Oral and IV Varespladib Rescue Experiments in Juvenile Pigs with Weakness Induced by Australian and Papuan Oxyuranus scutellatus Venoms
Abstract
1. Introduction
2. Results
2.1. Venom Dose Finding
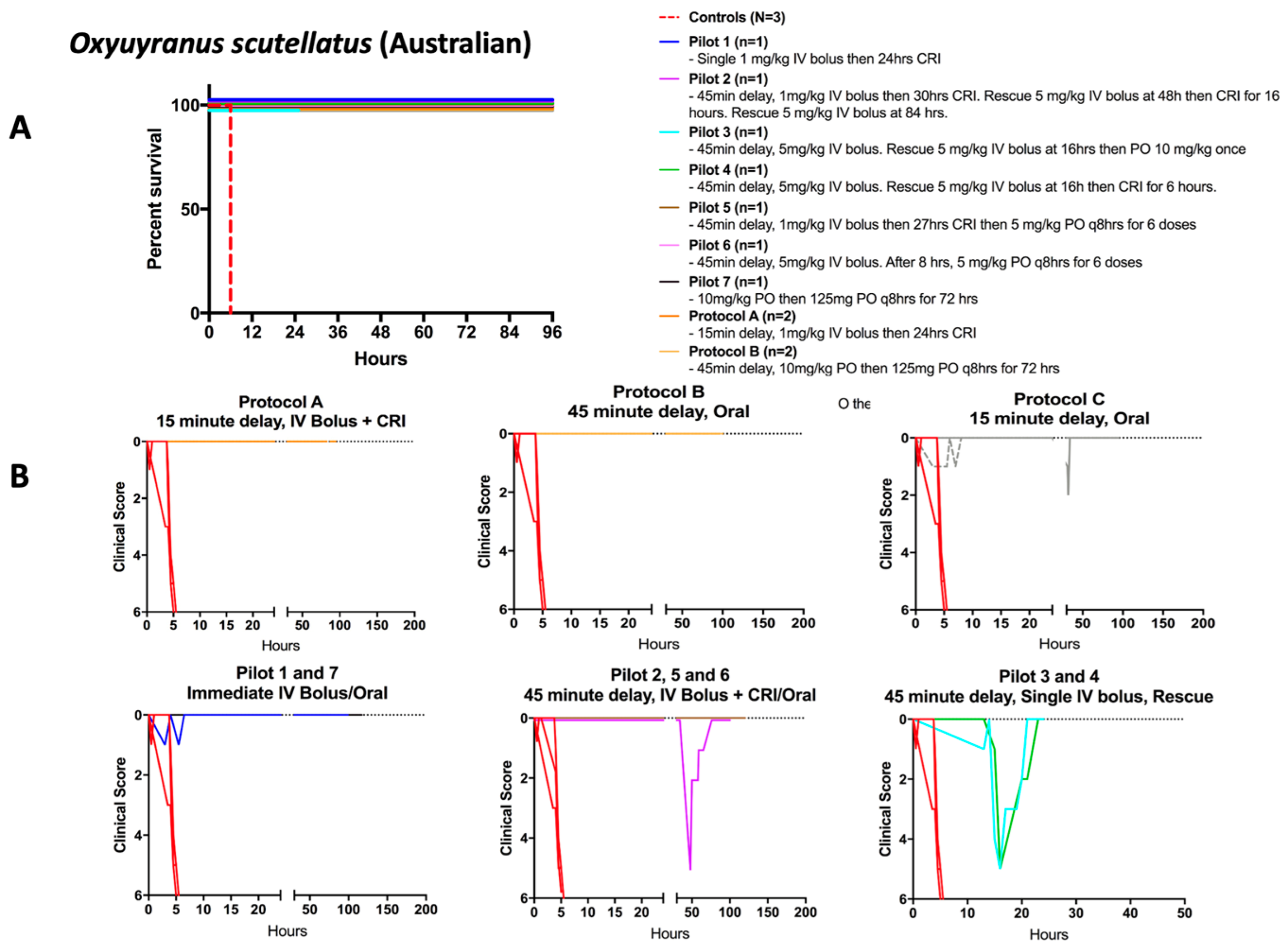
2.2. Rescue from Lethal Envenoming: Survival and Clinical Recovery from Weakness
2.3. Varespladib and Antivenom
2.4. Pulmonary Findings in Animals Experimentally Envenomed with Papuan Taipan Venom
3. Discussion
4. Materials and Methods
4.1. Venom, sPLA2 Inhibitor and Antivenom
4.2. Animal Methodology
4.2.1. Animals
4.2.2. Housing
4.2.3. Anesthesia, Instrumentation, and Monitoring
4.2.4. Intravenous Catheter Placement
4.2.5. Analgesia
4.2.6. Euthanasia
4.3. Envenoming
4.4. Lethality Dose Finding
4.5. Study Period Detail
- Every 15 min post-venom administration for the first 4 h following recovery from general anesthesia.
