Improvement in Quality-of-Life-Related Outcomes Following Treatment with IncobotulinumtoxinA in Adults with Limb Spasticity: A Pooled Analysis
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics
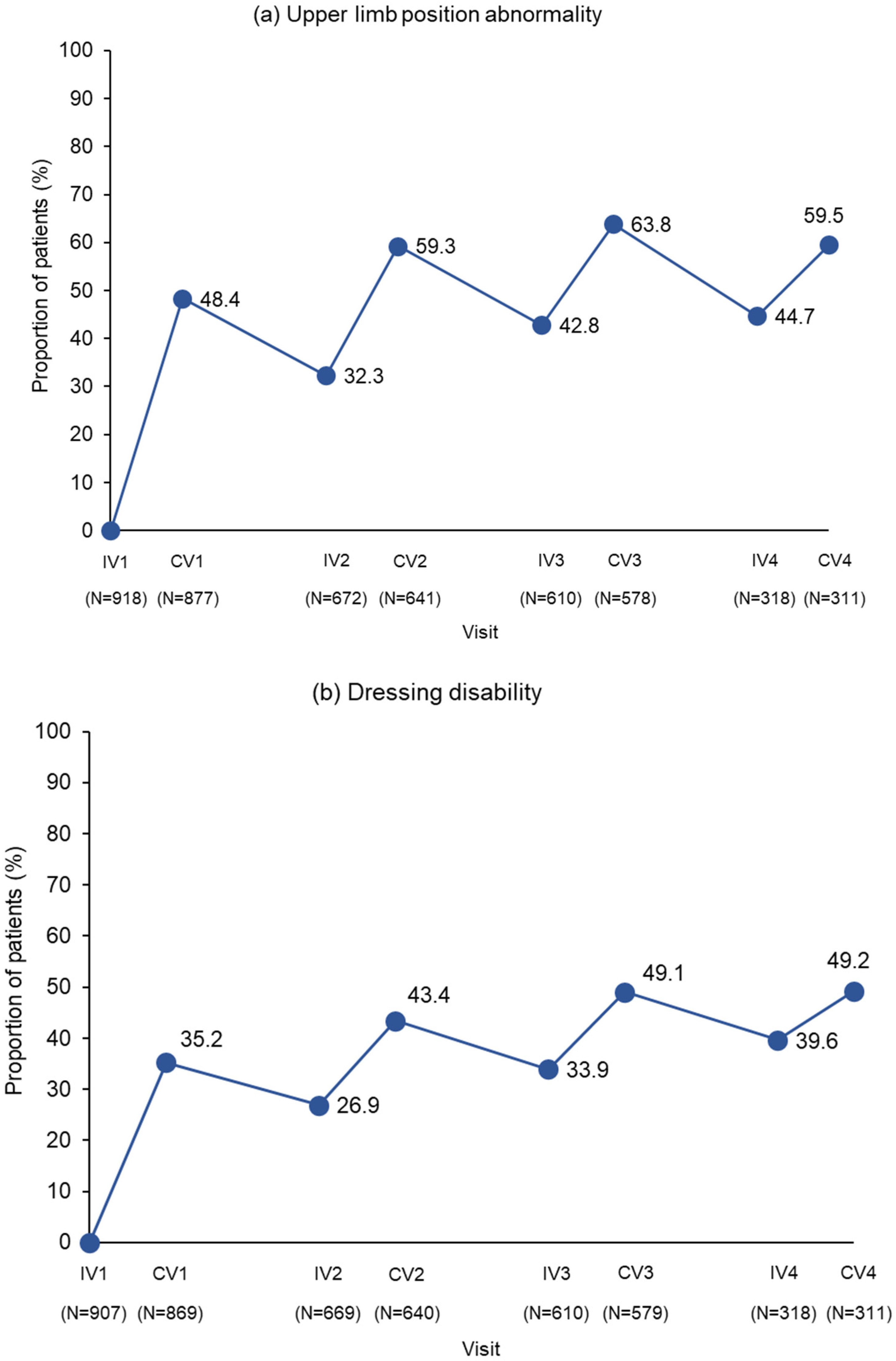
2.2. Response Rates at Week 4 Post First Injection
2.3. Response Rates Following Multiple Injection Cycles
2.4. Response Rates for the PTT
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Studies Included in the Analysis
5.2. Disability Assessment Scale
5.3. Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dressler, D.; Bhidayasiri, R.; Bohlega, S.; Chana, P.; Chien, H.F.; Chung, T.M.; Colosimo, C.; Ebke, M.; Fedoroff, K.; Frank, B.; et al. Defining spasticity: A new approach considering current movement disorders terminology and botulinum toxin therapy. J. Neurol. 2018, 265, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-L.; Hu, G.-C. Post-stroke spasticity: A review of epidemiology, pathophysiology, and treatments. Int. J. Gerontol. 2018, 12, 280–284. [Google Scholar] [CrossRef]
- Amatya, B.; Khan, F.; Galea, M. Rehabilitation for people with multiple sclerosis: An overview of Cochrane Reviews. Cochrane. Database. Syst. Rev. 2019, 1, CD012732. [Google Scholar] [CrossRef] [PubMed]
- Vitrikas, K.; Dalton, H.; Breish, D. Cerebral Palsy: An Overview. Am. Fam. Physician. 2020, 101, 213–220. [Google Scholar] [PubMed]
- Skoog, B.; Jakobsson, K.E. Prevalence of spasticity and below-level neuropathic pain related to spinal cord injury level and damage to the lower spinal segments. J. Rehabil. Med. Clin. Commun. 2020, 3, 1000039. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, V.; Amatya, B.; Khan, F. Overview of systematic reviews: Management of common traumatic brain injury-related complications. PLoS ONE 2022, 17, e0273998. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Chen, J.; Guo, Y.; Tan, S. Prevalence and risk factors for spasticity after stroke: A systematic review and meta-analysis. Front. Neurol. 2021, 11, 616097. [Google Scholar] [CrossRef] [PubMed]
- Pollock, A.; Farmer, S.E.; Brady, M.C.; Langhorne, P.; Mead, G.E.; Mehrholz, J.; van Wijck, F. Interventions for improving upper limb function after stroke. Cochrane Database Syst. Rev. 2014, 2014, CD010820. [Google Scholar] [CrossRef]
- Trompetto, C.; Marinelli, L.; Mori, L.; Pelosin, E.; Currà, A.; Molfetta, L.; Abbruzzese, G. Pathophysiology of spasticity: Implications for neurorehabilitation. Biomed. Res. Int. 2014, 2014, 354906. [Google Scholar] [CrossRef]
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef]
- Royal College of Physicians; British Society of Rehabilitation Medicine; Chartered Society of Physiotherapy; Association of Chartered Physiotherapists in Neurology; Royal College of Occupational Therapists. Spasticity in Adults: Management Using Botulinum Toxin. National Guidelines. 2018. Available online: https://www.rcplondon.ac.uk/file/12449/download (accessed on 6 November 2023).
