Reusable and Practical Biocomposite Based on Sphingopyxis sp. YF1 and Polyacrylonitrile-Based Carbon Fiber for the Efficient Bioremediation of Microcystin-LR-Contaminated Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of PAN-CF@YF1
2.2. The Selection of Immobilization Conditions for Sphingopyxis sp. YF1
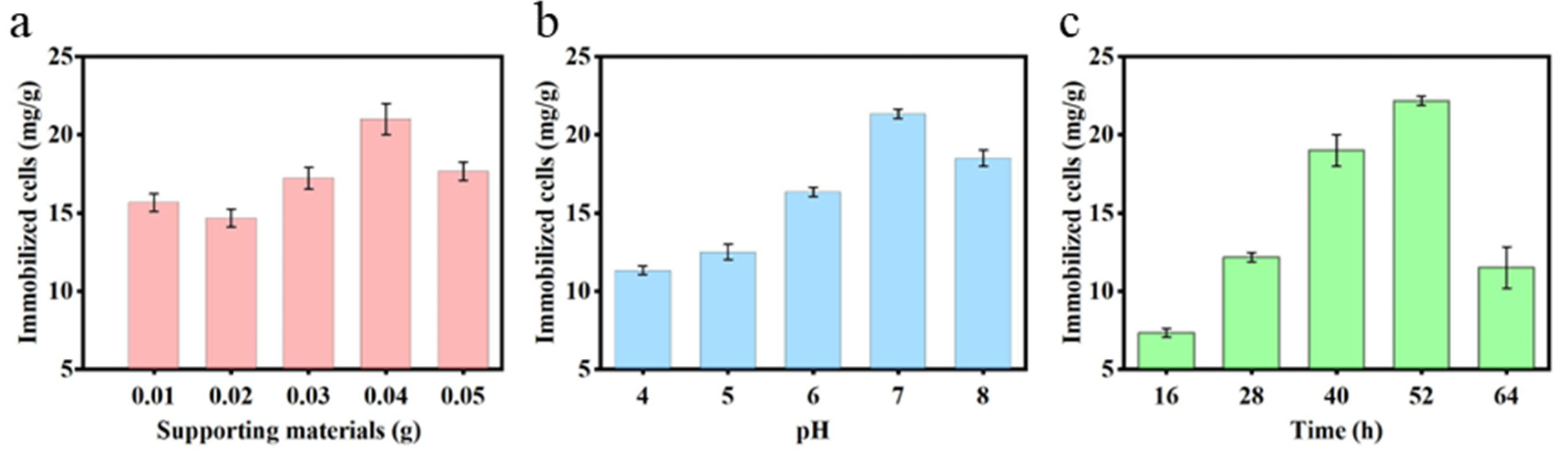
2.2.1. Effect of Supporting Materials
2.2.2. Effect of pH and Time
