendoza, E. Growth and domoic acid content of Pseudo-nitzschia australis isolated from northwestern Baja California, Mexico, cultured under batch conditions at different temperatures and two Si:NO3 ratios. Harmful Algae 2011, 12, 82–94. [Google Scholar] [CrossRef]
- Thorel, M.; Fauchot, J.; Morelle, J.; Raimbault, V.; Le Roy, B.; Miossec, C.; Kientz-Bouchart, V.; Claquin, P. Interactive effects of irradiance and temperature on growth and domoic acid production of the toxic diatom Pseudo-nitzschia australis (Bacillariophyceae). Harmful Algae 2014, 39, 232–241. [Google Scholar] [CrossRef]
- Clark, S.; Hubbard, K.A.; McGillicuddy, D.J.; Ralston, D.K.; Shankar, S. Investigating Pseudo-nitzschia australis introduction to the Gulf of Maine with observations and models. Cont. Shelf Res. 2021, 228, 104493. [Google Scholar] [CrossRef]
- Clark, S.; Hubbard, K.A.; Ralston, D.K.; McGillicuddy, D.J.; Stock, C.; Alexander, M.A.; Curchitser, E. Projected effects of climate change on Pseudo-nitzschia bloom dynamics in the Gulf of Maine. J. Mar. Syst. 2022, 230, 103737. [Google Scholar] [CrossRef]
- Hernández-Fariñas, T.; Soudant, D.; Barillé, L.; Belin, C.; Lefebvre, A.; Bacher, C. Temporal changes in the phytoplankton community along the French coast of the eastern English Channel and the southern Bight of the North Sea. ICES J. Mar. Sci. 2013, 71, 821–833. [Google Scholar] [CrossRef]
- McCabe, R.M.; Hickey, B.M.; Kudela, R.M.; Lefebvre, K.A.; Adams, N.G.; Bill, B.D.; Gulland, F.M.D.; Thomson, R.E.; Cochlan, W.P.; Trainer, V.L. An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophys. Res. Lett. 2016, 43, 10366–10376. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Qu, P.; Fu, F.; Tennenbaum, N.; Tatters, A.O.; Hutchins, D.A. Understanding the blob bloom: Warming increases toxicity and abundance of the harmful bloom diatom Pseudo-nitzschia in California coastal waters. Harmful Algae 2017, 67, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Martino, S.; Gianella, F.; Davidson, K. An approach for evaluating the economic impacts of harmful algal blooms; the effects of blooms of toxic Dinophysis spp. on the productivity of Scottish Shellfish farms. Harmful Algae 2020, 99, 101912. Available online: https//www.sciencedirect.com/science/article/abs/pll/51568988320301918 (accessed on 28 August 2023). [CrossRef]
- Adams, C.M.; Larkin, S.L.; Hoagland, P.; Sancewich, B. Assessing the Economic Consequences of Harmful Algal Blooms. In Harmful Algal Blooms; Shumway, S.E., Burkholder, J.M., Morton, S.L., Eds.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton- Methodik. Mitteilungen Int. Ver. Theor. Und Angew. Limnol. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Quilliam, M.A.; Xie, M.; Hardstaff, W.R. Rapid Extraction and Cleanup for Liquid Chromatographic Determination of Domoic Acid in Unsalted Seafood. J. AOAC Int. 2020, 78, 543–554. [Google Scholar] [CrossRef]