- Kaňovský, P.; Slawek, J.; Denes, Z.; Platz, T.; Sassin, I.; Comes, G.; Grafe, S. Efficacy and safety of botulinum neurotoxin NT 201 in poststroke upper limb spasticity. Clin. Neuropharmacol. 2009, 32, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Kaňovský, P.; Slawek, J.; Denes, Z.; Platz, T.; Comes, G.; Grafe, S.; Pulte, I. Efficacy and safety of treatment with incobotulinum toxin A (botulinum neurotoxin type A free from complexing proteins; NT 201) in post-stroke upper limb spasticity. J. Rehabil. Med. 2011, 43, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Elovic, E.P.; Munin, M.C.; Kaňovský, P.; Hanschmann, A.; Hiersemenzel, R.; Marciniak, C. Randomized, placebo-controlled trial of incobotulinumtoxinA for upper-limb post-stroke spasticity. Muscle. Nerve. 2016, 53, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Bensmail, D.; Ferreira, J.J.; Molteni, F.; Satkunam, L.; Moraleda, S.; Rekand, T.; McGuire, J.; Scheschonka, A.; Flatau-Baqué, B.; et al. TOWER study investigators. Safety and efficacy of incobotulinumtoxinA doses up to 800 U in limb spasticity: The TOWER study. Neurology 2017, 88, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, C.; Munin, M.C.; Brashear, A.; Rubin, B.S.; Patel, A.T.; Slawek, J.; Hanschmann, A.; Hiersemenzel, R.; Elovic, E.P. IncobotulinumtoxinA treatment in upper-limb poststroke spasticity in the open-label extension period of PURE: Efficacy in passive function, caregiver burden, and quality of life. PM R 2020, 12, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Masakado, Y.; Abo, M.; Kondo, K.; Saeki, S.; Saitoh, E.; Dekundy, A.; Hanschmann, A.; Kaji, R.; J-PURE Study Group. Efficacy and safety of incobotulinumtoxinA in post-stroke upper-limb spasticity in Japanese subjects: Results from a randomized, double-blind, placebo-controlled study (J-PURE). J. Neurol. 2020, 267, 2029–2041. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.; Schnitzler, A.; Medeiros, L.; Aguilar, M.; Lehnert-Batar, A.; Minnasch, P. Efficacy and safety of NT 201 for upper limb spasticity of various etiologies—a randomized parallel-group study. Acta Neurol. Scand. 2010, 122, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Elovic, E.P.; Brashear, A.; Kaelin, D.; Liu, J.; Millis, S.R.; Barron, R.; Turkel, C. Repeated treatments with botulinum toxin type a produce sustained decreases in the limitations associated with focal upper-limb poststroke spasticity for caregivers and patients. Arch. Phys. Med. Rehabil. 2008, 89, 799–806. [Google Scholar] [CrossRef]
- Brashear, A.; Zafonte, R.; Corcoran, M.; Galvez-Jimenez, N.; Gracies, J.M.; Gordon, M.F.; McAfee, A.; Ruffing, K.; Thompson, B.; Williams, M.; et al. Inter- and intrarater reliability of the Ashworth Scale and the Disability Assessment Scale in patients with upper-limb poststroke spasticity. Arch. Phys. Med. Rehabil. 2002, 83, 1349–1354. [Google Scholar] [CrossRef]
- Gracies, J.M.; Jech, R.; Valkovic, P.; Marque, P.; Vecchio, M.; Denes, Z.; Vilain, C.; Delafont, B.; Picaut, P. When can maximal efficacy occur with repeat botulinum toxin injection in upper limb spastic paresis? Brain. Commun. 2020, 3, fcaa201. [Google Scholar] [CrossRef]
- Francisco, G.E.; Bandari, D.S.; Bavikatte, G.; Jost, W.H.; McCusker, E.; Largent, J.; Zuzek, A.; Esquenazi, A. High clinician- and patient-reported satisfaction with individualized onabotulinumtoxinA treatment for spasticity across several etiologies from the ASPIRE study. Toxicon X 2020, 7, 100040. [Google Scholar] [CrossRef] [PubMed]
- Synnot, A.; Chau, M.; Pitt, V.; O’Connor, D.; Gruen, R.L.; Wasiak, J.; Clavisi, O.; Pattuwage, L.; Phillips, K. Interventions for managing skeletal muscle spasticity following traumatic brain injury. Cochrane Database Syst. Rev. 2017, 11, CD008929. [Google Scholar] [CrossRef] [PubMed]