2.3. Box–Behnken Design (BBD) for Sphingopyxis sp. YF1 Immobilization Improvement
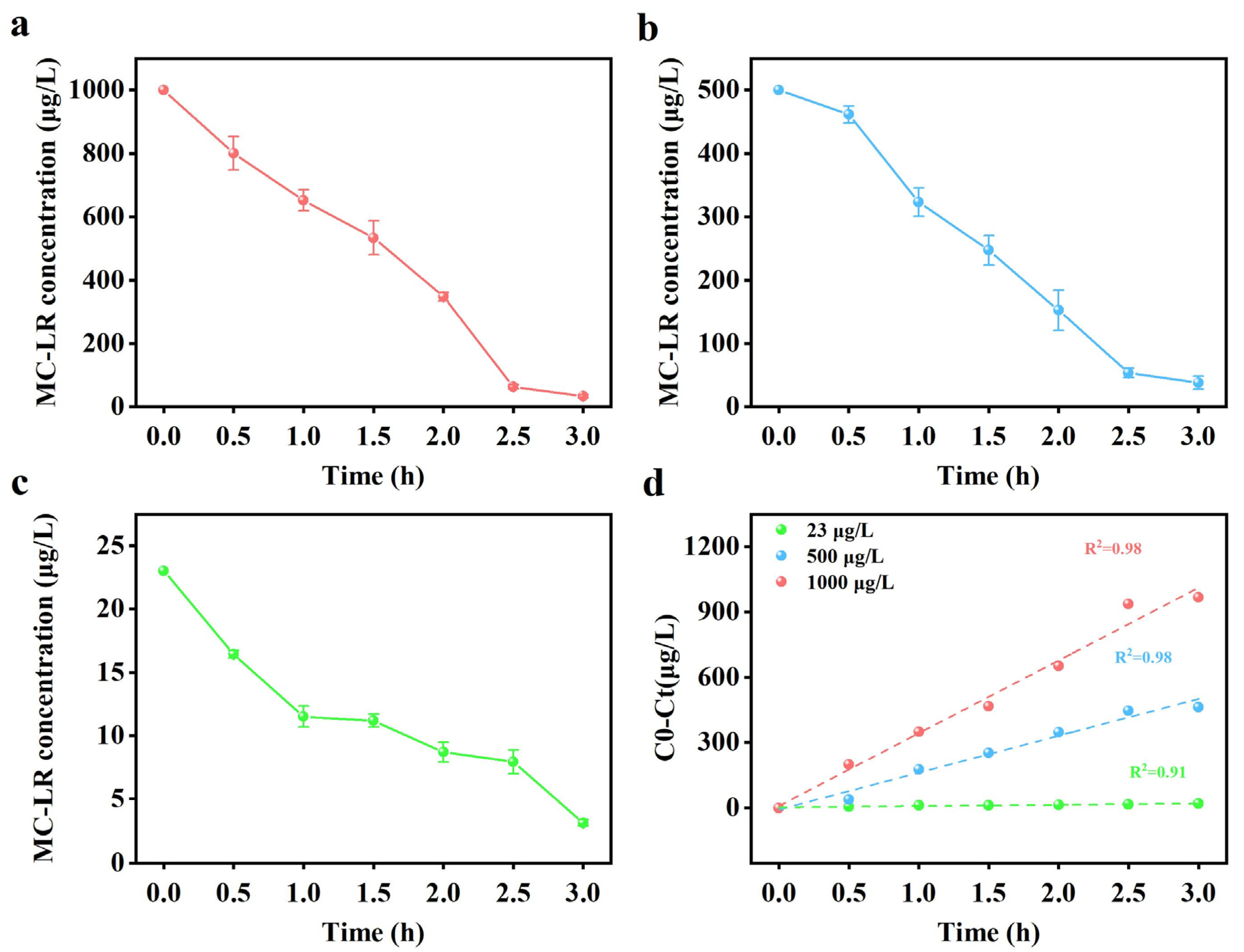
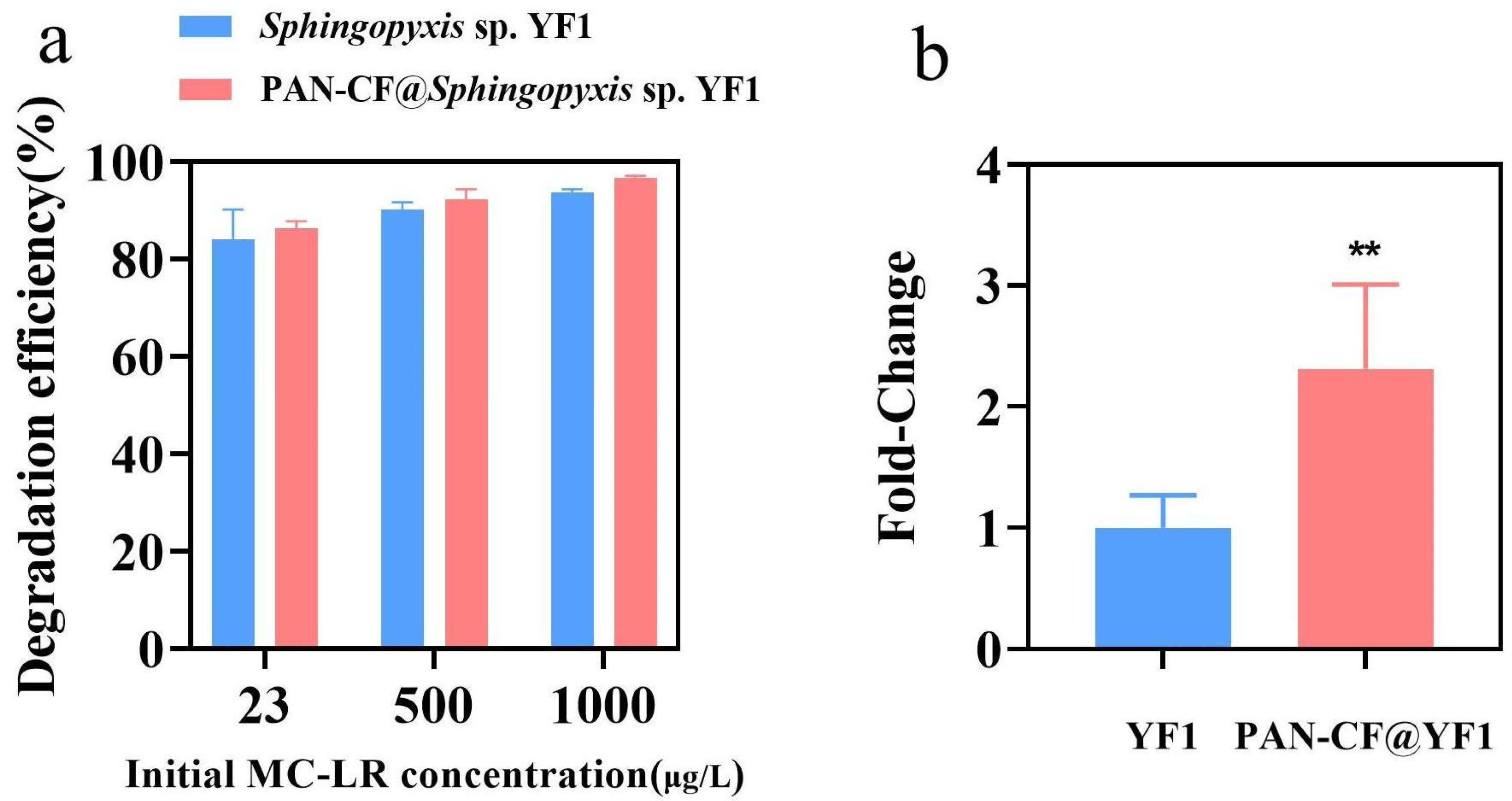
2.4. Removal of MC-LR by PAN-CF@YF1
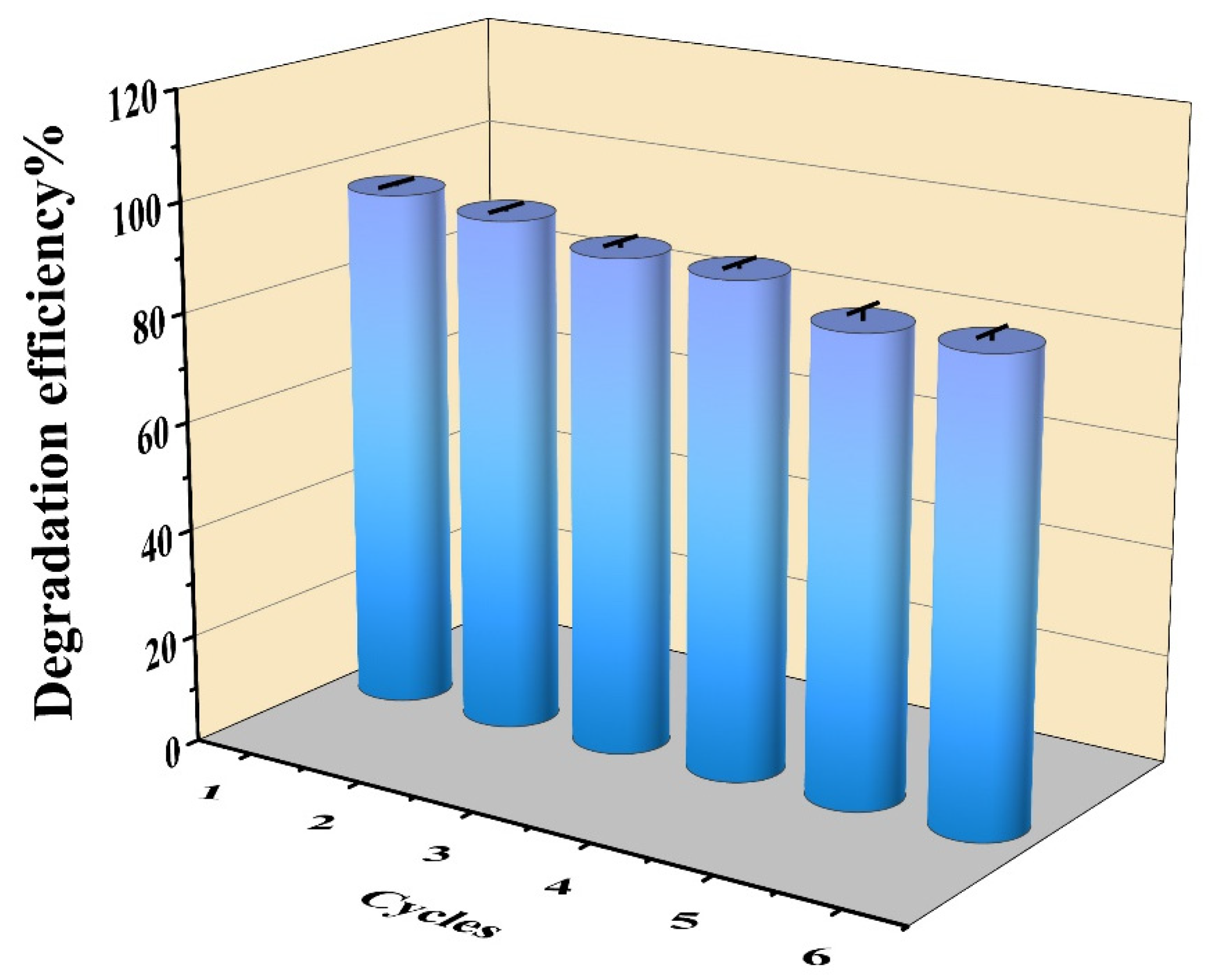