- Hasle, G.R. Nitzschia and Fragilariopsis species studied in the light and electron microscopes. II. The group Pseudonitzschia. Skrifter/Det Norske Videnskaps-Akademi I Oslo. I Mat.-Nat. Kl. Ny Serie 1965, 18, 1–45. [Google Scholar]
- Hatfield, R.G.; Bean, T.; Turner, A.D.; Lees, D.N.; Lowther, J.; Lewis, A.; Baker-Austin, C. Development of a TaqMan qPCR assay for detection of Alexandrium spp and application to harmful algal bloom monitoring. Toxicon X 2019, 2, 100011. [Google Scholar] [CrossRef]
- Hatfield, R.G.; Batista, F.M.; Bean, T.P.; Fonseca, V.G.; Santos, A.; Turner, A.D.; Lewis, A.; Dean, A.J.; Martinez-Urtaza, J. The Application of Nanopore Sequencing Technology to the Study of Dinoflagellates: A Proof of Concept Study for Rapid Sequence-Based Discrimination of Potentially Harmful Algae. Front. Microbiol. 2020, 11, 844. [Google Scholar] [CrossRef]
- Zhang, H.; Bhattacharya, D.; Lin, S. Phylogeny of dinoflagellates based on mitochondrial cytochrome b and nuclear small subunit rDNA sequence comparisons. J. Phycol. 2005, 41, 411–420. [Google Scholar] [CrossRef]
- Scholin, C.A.; Anderson, D.M.; Sogin, M.L. Two distinct small subunit Ribosomal RNA genes in the North American toxic dinoflagellate Alexandrium Fundyense (Dinophyceae). J. Phycol. 1993, 29, 209–216. [Google Scholar] [CrossRef]
- Scholin, C.A.; Buck, K.R.; Britschgi, T.; Cangelosi, G.; Chavez, F.P. Identification of Pseudo-nitzschia australis (Bacillariophyceae) using rRNA-targeted probes in whole cell and sandwich hybridization formats. Phycologia 1996, 35, 190–197. [Google Scholar] [CrossRef]
- Guillou, L.; Bachar, D.; Audic, S.; Bass, D.; Berney, C.; Bittner, L.; Boutte, C.; Burgaud, G.; de Vargas, C.; Decelle, J.; et al. The Protist Ribosomal Reference database (PR2): A catalog of unicellular eukaryote Small Sub-Unit rRNA sequences with curated taxonomy. Nucleic Acids Res. 2013, 41, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Barnett, D.W.; Garrison, E.K.; Quinlan, A.R.; Strömberg, M.P.; Marth, G.T. Bamtools: A C++ API and toolkit for analyzing and managing BAM files. Bioinformatics 2011, 27, 1691–1692. [Google Scholar] [CrossRef]
- Li, H. Seqtk; GitHub: San Francisco, CA, USA, 2015. [Google Scholar]
- Sahlin, K.; Lim, M.C.W.; Prost, S. NGSpeciesID: DNA barcode and amplicon consensus generation from long-read sequencing data. Ecol. Evol. 2021, 11, 1392–1398. [Google Scholar] [CrossRef]
- Vaser, R.; Sović, I.; Nagarajan, N.; Šikić, M. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res. 2017, 27, 737–746. [Google Scholar] [CrossRef]
- Medaka, Version 1.2.4; Github: San Francisco, CA, USA. Available online: Nanopretech.com(accessed on 28 August 2023).
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
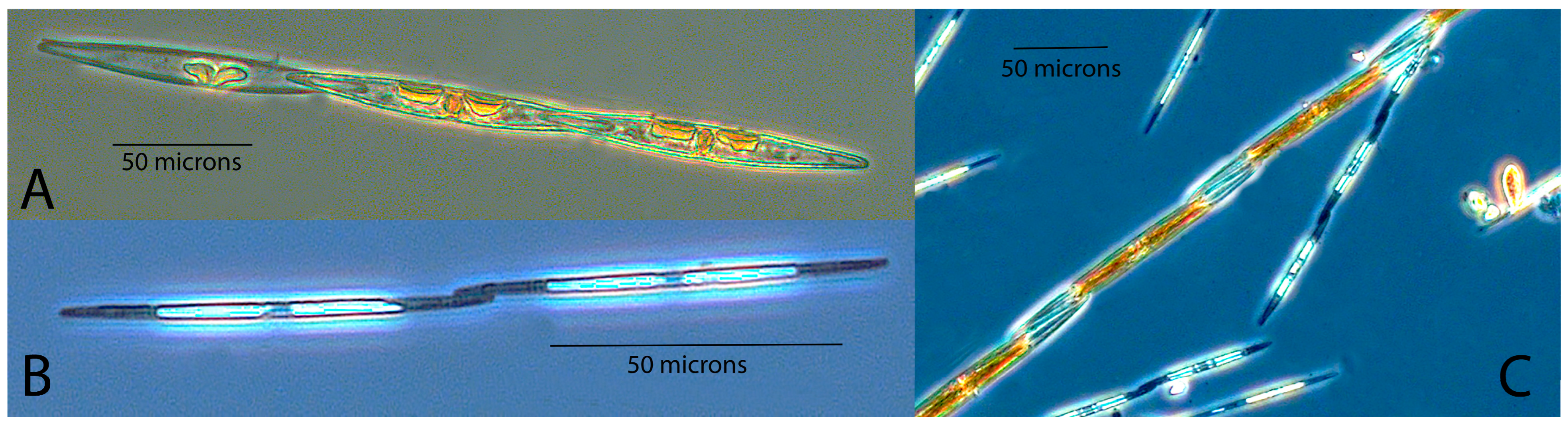












| Site | Collected Week | Valve Shape | Fibulae in 10 µm | Striae in 10 µm | Central Nodule | Poroid Rows | Poroids in 1 µm | Apical Axis (µm) | Transapical Axis (µm) | Band Striae in 10 µm | Probable Species | Possible Species | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slyde | 28 | Linear | 25/26 | 43/44 | Present | 1 | 5/6 | 58.0 | 1.3 | P. pseudodelicatissima | P. plurisecta | Sectors in poroids unclear | |