- Gillard, P.J.; Sucharew, H.; Kleindorfer, D.; Belagaje, S.; Varon, S.; Alwell, K.; Moomaw, C.J.; Woo, D.; Khatri, P.; Flaherty, M.L.; et al. The negative impact of spasticity on the health-related quality of life of stroke survivors: A longitudinal cohort study. Health Qual. Life Outcomes 2015, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, S.; Smith, J.G.; Cary, I.; Dalton, C.; Pinto, A.; Ward, C.; Saverino, A. The positive and the negative impacts of spasticity in patients with long-term neurological conditions: An observational study. Disabil. Rehabil. 2021, 43, 3357–3364. [Google Scholar] [CrossRef] [PubMed]
- Doan, Q.V.; Brashear, A.; Gillard, P.J.; Varon, S.F.; Vandenburgh, A.M.; Turkel, C.C.; Elovic, E.P. Relationship between disability and health-related quality of life and caregiver burden in patients with upper limb poststroke spasticity. PM R 2012, 4, 4–10. [Google Scholar] [CrossRef]
- Ganapathy, V.; Graham, G.D.; DiBonaventura, M.D.; Gillard, P.J.; Goren, A.; Zorowitz, R.D. Caregiver burden, productivity loss, and indirect costs associated with caring for patients with poststroke spasticity. Clin. Interv. Aging 2015, 10, 1793–1802. [Google Scholar]
- Zorowitz, R.D.; Gillard, P.J.; Brainin, M. Poststroke spasticity: Sequelae and burden on stroke survivors and caregivers. Neurology 2013, 80, S45–S52. [Google Scholar] [CrossRef]
- Wissel, J.; Camões-Barbosa, A.; Comes, G.; Althaus, M.; Scheschonka, A.; Simpson, D.M. Pain reduction in adults with limb spasticity following treatment with incobotulinumtoxinA: A pooled analysis. Toxins 2021, 13, 887. [Google Scholar] [CrossRef]
- Tennigkeit, F.; Durand-Lagarde, M.; Flatau-Baqué, B.; Merz Therapeutics GmbH, Frankfurt/Main, Germany. Unpublished work. 2008.
- Hung, J.W.; Wu, W.C.; Chen, Y.J.; Pong, Y.P.; Chang, K.C. Predictors of clinically important improvements in motor function and daily use of affected arm after a botulinum toxin A injection in patients with chronic stroke. Toxins 2021, 14, 13. [Google Scholar]
- Patel, A.T.; Ward, A.B.; Geis, C.; Jost, W.H.; Liu, C.; Dimitrova, R. Impact of early intervention with onabotulinumtoxinA treatment in adult patients with post-stroke lower limb spasticity: Results from the double-blind, placebo-controlled, phase 3 REFLEX study. J. Neural. Transm. 2020, 127, 1619–1629. [Google Scholar] [CrossRef]
- Wissel, J.; Fheodoroff, K.; Hoonhorst, M.; Müngersdorf, M.; Gallien, P.; Meier, N.; Hamacher, J.; Hefter, H.; Maisonobe, P.; Koch, M. Effectiveness of abobotulinumtoxinA in post-stroke upper limb spasticity in relation to timing of treatment. Front. Neurol. 2020, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Wissel, J.; Jost, W.H.; Castagna, A.; Althaus, M.; Comes, G.; Scheschonka, A.; Vacchelli, M.; Jinnah, H.A. Pain reduction in cervical dystonia following treatment with incobotulinumtoxinA: A pooled analysis. Toxins 2023, 15, 333. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.M.; O’Dell, M.; Vecchio, M.; Hedera, P.; Kocer, S.; Rudzinska-Bar, M.; Rubin, B.; Timerbaeva, S.L.; Lusakowska, A.; Boyer, F.C.; et al. International AbobotulinumtoxinA Adult Upper Limb Spasticity Study Group. Effects of repeated abobotulinumtoxinA injections in upper limb spasticity. Muscle. Nerve. 2018, 57, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kaňovský, P.; Elovic, E.P.; Munin, M.C.; Hanschmann, A.; Pulte, I.; Althaus, M.; Hiersemenzel, R.; Marciniak, C. Sustained efficacy of incobotulinumtoxinA repeated injections for upper-limb post-stroke spasticity: A post hoc analysis. J. Rehabil. Med. 2021, 53, jrm00138. [Google Scholar] [CrossRef] [PubMed]
- Brashear, A.; Gordon, M.F.; Elovic, E.; Kassicieh, V.D.; Marciniak, C.; Do, M.; Lee, C.H.; Jenkins, S.; Turkel, C.; Botox Post-Stroke Spasticity Study Group. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke. N. Engl. J. Med. 2002, 347, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.M.; Brashear, A.; Jech, R.; McAllister, P.; Banach, M.; Valkovic, P.; Walker, H.; Marciniak, C.; Deltombe, T.; Skoromets, A.; et al. International AbobotulinumtoxinA Adult Upper Limb Spasticity Study Group. Safety and efficacy of abobotulinumtoxinA for hemiparesis in adults with upper limb spasticity after stroke or traumatic brain injury: A double-blind randomised controlled trial. Lancet. Neurol. 2015, 14, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.C.; Chen, R.; Fu, C.; Chen, Y.; Wu, Q.; Chen, R.; Lin, X.; Luo, S. Efficacy and safety of botulinum toxin type A for limb spasticity after stroke: A meta-analysis of randomized controlled trials. Biomed. Res. Int. 2019, 2019, 8329306. [Google Scholar] [CrossRef]