- Every 30 min from 4–8 h post-venom administration.
- Hourly from 8 h to 48 h post-venom administration.
- Every 6 h from 48 h through 96 h or study end.
4.6. Rescue Experiments with Varespladib and Antivenom
4.7. Tissue Pathology
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Longbottom, J.; Shearer, F.M.; Devine, M.; Alcoba, G.; Chappuis, F.; Weiss, D.J.; Ray, S.E.; Ray, N.; Warrell, D.A.; Ruiz de Castañeda, R.R.; et al. Vulnerability to snakebite envenoming: A global mapping of hotspots. Lancet 2018, 392, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Dandona, R.; Kumar, G.A.; Kharyal, A.; George, S.; Akbar, M.; Dandona, L. Mortality due to snakebite and other venomous animals in the Indian state of Bihar: Findings from a representative mortality study. PLoS ONE 2018, 13, e0198900. [Google Scholar] [CrossRef]
- Prasarnpun, S.; Walsh, J.; Harris, J.B. β-bungarotoxin-induced depletion of synaptic vesicles at the mammalian neuromuscular junction. Neuropharmacology 2004, 47, 304–314. [Google Scholar] [CrossRef]
- Prasarnpun, S.; Walsh, J.; Awad, S.S.; Harris, J.B. Envenoming bites by kraits: The biological basis of treatment-resistant neuromuscular paralysis. Brain 2005, 128, 2987–2996. [Google Scholar] [CrossRef] [PubMed]
- Anil, A.; Singh, S.; Bhalla, A.; Sharma, N.; Agarwal, R.; Simpson, I.D. Role of neostigmine and polyvalent antivenom in Indian common krait (Bungarus caeruleus) bite. J. Infect. Public Health 2010, 3, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, I.S.; Gandra, R.R.; Dhooria, S.; Aggarwal, A.N.; Prasad, K.T.; Muthu, V.; Sharma, N.; Agarwal, R. A randomised trial of adaptive support ventilation in patients with neuroparalytic snake envenomation. Br. J. Anaesth. 2022, 128, e232–e234. [Google Scholar] [CrossRef]
- Williams, D.J.; Faiz, M.A.; Abela-Ridder, B.; Ainsworth, S.; Bulfone, T.C.; Nickerson, A.D.; Habib, A.G.; Junghanss, T.; Fan, H.W.; Turner, M.; et al. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLoS Neglected Trop. Dis. 2019, 13, e0007059. [Google Scholar] [CrossRef]
- Lalloo, D.G.; Trevett, A.J.; Korinhona, A.; Nwokolo, N.; Laurenson, I.F.; Paul, M.; Black, J.; Naraqi, S.; Mavo, B.; Saweri, A.; et al. Snake Bites by the Papuan Taipan (Oxyuranus scutellatus canni): Paralysis, Hemostatic and Electrocardiographic Abnormalities, and Effects of Antivenom. Am. J. Trop. Med. Hyg. 1995, 52, 525–531. [Google Scholar] [CrossRef]
- Lewin, M.; Bulfone, T. Varespladib (LY315920) Rescues Mice from Rapidly Lethal Doses of Unfractionated Viper Venom. In Proceedings of the 19th World Congress of the International Society on Toxinology, Haikou, China, 24–31 October 2017. [Google Scholar]
- Bell, D.; Wijegunasinghe, D.; Samarakoon, S.; Palipana, H.; Gunasekera, S.; de Silva, H.A.; Lalloo, D.G.; Ranawaka, U.K.; de Silva, H.J. Neurophysiological findings in patients 1 year after snake bite induced neurotoxicity in Sri Lanka. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 351–356. [Google Scholar] [CrossRef][Green Version]