| Slyde | 28 | Linear | 26 | 43/44 | Present | 1 | 5/6 | 61.2 | 1.2 | 45 | P. pseudodelicatissima | P. plurisecta | Sectors in poroids unclear |
| Slyde | 28 | Lanceolate | 19/20 | 20 | Absent | 2 (+1?) | 8 | 87.5 | 6.1 | P. seriata | P. australis | Rows of poroids unclear ** | |
| Bunya Sand | 28 | Linear | 23 | 42 | Present | 1 | 5/6 | 3.1 | 44 | P. pseudodelicatissima | P. plurisecta | Sectors in poroids unclear | |
| East of Linga | 29 | Linear | 24 | 43 | Present | 1 | 6 | 1.6 | 42 | P. pseudodelicatissima | P. plurisecta | Sectors in poroids unclear | |
| East of Linga | 29 | Linear-lanceolate | 23/25 | 43/44 | Present | 1 (+1?) | 7? | 57.8 | 1.7 | 42/43 | P. delicatissima | P. pseudodelicatissima * | Rows of poroids unclear |
| East of Linga | 29 | Linear-lanceolate | 24/26 | 40/41 | Present | 2 | 8 | 1.5 | 43 | P. delicatissima | |||
| Busta Voe Lee | 29 | Linear | 23 | 41 | Present | 1 | 6/7 | 67.0 | 1.6 | P. pseudodelicatissima | P. plurisecta | Sectors in poroids unclear | |
| Slyde | 31 | Linear | 23 | 42 | Not visible | 1 | 6/7 | 1.2 | 44 | P. pseudodelicatissima | P. plurisecta | Sectors in poroids unclear | |
| Slyde | 31 | Linear | 24 | 43 | Present | 1 | 6 | 1.7 | 44 | P. pseudodelicatissima | P. plurisecta | Sectors in poroids unclear | |
| Slyde | 31 | Linear | 24 | 44 | Not visible | 1 | 6/7 | 1.6 | P. pseudodelicatissima | P. plurisecta | Sectors in poroids unclear | ||
| Slyde | 31 | Linear | 23 | 41/42 | Present | 1 | 5/6 | 57.0 | 1.7 | 42 | P. pseudodelicatissima | P. plurisecta | Sectors in poroids unclear |
| Slyde | 31 | Linear-lanceolate | 24 | 43 | Not visible | 2 | 8/10 | 1.8 | 42 | P. delicatissima | Cell broken—nodule? | ||
| Slyde | 31 | Lanceolate | 21/22 | 22/23 | Present | 2 | 6 | 84.6 | 6.1 | 38 | P. fraudulenta | ||
| Slyde | 31 | Lanceolate | 24 | 22/24 | Present | 2 | 6 | 86.7 | 5.8 | 37 | P. fraudulenta | ||
| Slyde | 31 | Lanceolate | 18 | 14/15 | Not visible | 2 (+1?) | 6/7 | 103.0 | 4.8 | P. seriata | P. australis | Cell broken—nodule? | |
| Slyde | 31 | Lanceolate | 15 | 17/18 | Absent | 2 | 5/6 | 97.5 | 7.5 | 19 | P. australis | ||
| Slyde | 31 | Lanceolate | 16 | 16/17 | Absent | 2 | 5/6 | 96.0 | 6.7 | P. australis | |||
| Slyde | 31 | Lanceolate | 18/20 | 15/16 | Absent | 2 | 4/5 | 81.8 | 7.7 | 18/20 | P. australis | ||
| Slyde | 31 | Lanceolate | 20 | 17/18 | Absent | 2 | 5/6 | 80.0 | 6.9 | 21 | P. australis | ||
| Braewick Voe | 32 | Lanceolate | 17 | 17 | Absent | 2 | 5/6 | 93.0 | 7.0 | 17 | P. australis | ||
| Braewick Voe | 32 | Lanceolate | 19 | 17/18 | Absent | 2 | 5/6 | 95.3 | 8.2 | 19/20 | P. australis | ||
| Braewick Voe | 32 | Lanceolate | 20 | 16/17 | Absent | 2 | 5/6 | 80.0 | 8.2 | 20 | P. australis | ||
| Inner Site 1 | 34 | Lanceolate | 18 | 15 | Absent | 8.9 | 20 | P. australis | P. seriata | Rows of poroids unclear | |||
| Bunya Sand | 34 | Linear-lanceolate | 28 | 38 | Present | 1 (+1?) | 5 | 46.5 | 2.0 | P. delicatissima | P. pseudodelicatissima * | Rows of poroids unclear | |