- Kaji, R.; Osako, Y.; Suyama, K.; Maeda, T.; Uechi, Y.; Iwasaki, M. GSK1358820 Spasticity Study Group. Botulinum toxin type A in post-stroke upper limb spasticity. Curr. Med. Res. Opin. 2010, 26, 1983–1992. [Google Scholar] [CrossRef]
- Fujimura, K.; Kagaya, H.; Onaka, H.; Okochi, Y.; Yamada, M.; Ternanashi, T.; Kanada, Y.; Saitoh, E. Improvement in Disability Assessment Scale after Botulinum toxin A treatment for upper limb spasticity. Japan J. Comp. Rehab. Sci. 2017, 8, 4–9. [Google Scholar] [CrossRef]
- Walter, U.; Mühlenhoff, C.; Benecke, R.; Dressler, D.; Mix, E.; Alt, J.; Wittstock, M.; Dudesek, A.; Storch, A.; Kamm, C. Frequency and risk factors of antibody-induced secondary failure of botulinum neurotoxin therapy. Neurology 2020, 94, e2109–e2120. [Google Scholar] [CrossRef]
- LaCroix-Desmazes, S.; Mouly, S.; Popoff, M.-R.; Colosio, C. Systematic analysis of botulinum neurotoxin type A immunogenicity in clinical studies. Basal. Ganglia. 2017, 9, 12–17. [Google Scholar] [CrossRef]
- Naumann, M.; Boo, L.M.; Ackerman, A.H.; Gallagher, C.J. Immunogenicity of botulinum toxins. J. Neural. Transm. 2013, 120, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Zakin, E.; Simpson, D. Evidence on botulinum toxin in selected disorders. Toxicon 2018, 147, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N.; Scott, L.J. IncobotulinumtoxinA: A review in upper limb spasticity. Drugs 2016, 76, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Petracca, M.; Lo Monaco, M.R.; Ialongo, T.; Di Stasio, E.; Cerbarano, M.L.; Maggi, L.; De Biase, A.; Di Lazzaro, G.; Calabresi, P.; Bentivoglio, A.R. Efficacy and safety of long-term botulinum toxin treatment for acquired cervical dystonia: A 25-year follow-up. J. Neurol. 2023, 270, 340–347. [Google Scholar] [CrossRef]
- Rodrigues, F.B.; Duarte, G.S.; Marques, R.E.; Castelão, M.; Ferreira, J.; Sampaio, C.; Moore, A.P.; Costa, J. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst. Rev. 2020, 11, CD003633. [Google Scholar]
- Trevidic, P.; Connolly, S.A.; Biwer, B.; Ellers-Lenz, B.; Harrington, L.S.; Kestemont, P.; Noah, E.M.; Sattler, G.; Weissenberger, P.; Kerscher, M. IncobotulinumtoxinA is an effective and well-tolerated treatment for upper facial lines: Results from an open-label extension period of a Phase III study. Dermatol. Surg. 2017, 43, S285–S292. [Google Scholar] [CrossRef]
- Han, Y.; Stevens, A.L.; Dashtipour, K.; Hauser, R.A.; Mari, Z. A mixed treatment comparison to compare the efficacy and safety of botulinum toxin treatments for cervical dystonia. J. Neurol. 2016, 263, 772–780. [Google Scholar] [CrossRef]
- Dressler, D.; Rychlik, R.; Kreimendahl, F.; Schnur, N.; Lambert-Baumann, J. Long-term efficacy and safety of incobotulinumtoxinA and conventional treatment of poststroke arm spasticity: A prospective, non-interventional, open-label, parallel-group study. BMJ Open 2015, 30, e009358. [Google Scholar] [CrossRef]
- Kerscher, M.; Rzany, B.; Prager, W.; Turnbull, C.; Trevidic, P.; Inglefield, C. Efficacy and safety of incobotulinumtoxinA in the treatment of upper facial lines: Results from a randomized, double-blind, placebo-controlled, Phase III study. Dermatol. Surg. 2015, 41, 1149–1157. [Google Scholar] [CrossRef]
- Jost, W.H.; Benecke, R.; Hauschke, D.; Jankovic, J.; Kaňovský, P.; Roggenkämper, P.; Simpson, D.M.; Comella, C.L. Clinical and pharmacological properties of incobotulinumtoxinA and its use in neurological disorders. Drug Des. Devel. Ther. 2015, 9, 1913–1926. [Google Scholar] [CrossRef]