- Ranawaka, U.K.; Lalloo, D.G.; De Silva, H.J. Neurotoxicity in Snakebite—The Limits of Our Knowledge. PLoS Negl. Trop. Dis. 2013, 7, e2302. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Lohse, B.; Lomonte, B.; Engmark, M.; Gutiérrez, J.M. Selecting key toxins for focused development of elapid snake antivenoms and inhibitors guided by a Toxicity Score. Toxicon 2015, 104, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Gutiérrez, J.M.; Knudsen, C.; Johansen, K.H.; Bermúdez-Méndez, E.; Cerni, F.A.; Jürgensen, J.A.; Ledsgaard, L.; Martos-Esteban, A.; Øhlenschlæger, M.; et al. Pros and cons of different therapeutic antibody formats for recombinant antivenom development. Toxicon 2018, 146, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Sanz, L.; Quesada-Bernat, S.; Ramos, T.; Casais-E-Silva, L.L.; Corrêa-Netto, C.; Silva-Haad, J.J.; Sasa, M.; Lomonte, B.; Calvete, J.J. New insights into the phylogeographic distribution of the 3FTx/PLA2 venom dichotomy across genus Micrurus in South America. J. Proteom. 2019, 200, 90–101. [Google Scholar] [CrossRef]
- Bickler, P.E.; Abouyannis, M.; Bhalla, A.; Lewin, M.R. Neuromuscular Weakness and Paralysis Produced by Snakebite Envenoming: Mechanisms and Proposed Standards for Clinical Assessment. Toxins 2023, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.B.; Scott-Davey, T. Secreted Phospholipases A2 of Snake Venoms: Effects on the Peripheral Neuromuscular System with Comments on the Role of Phospholipases A2 in Disorders of the CNS and Their Uses in Industry. Toxins 2013, 5, 2533–2571. [Google Scholar] [CrossRef]
- Lalloo, D.G.; Trevett, A.J.; Nwokolo, N.; Laurenson, I.F.; Naraqi, S.; Kevau, I.; Kemp, M.W.; Hooper, R.J.L.; Theakston, R.D.G.; Warrell, D. Electrocardiographic abnormalities in patients bitten by taipans (Oxyuranus scutellatus canni) and other elapid snakes in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 53–56. [Google Scholar] [CrossRef]
- Crachi, M.T.; Hammer, L.W.; Hodgson, W.C. The effects of antivenom on the in vitro neurotoxicity of venoms from the taipans Oxyuranus scutellatus, Oxyuranus microlepidotus and Oxyuranus scutellatus canni. Toxicon 1999, 37, 1771–1778. [Google Scholar] [CrossRef]
- Kornhauser, R.; Hart, A.J.; Reeve, S.; Smith, A.I.; Fry, B.G.; Hodgson, W.C. Variations in the pharmacological profile of post-synaptic neurotoxins isolated from the venoms of the Papuan (Oxyuranus scutellatus canni) and coastal (Oxyuranus scutellatus scutellatus) taipans. Neurotoxicology 2010, 31, 239–243. [Google Scholar] [CrossRef]
- Connolly, S.; Trevett, A.J.; Nwokolo, N.C.; Lalloo, D.G.; Naraqi, S.; Mantle, D.; Schofield, I.S.; Fawcett, P.R.W.; Harris, J.B.; Warrell, D.A. Neuromuscular effects of papuan taipan snake venom. Ann. Neurol. 1995, 38, 916–920. [Google Scholar] [CrossRef]
- Trevett, A.J.; Lalloo, D.G.; Nwokolo, N.C.; Naraqi, S.; Kevau, I.H.; Theakston, R.D.G.; Warrell, D.A. Electrophysiological findings in patients envenomed following the bite of a Papuan taipan (Oxyuranus scutellatus canni). Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 415–417. [Google Scholar] [CrossRef]
- Chaisakul, J.; Isbister, G.K.; Tare, M.; Parkington, H.C.; Hodgson, W.C. Hypotensive and vascular relaxant effects of phospholipase A2 toxins from Papuan taipan (Oxyuranus scutellatus) venom. Eur. J. Pharmacol. 2014, 723, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Trevett, A.J.; Lalloo, D.G.; Nwokolo, N.C.; Naraqi, S.; Kevau, I.H.; Theakston, R.D.G.; Warrell, D.A. The efficacy of antivenom in the treatment of bites by the Papuan taipan (Oxyuranus scutellatus canni). Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 322–325. [Google Scholar] [CrossRef]
- Chaisakul, J.; Isbister, G.K.; Konstantakopoulos, N.; Tare, M.; Parkington, H.C.; Hodgson, W.C. In vivo and in vitro cardiovascular effects of Papuan taipan (Oxyuranus scutellatus) venom: Exploring “sudden collapse”. Toxicol. Lett. 2012, 213, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; de Cássia de O Collaço, R.; Villalta, M.; Segura, Á.; Vargas, M.; Wright, C.E.; Paiva, O.K.; Matainaho, T.; Jensen, S.D.; Leon, G.; et al. Neutralization of the neuromuscular inhibition of venom and taipoxin from the taipan (Oxyuranus scutellatus) by F(ab′) 2 and whole IgG antivenoms. Toxicol. Lett. 2016, 241, 175–183. [Google Scholar] [CrossRef]