| Bunya Sand | 34 | Linear-lanceolate | 12 | 11/12 | Absent | 2 | 3 | 100.0 | 3.8 | 21 | P. pungens | ||
| Bunya Sand | 34 | Lanceolate | 17 | 17 | Not visible | 2 (+1) | 6/7 | 7.8 | 21 | P. seriata | P. australis | Rows of poroids unclear ** | |
| Bunya Sand | 34 | Lanceolate | 18 | 18 | Absent | 2 (+1?) | 5/6 | 95.8 | 8.4 | 21 | P. australis | P. seriata | Rows of poroids unclear |
| Bunya Sand | 34 | Lanceolate | 16 | 17/18 | Not visible | 2 | 5/6 | 7.8 | P. australis | ||||
| Bunya Sand | 34 | Lanceolate | 15 | 15 | Absent | 2 | 5 | 8.2 | 20 | P. australis | |||
| Bunya Sand | 34 | Lanceolate | 15/16 | 17/18 | Absent | 2 | 5 | 93.6 | 8.2 | 20 | P. australis | ||
| Bunya Sand | 34 | Lanceolate | 16 | 17 | Not visible | 2 | 5/6 | 8.0 | 19 | P. australis | Cell broken—nodule? | ||
| Bunya Sand | 34 | Lanceolate | 16 | 16 | Not visible | 2 | 5/6 | 81.5 | 8.1 | 21 | P. australis | ||
| Bunya Sand | 34 | Lanceolate | 17 | 17/18 | Absent | 2 | 5/6 | 82.2 | 7.7 | 20 | P. australis | ||
| Bunya Sand | 34 | Lanceolate | 18 | 17 | Absent | 2 | 6 | 91.0 | 7.9 | 20 | P. australis | ||
| Bunya Sand | 34 | Lanceolate | 19/20 | 18 | Absent | 2 | 5 | 94.9 | 8.5 | 20 | P. australis | ||
| Bunya Sand | 34 | Lanceolate | 20 | 17/18 | Absent | 2 | 5/6 | 101.5 | 8.3 | 20/21 | P. australis | ||
| Braewick Voe | 37 | Lanceolate | 22 | 22/23 | Present | 2 | 6 | 95.3 | 5.9 | P. fraudulenta | |||
| Braewick Voe | 37 | Lanceolate | 22 | 23/24 | Present | 2 | 5/6 | 63.3 | 6.2 | P. fraudulenta | P. plurisecta | Sectors in poroids unclear |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whyte, C.; Swan, S.C.; Turner, A.D.; Hatfield, R.G.; Mitchell, E.; Lafferty, S.; Morrell, N.; Rowland-Pilgrim, S.; Davidson, K. The Presence of Pseudo-nitzschia australis in North Atlantic Aquaculture Sites, Implications for Monitoring Amnesic Shellfish Toxins. Toxins 2023, 15, 554. https://doi.org/10.3390/toxins15090554
Whyte C, Swan SC, Turner AD, Hatfield RG, Mitchell E, Lafferty S, Morrell N, Rowland-Pilgrim S, Davidson K. The Presence of Pseudo-nitzschia australis in North Atlantic Aquaculture Sites, Implications for Monitoring Amnesic Shellfish Toxins. Toxins. 2023; 15(9):554. https://doi.org/10.3390/toxins15090554
Chicago/Turabian StyleWhyte, Callum, Sarah C. Swan, Andrew D. Turner, Robert G. Hatfield, Elaine Mitchell, Shannon Lafferty, Nadine Morrell, Stepahanie Rowland-Pilgrim, and Keith Davidson. 2023. "The Presence of Pseudo-nitzschia australis in North Atlantic Aquaculture Sites, Implications for Monitoring Amnesic Shellfish Toxins" Toxins 15, no. 9: 554. https://doi.org/10.3390/toxins15090554
APA StyleWhyte, C., Swan, S. C., Turner, A. D., Hatfield, R. G., Mitchell, E., Lafferty, S., Morrell, N., Rowland-Pilgrim, S., & Davidson, K. (2023). The Presence of Pseudo-nitzschia australis in North Atlantic Aquaculture Sites, Implications for Monitoring Amnesic Shellfish Toxins. Toxins, 15(9), 554. https://doi.org/10.3390/toxins15090554