- Evidente, V.G.; Truong, D.; Jankovic, J.; Comella, C.L.; Grafe, S.; Hanschmann, A. IncobotulinumtoxinA (Xeomin®) injected for blepharospasm or cervical dystonia according to patient needs is well tolerated. J. Neurol. Sci. 2014, 346, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, M.; Cirillo, P.; Fundarò, S.P.; Quartucci, S.; Sciuto, C.; Sito, G.; Tonini, D.; Trocchi, G.; Signorini, M. Safety of botulinum toxin A in aesthetic treatments: A systematic review of clinical studies. Dermatol. Surg. 2014, 40, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Evidente, V.G.; Fernandez, H.H.; LeDoux, M.S.; Brashear, A.; Grafe, S.; Hanschmann, A.; Comella, C.L. A randomized, double-blind study of repeated incobotulinumtoxinA (Xeomin(®)) in cervical dystonia. J. Neural. Transm. 2013, 120, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Dressler, D.; Paus, S.; Seitzinger, A.; Gebhardt, B.; Kupsch, A. Long-term efficacy and safety of incobotulinumtoxinA injections in patients with cervical dystonia. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, H.H.; Pappert, E.J.; Comella, C.L.; Evidente, V.G.; Truong, D.D.; Verma, A.; Jankovic, J. Efficacy and safety of incobotulinumtoxinA in subjects previously treated with botulinum toxin versus toxin-naïve subjects with cervical dystonia. Tremor. Other Hyperkinet. Mov. 2013, 3, tre-03-140-2921-1. [Google Scholar] [CrossRef]
- Truong, D.D.; Gollomp, S.M.; Jankovic, J.; LeWitt, P.A.; Marx, M.; Hanschmann, A.; Fernandez, H.H.; Xeomin US Blepharospasm Study Group. Sustained efficacy and safety of repeated incobotulinumtoxinA (Xeomin(®)) injections in blepharospasm. J. Neural. Transm. 2013, 120, 1345–1353. [Google Scholar] [CrossRef]
- Hallett, M.; Albanese, A.; Dressler, D.; Segal, K.R.; Simpson, D.M.; Truong, D.; Jankovic, J. Evidence-based review and assessment of botulinum neurotoxin for the treatment of movement disorders. Toxicon 2013, 67, 94–114. [Google Scholar] [CrossRef]
- Esquenazi, A.; Albanese, A.; Chancellor, M.B.; Elovic, E.; Segal, K.R.; Simpson, D.M.; Smith, C.P.; Ward, A.B. Evidence-based review and assessment of botulinum neurotoxin for the treatment of adult spasticity in the upper motor neuron syndrome. Toxicon 2013, 67, 115–128. [Google Scholar] [CrossRef]
- Pagan, F.L.; Harrison, A. A guide to dosing in the treatment of cervical dystonia and blepharospasm with Xeomin®: A new botulinum neurotoxin A. Parkinsonism. Relat. Disord. 2012, 18, 441–445. [Google Scholar] [CrossRef]
- Comella, C.L.; Jankovic, J.; Truong, D.D.; Hanschmann, A.; Grafe, S.U.S. XEOMIN Cervical Dystonia Study Group. Efficacy and safety of incobotulinumtoxinA (NT 201, XEOMIN®, botulinum neurotoxin type A, without accessory proteins) in patients with cervical dystonia. J. Neurol. Sci. 2011, 308, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Comella, C.; Hanschmann, A.; Grafe, S. Efficacy and safety of incobotulinumtoxinA (NT 201, Xeomin) in the treatment of blepharospasm-a randomized trial. Mov. Disord. 2011, 26, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Samadzadeh, S.; Ürer, B.; Brauns, R.; Rosenthal, D.; Lee, J.I.; Albrecht, P.; Hefter, H. Clinical implications of difference in antigenicity of different botulinum neurotoxin type a preparations: Clinical take-home messages from our research pool and literature. Toxins 2020, 12, 499. [Google Scholar] [CrossRef] [PubMed]
- Samizadeh, S.; De Boulle, K. Botulinum neurotoxin formulations: Overcoming the confusion. Clin. Cosmet. Investig. Dermatol. 2018, 11, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Hefter, H.; Ürer, B.; Brauns, R.; Rosenthal, D.; Meuth, S.G.; Lee, J.I.; Albrecht, P.; Samadzadeh, S. Significant long-lasting improvement after switch to incobotulinum toxin in cervical dystonia patients with secondary treatment failure. Toxins 2022, 14, 44. [Google Scholar] [CrossRef]
- Carr, W.W.; Jain, N.; Sublett, J.W. Immunogenicity of botulinum toxin formulations: Potential therapeutic implications. Adv. Ther. 2021, 38, 5046–5064. [Google Scholar] [CrossRef]
- Bellows, S.; Jankovic, J. Immunogenicity associated with botulinum toxin treatment. Toxins 2019, 11, 491. [Google Scholar] [CrossRef]
- Kerscher, M.; Wanitphakdeedecha, R.; Trindade de Almeida, A.; Maas, C.; Frevert, J. IncobotulinumtoxinA: A highly purified and precisely manufactured botulinum neurotoxin type A. J. Drugs Dermatol. 2019, 18, 52–57. [Google Scholar]