- Fohlman, J.; Eaker, D.; Karlsson, E.; Thesleff, S. Taipoxin, an Extremely Potent Presynaptic Neurotoxin from the Venom of the Australian Snake Taipan (Oxyuranus s. scutellatus). Isolation, Characterization, Quaternary Structure and Pharmacological Properties. Eur. J. Biochem. 1976, 68, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, C.; Gutiørrez, J.M.; Lomonte, B.; Gutiérrez, J.M.; Lomonte, B. Cellular pathology induced by snake venom phospholipase A2 myotoxins and neurotoxins: Common aspects of their mechanisms of action. Cell. Mol. Life Sci. 2008, 65, 2897–2912. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Lomonte, B. Phospholipases A2: Unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon 2013, 62, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Vuong, N.T.; Jackson, T.N.W.; Wright, C.E. Role of Phospholipases A2 in Vascular Relaxation and Sympatholytic Effects of Five Australian Brown Snake, Pseudonaja spp., Venoms in Rat Isolated Tissues. Front. Pharmacol. 2021, 12, 754304. [Google Scholar] [CrossRef]
- Arias, A.S.; Rucavado, A.; Gutiérrez, J.M. Peptidomimetic hydroxamate metalloproteinase inhibitors abrogate local and systemic toxicity induced by Echis ocellatus (saw-scaled) snake venom. Toxicon 2017, 132, 40–49. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Engmark, M.; Milbo, C.; Johannesen, J.J.; Lomonte, B.; Gutiérrez, J.M.; Lohse, B.; Gutiérrez, J. From Fangs to Pharmacology: The Future of Snakebite Envenoming Therapy. Curr. Pharm. Des. 2016, 22, 5270–5293. [Google Scholar] [CrossRef]
- Lewin, M.; Samuel, S.; Merkel, J.; Bickler, P. Varespladib (LY315920) Appears to Be a Potent, Broad-Spectrum, Inhibitor of Snake Venom Phospholipase A2 and a Possible Pre-Referral Treatment for Envenomation. Toxins 2016, 8, 248. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhang, D.; Xiao, H.; Xiong, S.; Huang, C. Exploration of the Inhibitory Potential of Varespladib for Snakebite Envenomation. Molecules 2018, 23, 391. [Google Scholar] [CrossRef]
- Bittenbinder, M.A.; Zdenek, C.N.; op den Brouw, B.; Youngman, N.J.; Dobson, J.S.; Naude, A.; Vonk, F.J.; Fry, B.G. Coagulotoxic Cobras: Clinical Implications of Strong Anticoagulant Actions of African Spitting Naja Venoms That Are Not Neutralised by Antivenom but Are by LY315920 (Varespladib). Toxins 2018, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Lewin, M.R.; María Gutiérrez, J.; Samuel, S.P.; Herrera, M.; Bryan-Quirós, W.; Lomonte, B.; Bickler, P.E.; Bulfone, T.C.; Williams, D.J. Delayed Oral LY333013 Rescues Mice from Highly Neurotoxic, Lethal Doses of Papuan Taipan (Oxyuranus scutellatus) Venom. Toxins 2018, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Lewin, M.R.; Gilliam, L.L.; Gilliam, J.; Samuel, S.P.; Bulfone, T.C.; Bickler, P.E.; Gutiérrez, J.M. Delayed LY333013 (Oral) and LY315920 (Intravenous) Reverse Severe Neurotoxicity and Rescue Juvenile Pigs from Lethal Doses of Micrurus fulvius (Eastern Coral Snake) Venom. Toxins 2018, 10, 479. [Google Scholar] [CrossRef]
- Fontana Oliveira, I.C.; Gutiérrez, J.M.; Lewin, M.R.; Oshima-Franco, Y. Varespladib (LY315920) inhibits neuromuscular blockade induced by Oxyuranus scutellatus venom in a nerve-muscle preparation. Toxicon 2020, 187, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Lind, P.; Eaker, D. Amino-Acid Sequence of the Alpha-Subunit of Taipoxin, an Extremely Potent Presynaptic Neurotoxin from the Australian Snake Taipan (Oxyuranus s. Scutellatus). Eur. J. Biochem. 1982, 124, 441–447. [Google Scholar] [CrossRef]
- Lipps, B.V. Isolation of Subunits, Alpha, Beta and Gamma of the Complex Taipoxin from the Venom of Australian Taipan Snake (Oxyuranus s. Scutellatus): Characterization of Beta Taipoxin as a Potent Mitogen. Toxicon 2000, 38, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.B.; Grubb, B.D.; Maltin, C.A.; Dixon, R. The Neurotoxicity of the Venom Phospholipases A2, Notexin and Taipoxin. Exp. Neurol. 2000, 161, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Fernández, J.; Vargas, M.; Villalta, M.; Segura, Á.; León, G.; Angulo, Y.; Paiva, O.; Matainaho, T.; Jensen, S.D.; et al. Comparative proteomic analysis of the venom of the taipan snake, Oxyuranus scutellatus, from Papua New Guinea and Australia: Role of neurotoxic and procoagulant effects in venom toxicity. J. Proteom. 2012, 75, 2128–2140. [Google Scholar] [CrossRef]
- Cendron, L.; Mičetić, I.; Polverino de Laureto, P.; Paoli, M. Structural analysis of trimeric phospholipase A2 neurotoxin from the Australian taipan snake venom. FEBS J. 2012, 279, 3121–3135. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, N.J.; Stevens, K.B.; Sepulveda, M.F.; Jarvis, N.; Marr, C.M. Repeatability and reproducibility of the Obel grading system for equine laminitis. Veter. Rec. 2010, 167, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Burnouf, T.; Harrison, R.A.; Calvete, J.J.; Kuch, U.; Warrell, D.A.; Williams, D.J. A multicomponent strategy to improve the availability of antivenom for treating snakebite envenoming. Bull. World Health Organ. 2014, 92, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.; Segura, A.; Herrera, M.; Villalta, M.; Estrada, R.; Cerdas, M.; Paiva, O.; Matainaho, T.; Jensen, S.D.; Winkel, K.D.; et al. Preclinical Evaluation of Caprylic Acid-Fractionated IgG Antivenom for the Treatment of Taipan (Oxyuranus scutellatus) Envenoming in Papua New Guinea. PLoS Neglected Trop. Dis. 2011, 5, e1144. [Google Scholar] [CrossRef]
- Herrera, M.; Paiva, O.K.; Pagotto, A.H.; Segura, Á.; Serrano, S.M.T.; Vargas, M.; Villalta, M.; Jensen, S.D.; León, G.; Williams, D.J.; et al. Antivenomic Characterization of Two Antivenoms Against the Venom of the Taipan, Oxyuranus scutellatus, from Papua New Guinea and Australia. Am. J. Trop. Med. Hyg. 2014, 91, 887–894. [Google Scholar] [CrossRef]
- Navarro, D.; Vargas, M.; Herrera, M.; Segura, Á.; Gómez, A.; Villalta, M.; Ramírez, N.; Williams, D.; Gutiérrez, J.M.; León, G. Development of a chicken-derived antivenom against the taipan snake (Oxyuranus scutellatus) venom and comparison with an equine antivenom. Toxicon 2016, 120, 1–8. [Google Scholar] [CrossRef]
- Campbell, C.H. The taipan (Oxyuranus scutellatus) and the effect of its bite. Med. J. Aust. 1967, 1, 735–739. [Google Scholar] [CrossRef]
- Lalloo, D.G.; Trevett, A.J.; Owens, D.; Minei, J.; Naraqi, S.; Saweri, A.; Hutton, R.A.; Theakston, R.D.G.; Warrell, D.A. Coagulopathy following bites by the Papuan taipan (Oxyuranus scutellatus canni). Blood Coagul. Fibrinolysis 1995, 6, 65–72. [Google Scholar] [CrossRef]
- Zdenek, C.N.; Hay, C.; Arbuckle, K.; Jackson, T.N.W.; Bos, M.H.A.; op den Brouw, B.; Debono, J.; Allen, L.; Dunstan, N.; Morley, T.; et al. Coagulotoxic effects by brown snake (Pseudonaja) and taipan (Oxyuranus) venoms, and the efficacy of a new antivenom. Toxicol. Vitr. 2019, 58, 97–109. [Google Scholar] [CrossRef]