- Jimenez-Shahed, J. A new treatment for focal dystonias: IncobotulinumtoxinA (Xeomin®), a botulinum neurotoxin type A free from complexing proteins. Neuropsychiatr. Dis. Treat. 2012, 8, 13–25. [Google Scholar] [CrossRef]
- Albrecht, P.; Jansen, A.; Lee, J.I.; Moll, M.; Ringelstein, M.; Rosenthal, D.; Bigalke, H.; Aktas, O.; Hartung, H.P.; Hefter, H. High prevalence of neutralizing antibodies after long-term botulinum neurotoxin therapy. Neurology 2019, 92, e48–e54. [Google Scholar] [CrossRef]
- Benecke, R. Clinical relevance of botulinum toxin immunogenicity. BioDrugs 2012, 26, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Car, H.; Bogucki, A.; Bonikowski, M.; Dec-Ćwiek, M.; Drużdż, A.; Koziorowski, D.; Rudzińska-Bar, M.; Sarzyńska-Długosz, I.; Sławek, J. Botulinum toxin type-A preparations are not the same medications—Basic science (Part 1). Neurol. Neurochir. Pol. 2021, 55, 133140. [Google Scholar] [CrossRef] [PubMed]
- Hefter, H.; Brauns, R.; Ürer, B.; Rosenthal, D.; Albrecht, P. Effective long-term treatment with incobotulinumtoxin (Xeomin®) without neutralizing antibody induction: A monocentric, cross-sectional study. J. Neurol. 2020, 267, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Hefter, H.; Hartmann, C.J.; Kahlen, U.; Samadzadeh, S.; Rosenthal, D.; Moll, M. Clinical improvement after treatment with incobotulinumtoxinA (XEOMIN) in patients with cervical dystonia resistant to botulinum toxin preparations containing complexing proteins. Front. Neurol. 2021, 12, 636590. [Google Scholar] [CrossRef] [PubMed]
- Hefter, H.; Ürer, B.; Brauns, R.; Rosenthal, D.; Meuth, S.G.; Lee, J.I.; Albrecht, P.; Samadzadeh, S. The complex relationship between antibody titers and clinical outcome in botulinum toxin type A long-term treated patients with cervical dystonia. J. Neurol. 2022, 269, 5991–6002. [Google Scholar] [CrossRef]
- Hefter, H.; Rosenthal, D.; Jansen, A.; Brauns, R.; Ürer, B.; Bigalke, H.; Hartung, H.P.; Meuth, S.G.; Lee, J.I.; Albrecht, P.; et al. Significantly lower antigenicity of incobotulinumtoxin than abo- or onabotulinumtoxin. J. Neurol. 2023, 270, 788–796. [Google Scholar] [CrossRef]
- Marciniak, C.; Munin, M.C.; Brashear, A.; Rubin, B.S.; Patel, A.T.; Slawek, J.; Hanschmann, A.; Hiersemenzel, R.; Elovic, E.P. IncobotulinumtoxinA efficacy and safety in adults with upper-limb spasticity following stroke: Results from the open-label extension period of a phase 3 study. Adv. Ther. 2019, 36, 187–199. [Google Scholar] [CrossRef]



| DAS Domain | Limb Position | Dressing | Hygiene | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | INCO (n = 699) | Placebo (n = 219) | Total (n = 918) | INCO (n = 690) | Placebo (n = 217) | Total (n = 907) | INCO (n = 655) | Placebo (n = 210) | Total (n = 865) |
| Mean ± SD age, years | 55.9 ± 12.8 | 56.3 ± 12.2 | 56.0 ± 12.6 | 56.1 ± 12.6 | 56.2 ± 1 2.3 | 56.1 ± 12.5 | 56.2 ± 12.6 | 56.4 ± 12.3 | 56.2 ± 12.5 |
| Sex, n (%) | |||||||||
| Male | 435 (62.2) | 136 (62.1) | 571 (62.2) | 430 (62.3) | 134 (61.8) | 564 (62.2) | 403 (61.5) | 128 (61.0) | 531 (61.4) |
| Female | 264 (37.8) | 83 (37.9) | 347 (37.8) | 260 (37.7) | 83 (38.2) | 343 (37.8) | 252 (38.5) | 82 (39.0) | 334 (38.6) |
| Ethnicity, n (%) | |||||||||
| White | 535 (76.5) | 170 (77.6) | 705 (76.8) | 530 (76.8) | 169 (77.9) | 699 (77.1) | 506 (77.3) | 166 (79.1) | 672 (77.7) |
| Black or African American | 11 (1.6) | 3 (1.4) | 14 (1.5) | 10 (1.5) | 3 (1.4) | 13 (1.4) | 10 (1.5) | 3 (1.4) | 13 (1.5) |
| Asian | 95 (13.6) | 46 (21.0) | 141 (15.4) | 95 (13.8) | 45 (20.7) | 140 (15.4) | 89 (13.6) | 41 (19.5) | 130 (15.0) |
| Other | 6 (0.9) | 0 | 6 (0.7) | 6 (0.9) | 0 | 6 (0.7) | 5 (0.8) | 0 | 5 (0.6) |
| Missing | 52 (7.4) | 0 | 52 (5.7) | 49 (7.1) | 0 | 49 (5.4) | 45 (6.9) | 0 | 45 (5.2) |
| Mean ± SD height, cm | 168.3 ± 9.5 a | 168.9 ± 8.2 | 168.5 ± 9.2 | 168.3 ± 9.5 b | 168.7 ± 8.2 | 168.4 ± 9.2 | 168.2 ± 9.5 c | 168.6 ± 8.3 | 168.3 ± 9.2 |
| Mean ± SD weight, kg | 75.4 ± 14.9 d | 75.3 ± 14.5 | 75.3 ± 14.8 | 75.4 ± 14.9 e | 75.2 ± 14.6 | 75.4 ± 14.8 | 75.5 ± 14.8 f | 75.4 ± 14.7 | 75.5 ± 14.8 |
| BoNT-A naïve, n (%) | 324 (46.4) | 154 (70.3) | 478 (52.1) | 321 (46.5) | 155 (71.4) | 476 (52.5) | 310 (47.3) | 150 (71.4) | 460 (53.2) |