- Johnston, C.I.; Ryan, N.M.; O’Leary, M.A.; Brown, S.G.A.; Isbister, G.K. Australian taipan (Oxyuranus spp.) envenoming: Clinical effects and potential benefits of early antivenom therapy—Australian Snakebite Project (ASP-25). Clin. Toxicol. 2017, 55, 115–122. [Google Scholar] [CrossRef]
- Johnston, C.I.; Brown, S.G.A.; O’leary, M.A.; Currie, B.J.; Greenberg, R.; Taylor, M.; Barnes, C.; White, J.; Isbister, G.K. Mulga snake (Pseudechis australis) envenoming: A spectrum of myotoxicity, anticoagulant coagulopathy, haemolysis and the role of early antivenom therapy—Australian Snakebite Project (ASP-19). Clin. Toxicol. 2013, 51, 417–424. [Google Scholar] [CrossRef]
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Prim. 2017, 3, 1763. [Google Scholar] [CrossRef]
- Harrison, R.A.; Hargreaves, A.; Wagstaff, S.C.; Faragher, B.; Lalloo, D.G. Snake Envenoming: A Disease of Poverty. PLoS Negl. Trop. Dis. 2009, 3, e569. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, B.; Warrell, D.A.; Suraweera, W.; Bhatia, P.; Dhingra, N.; Jotkar, R.M.; Rodriguez, P.S.; Mishra, K.; Whitaker, R.; Jha, P.; et al. Snakebite Mortality in India: A Nationally Representative Mortality Survey. PLoS Neglected Trop. Dis. 2011, 5, e1018. [Google Scholar] [CrossRef] [PubMed]
- Lewin, M.R.; Carter, R.W.; Matteo, I.A.; Samuel, S.P.; Rao, S.; Fry, B.G.; Bickler, P.E. Varespladib in the Treatment of Snakebite Envenoming: Development History and Preclinical Evidence Supporting Advancement to Clinical Trials in Patients Bitten by Venomous Snakes. Toxins 2022, 14, 783. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.W.; Gerardo, C.J.; Samuel, S.P.; Kumar, S.; Kotehal, S.D.; Mukherjee, P.P.; Shirazi, F.M.; Akpunonu, P.D.; Bammigatti, C.; Bhalla, A.; et al. The BRAVO Clinical Study Protocol: Oral Varespladib for Inhibition of Secretory Phospholipase A2 in the Treatment of Snakebite Envenoming. Toxins 2022, 15, 22. [Google Scholar] [CrossRef]
- Davis, S.S.; Illum, L.; Hinchcliffe, M. Gastrointestinal transit of dosage forms in the pig. J. Pharm. Pharmacol. 2001, 53, 33–39. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Abouyannis, M.; Aggarwal, D.; Lalloo, D.G.; Casewell, N.R.; Hamaluba, M.; Esmail, H. Clinical outcomes and outcome measurement tools reported in randomised controlled trials of treatment for snakebite envenoming: A systematic review. PLOS Neglected Trop. Dis. 2021, 15, e0009589. [Google Scholar] [CrossRef]
- Abouyannis, M.; Esmail, H.; Hamaluba, M.; Ngama, M.; Mwangudzah, H.; Mumba, N.; Yeri, B.K.; Mwalukore, S.; Alphan, H.J.; Aggarwal, D.; et al. A global core outcome measurement set for snakebite clinical trials. Lancet Glob. Health 2023, 11, e296–e300. [Google Scholar] [CrossRef]
- Villalta-Romero, F.; Gortat, A.; Herrera, A.E.; Arguedas, R.; Quesada, J.; de Melo, R.L.; Calvete, J.J.; Montero, M.; Murillo, R.; Rucavado, A.; et al. Identification of New Snake Venom Metalloproteinase Inhibitors Using Compound Screening and Rational Peptide Design. ACS Med. Chem. Lett. 2012, 3, 540–543. [Google Scholar] [CrossRef]
- Salvador, G.H.M.; Gomes, A.A.S.; Bryan-Quirós, W.; Fernández, J.; Lewin, M.R.; Gutiérrez, J.M.; Lomonte, B.; Fontes, M.R.M. Structural basis for phospholipase A2-like toxin inhibition by the synthetic compound Varespladib (LY315920). Sci. Rep. 2019, 9, 17203. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.D.; Lima Neto, J.X.; Bezerra, K.S.; Oliveira, J.I.N.; Machado, L.D.; Fulco, U.L. Quantum Biochemical Investigation of Lys49-PLA2 from Bothrops moojeni. J. Phys. Chem. B 2021, 125, 12972–12980. [Google Scholar] [CrossRef] [PubMed]