| Etiology of spasticity, n (%) | |||||||||
| Stroke | 652 (93.3) | 218 (99.5) | 870 (94.8) | 648 (93.9) | 216 (99.5) | 864 (95.3) | 616 (94.1) | 210 (100.0) | 826 (95.5) |
| Multiple sclerosis | 1 (0.1) | 1 (0.5) | 2 (0.2) | 1 (0.1) | 1 (0.5) | 2 (0.2) | 1 (0.2) | 0 | 1 (0.1) |
| Infantile cerebral palsy | 6 (0.9) | 0 | 6 (0.7) | 6 (0.9) | 0 | 6 (0.7) | 3 (0.5) | 0 | 3 (0.4) |
| Brain injury | 22 (3.2) | 0 | 22 (2.4) | 20 (2.9) | 0 | 20 (2.2) | 19 (2.9) | 0 | 19 (2.2) |
| Other | 18 (2.6) | 0 | 18 (2.0) | 15 (2.2) | 0 | 15 (1.7) | 16 (2.4) | 0 | 16 (1.9) |
| DAS score g at baseline, n (%) | |||||||||
| 1 = mild | 74 (10.6) | 22 (10.0) | 96 (10.5) | 114 (16.5) | 27 (12.4) | 141 (15.6) | 140 (21.4) | 25 (11.9) | 165 (19.1) |
| 2 = moderate | 345 (49.4) | 123 (56.2) | 468 (51.0) | 380 (55.1) | 128 (59.0) | 508 (56.0) | 340 (51.9) | 122 (58.1) | 462 (53.4) |
| 3 = severe | 280 (40.1) | 74 (33.8) | 354 (38.6) | 196 (28.4) | 62 (28.6) | 258 (28.5) | 175 (26.7) | 63 (30.0) | 238 (27.5) |
| Mean ± SD time since diagnosis of spasticity, years | 5.5 ± 6.5 | 3.9 ± 5.0 | 5.1 ± 6.2 | 5.5 ± 6.4 | 3.9 ± 5.0 | 5.1 ± 6.1 | 5.3 ± 6.2 | 4.0 ± 5.0 | 5.0 ± 6.0 |
| Time Since Stroke (Years) | IncobotulinumtoxinA | Placebo | Difference (IncobotulinumtoxinA–Placebo) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Participants with Data (n) | Response Rate (%) | 95% CI | Participants with Data (n) | Response Rate (%) | 95% CI | Response Rate (%) | 95% CI | p-Value | |
| Limb position abnormality | |||||||||
| 0–2 | 262 | 44.3 | [38.3–50.3] | 115 | 23.5 | [15.7–31.2] | 20.8 | [11.0–30.6] | <0.0001 |
| 3–5 | 169 | 45.0 | [37.5–52.5] | 54 | 16.7 | [6.7–26.6] | 28.3 | [15.9–40.8] | <0.0001 |
| 6–10 | 143 | 45.5 | [37.3–53.6] | 31 | 12.9 | [1.1–24.7] | 32.6 | [18.2–46.9] | <0.0001 |
| >10 | 94 | 53.2 | [43.1–63.3] | 17 | 11.8 | [0.0–27.1] | 41.4 | [23.1–59.8] | <0.0001 |
| Dressing disability | |||||||||
| 0–2 | 261 | 34.5 | [28.7–40.2] | 113 | 21.2 | [13.7–28.8] | 13.2 | [3.8–22.7] | 0.0063 |
| 3–5 | 167 | 26.9 | [20.2–33.7] | 54 | 13.0 | [4.0–21.9] | 14.0 | [2.8–25.2] | 0.0144 |
| 6–10 | 141 | 29.8 | [22.2–37.3] | 31 | 19.4 | [5.4–33.3] | 10.4 | [−5.4–26.3] | 0.1963 |
| >10 | 91 | 36.3 | [26.4–46.1] | 17 | 17.6 | [0.0–35.8] | 18.6 | [−2.0–39.3] | 0.0771 |
| Hygiene-related disability | |||||||||
| 0–2 | 251 | 31.1 | [25.4–36.8] | 106 | 17.0 | [9.8–24.1] | 14.1 | [4.9–23.3] | 0.0026 |
| 3–5 | 159 | 32.1 | [24.8–39.3] | 54 | 20.4 | [9.6–31.1] | 11.7 | [−1.3–24.7] | 0.0768 |
| 6–10 | 134 | 38.1 | [29.8–46.3] | 31 | 22.6 | [7.9–37.3] | 15.5 | [−1.4–32.3] | 0.0719 |
| >10 | 83 | 32.5 | [22.5–42.6] | 17 | 23.5 | [3.4–43.7] | 9.0 | [−13.5–31.5] | 0.4339 |
| Study Name/NCT Number (Merz ID) (Reference) | Phase | Countries | Study Design and Objective | Study Period | Primary Outcome | Treatment (Total Body Dose) | Subjects and Indication |
|---|---|---|---|---|---|---|---|
| (MRZ_60201_03071) [30] Not published (study terminated due to low recruitment) | 2 | Germany | Prospective, randomized, double-blind, placebo-controlled, parallel-group, multicenter pilot study (12 weeks) to investigate the efficacy and safety of incobotulinumtoxinA in the treatment of pain in upper limb spasticity | Main period | Mean evening pain intensity measured using the 11-point Box Scale | One treatment cycle: IncobotulinumtoxinA (up to 400 U; range: 240–400 U) Placebo | n = 14 adults with pain caused by upper limb spasticity due to multiple etiologies |
| NCT00432666 (MRZ_60201_0410) (Kanovsky et al. [12]; Kanovsky et al. [13]) | 3 | Czech Republic, Hungary, Poland | Prospective, randomized, double-blind, placebo-controlled, parallel-group, multicenter trial (20 weeks) with an open-label extension period (69 weeks) to investigate the efficacy and safety of incobotulinumtoxinA in the treatment of poststroke upper limb spasticity | Main Period | Wrist flexor response rate (≥1-point improvement in AS score) at week 4 | One treatment cycle incobotulinumtoxinA (intended up to 400 U; median 320 U; range: 80–435 U) Placebo | n = 148 adults with poststroke upper limb spasticity |