- Bickler, P.E. Amplification of Snake Venom Toxicity by Endogenous Signaling Pathways. Toxins 2020, 12, 68. [Google Scholar] [CrossRef] [PubMed]



| Score | Clinical Condition |
|---|---|
| 0 | Normal activity, interested in food/water/toys, normal grunting, curious about environment, responds normally to stimulation by moving away, rises easily and quickly from recumbency when stimulated. |
| 1 | Normal movement around pen, reduced interest in food/water/toys, reduced interaction with environment or caretakers, rises from recumbency and moves away when stimulated. |
| 2 | Evidence of weakness in one or more limbs, reduced interest in food/water/toys, reduced responsiveness to stimulation but still able to rise normally from recumbency. |
| 3 | Significant evidence of weakness (dog sitting) but able to rise unassisted when stimulated, some interest in food/water/toys, can stay up for >3 min. |
| 4 | Significant evidence of weakness (dog sitting), requires assistance to rise but can still stand, stays up >15 s but less than 3 min. |
| 5 | Significant evidence of weakness (dog sitting), requires assistance to stand but unable to remain standing for >15 s. |
| 6 | Marked evidence of weakness (sternal or lateral recumbency) unable to rise even with assistance. |
| Protocol (# of Animals) | Treatment | Initial Dosage Administration | Result |
|---|---|---|---|
| Pilot 1 (n = 1) | Venom + IV varespladib | -Immediate loading dose -1 mg/kg over 15 min -CRI for 20 h | -Survival to end of study period |
| Pilot 2 (n = 1) | Venom + IV varespladib | -45 min delay -loading dose 1 mg/kg over 15 min -CRI for 30 h | -Relapse at 36 h, rescue IV bolus 5 mg/kg -Relapse at 48 h, rescue IV bolus 5 mg/kg followed by 16 h CRI -Survival to end of study period |
| Pilot 3 (n = 1) | Venom + IV varespladib with switch to oral | -45 min delay, -loading dose 5 mg/kg over 15 min | -Relapse at 16 h, rescue IV bolus 5 mg/kg and Oral 10 mg/kg × 1 -Survival to end of study period |
| Pilot 4 (n = 1) | Venom + IV varespladib | -45 min delay -loading dose 5 mg/kg over 15 min | -Relapse at 16 h, rescue IV bolus 5 mg/kg followed by 6 h CRI -Survival to end of study period |
| Pilot 5 (n = 1) | Venom + IV varespladib and switch to oral varespladib | -45 min delay, loading dose 1 mg/kg over 15 min -CRI for ~27 h -Oral 5 mg/kg every 8 h for 48 h | -Survival to end of study period |
| Pilot 6 (n = 1) | Venom + IV varespladib and switch to oral varespladib | -45 min delay, loading dose 5 mg/kg over 15 min -Wait 8 h -Oral 5 mg/kg every 8 h | -Relapse at 105 h, rescue IV bolus 5 mg/kg and oral 5 mg/kg q8 h x6 -Survival to end of study period |
| Pilot 7 (n = 1) | Venom + Oral varespladib | -Immediate orogastric (OG) tube 10 mg/kg -Wait 8 h -Oral 5 mg/kg every 8 h for 72 h | -Survival to end of study period |
| Protocol A (n = 2) | Venom + IV varespladib | -15 min delay, loading dose 1 mg/kg over 15 min -CRI for 23 h and 25 h | -Survival to end of study period |
| Protocol B (n = 2) | Venom + Oral varespladib | -45 min delay, OG tube 10 mg/kg -Wait 8 h -Oral 5 mg/kg every 8 h for 72 h | -Survival to end of study period |
| Protocol C (n = 2) | Venom + Oral varespladib | -15 min delay, OG tube 5 mg/kg -Wait 8 h -Oral 5 mg/kg every 8 h for 72 h | -Survival to end of study period |
| Protocol (# of Animals) | Treatment | Initial Dosage Administration | Results |