| OLEX | Five treatment cycles: IncobotulinumtoxinA (intended up to 400 U; 1st cycle median 385 U, others 400 U) | n = 145 adults (from main period) | |||||
| NT-SPIN NCT00465738 (MRZ_60201_06071) (Barnes et al. [18]) | 3 | Austria, France, Germany, Italy, Portugal, Spain, Switzerland, United Kingdom | Prospective, randomized, observer-blind, parallel-group, multicenter trial (20 weeks) to assess efficacy and safety of two different dilutions of incobotulinumtoxinA in patients with upper limb spasticity | Main Period | DAS response rate (≥1-point improvement) at week 4 a | One treatment cycle: IncobotulinumtoxinA (two dilutions: (20 or 50 U/mL) (intended up to 400 U; median 300 U; actual up to 495 U) | n = 192 adults with stable upper limb spasticity of diverse etiology |
| PURE NCT01392300 (MRZ_60201_SP3001) (Elovic et al. [14]; Marciniak et al. [79]; Marciniak et al. [16]) | 3 | Czech Republic, Germany, Hungary, India, Poland, Russian Federation, United States of America | Prospective, randomized, double-blind, placebo-controlled, parallel-group, multicenter study (12 weeks) with an open-label extension period (36 weeks) to investigate the efficacy and safety of incobotulinumtoxinA in the treatment of poststroke upper limb spasticity | Main Period | Change in muscle tone from baseline to week 4, measured using the AS b | One treatment cycle: IncobotulinumtoxinA (400 U) Placebo | n = 317 adults with poststroke upper limb spasticity |
| OLEX | Three treatment cycles: IncobotulinumtoxinA (400 U) | n = 299 (from main period) | |||||
| TOWER NCT01603459 (MRZ_60201_3053) (Wissel et al. [15]) | 3 | Canada, France, Germany, Italy, Norway, Portugal, Spain, United States of America | Prospective, nonrandomized, open-label, single-arm, multicenter dose-titration study (48 weeks) to investigate the safety and efficacy of incobotulinumtoxinA in subjects requiring doses of 800 U during the course of the study for the treatment of upper and lower limb spasticity | Main Period | Safety | Three treatment cycles: IncobotulinumtoxinA (IC1: 400, IC2: 600, IC3: ≤800 U c), | n = 155 adults with chronic upper and lower limb spasticity of the same body side due to cerebral causes |
| J-PURE JapicCTI Number: CTI-153029 (MRZ_60201_30991) (Masakado et al. [17]) | 3 | Japan | Prospective, randomized, double-blind, placebo-controlled, parallel-group, multicenter study (52 weeks in total), with an open-label lead-in tolerability period (1 week), a main study period (12 weeks) and an open-label extension period (32–40 weeks), to investigate the efficacy and safety of two different doses of incobotulinumtoxinA in the treatment of poststroke upper limb spasticity | Main Period | Change in muscle tone from baseline to week 4, measured using the modified AS | One treatment cycle: IncobotulinumtoxinA (400 U or 250 U) Placebo | n = 100 adults with poststroke upper limb spasticity |
| OLEX | Three treatment cycles: IncobotulinumtoxinA (400 U) | n = 90 (from main period) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molteni, F.; Wissel, J.; Fheodoroff, K.; Munin, M.C.; Patel, A.T.; Althaus, M.; Comes, G.; Dekundy, A.; Pulte, I.; Scheschonka, A.; et al. Improvement in Quality-of-Life-Related Outcomes Following Treatment with IncobotulinumtoxinA in Adults with Limb Spasticity: A Pooled Analysis. Toxins 2024, 16, 19. https://doi.org/10.3390/toxins16010019
Molteni F, Wissel J, Fheodoroff K, Munin MC, Patel AT, Althaus M, Comes G, Dekundy A, Pulte I, Scheschonka A, et al. Improvement in Quality-of-Life-Related Outcomes Following Treatment with IncobotulinumtoxinA in Adults with Limb Spasticity: A Pooled Analysis. Toxins. 2024; 16(1):19. https://doi.org/10.3390/toxins16010019
Chicago/Turabian StyleMolteni, Franco, Jörg Wissel, Klemens Fheodoroff, Michael C. Munin, Atul T. Patel, Michael Althaus, Georg Comes, Andrzej Dekundy, Irena Pulte, Astrid Scheschonka, and et al. 2024. "Improvement in Quality-of-Life-Related Outcomes Following Treatment with IncobotulinumtoxinA in Adults with Limb Spasticity: A Pooled Analysis" Toxins 16, no. 1: 19. https://doi.org/10.3390/toxins16010019