|---|---|---|---|
| Protocol D (n = 2) | Venom + antivenom | -45 min delay -Antivenom 5 mL diluted in 25 mL LRS given over 10 min per manufacturer’s instructions | Euthanasia at 2.8 and 3.1 h, respectively |
| Protocol E (n = 2) | Venom + IV varespladib | -45 min delay, loading dose 1 mg/kg IV over 15 min -CRI (0.25–0.5 mg/kg/h) for 140–167 h | -Relapse 17–18 h later, rescue with IV bolus 1 mg/kg over 10 min -Survival to end of study |
| Protocol F (n = 2) | Venom + antivenom | -Immediate antivenom 5 mL diluted in 25 mL LRS given over 15 min | -Survival to end of study |
| Protocol G (n = 2) | Venom + antivenom + IV varespladib (rescue) | -45 min delay, antivenom 5 mL diluted in 25 mL LRS given over 28 min -CRI until 145 h post venom with rescue IV bolus prn weakness | Surviving animal: Severe signs/symptoms at 4.2 h post venom and 3.5 h after antivenom, rescue with single IV rescue bolus 5 mg/kg at 81 h and continuation of CRI. Survival to end of study. Euthanized animal: Milder signs/symptoms at approximately 4 h. Euthanized at 22 h for confirmed S. suis infection |
| Treatment Group | Protocol | Animals with Identified Pulmonary Emboli |
|---|---|---|
| Venom only (Control) | Control | 2 of 3 |
| Venom + antivenom (45 min delay) | D | 2 of 2 |
| Venom + varespladib (45 min delay) | E | 0 of 2 |
| Venom + antivenom (immediate) | F | 0 of 2 |
| Venom + antivenom (45 min delay) + varespladib rescue | G | 1 of 2 |
| Lameness Score | Clinical Picture |
|---|---|
| 0 | No abnormalities noted in ability to stand or walk |
| 1 | Slight limp or favoring of limb(s) noted while walking but stands normally |
| 2 | Slight limp or favoring of limb(s) noted while walking and favoring of limb(s) apparent when standing |
| 3 | Significant limp or favoring of limb (s) when walking, bears minimal weight on limb(s) when standing |
| 4 | Reluctant, but able/willing to bear weight when standing or walking |
| 5 | Unable/unwilling to bear weight on limb (s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilliam, L.L.; Gilliam, J.; Samuel, S.P.; Carter, R.W.; Ritchey, J.; Bulfone, T.; Gutiérrez, J.M.; Williams, D.J.; Durkin, D.M.; Stephens, S.I.; et al. Oral and IV Varespladib Rescue Experiments in Juvenile Pigs with Weakness Induced by Australian and Papuan Oxyuranus scutellatus Venoms. Toxins 2023, 15, 557. https://doi.org/10.3390/toxins15090557
Gilliam LL, Gilliam J, Samuel SP, Carter RW, Ritchey J, Bulfone T, Gutiérrez JM, Williams DJ, Durkin DM, Stephens SI, et al. Oral and IV Varespladib Rescue Experiments in Juvenile Pigs with Weakness Induced by Australian and Papuan Oxyuranus scutellatus Venoms. Toxins. 2023; 15(9):557. https://doi.org/10.3390/toxins15090557
Chicago/Turabian StyleGilliam, Lyndi L., John Gilliam, Stephen P. Samuel, Rebecca W. Carter, Jerry Ritchey, Tommaso Bulfone, José María Gutiérrez, David J. Williams, Daniela M. Durkin, Sally I. Stephens, and et al. 2023. "Oral and IV Varespladib Rescue Experiments in Juvenile Pigs with Weakness Induced by Australian and Papuan Oxyuranus scutellatus Venoms" Toxins 15, no. 9: 557. https://doi.org/10.3390/toxins15090557
APA StyleGilliam, L. L., Gilliam, J., Samuel, S. P., Carter, R. W., Ritchey, J., Bulfone, T., Gutiérrez, J. M., Williams, D. J., Durkin, D. M., Stephens, S. I., & Lewin, M. R. (2023). Oral and IV Varespladib Rescue Experiments in Juvenile Pigs with Weakness Induced by Australian and Papuan Oxyuranus scutellatus Venoms. Toxins, 15(9), 557. https://doi.org/10.3390/toxins15090557