2.5. Reusability of PAN-CF@YF1
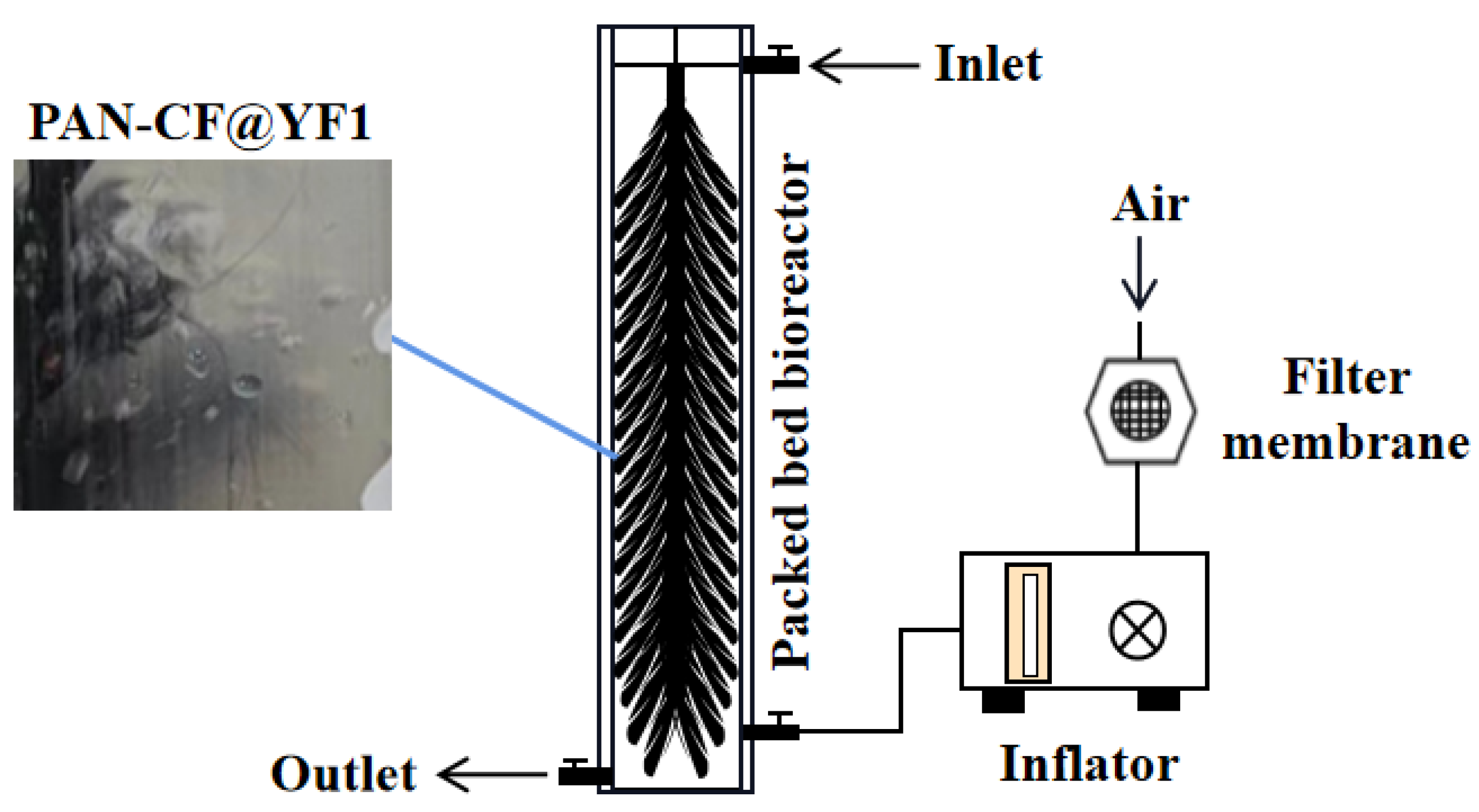
2.6. Degradation of MC-LR by PBBR
3. Conclusions
4. Material and Methods
4.1. Materials and Reagents
4.2. The MC-LR Degrading Bacterial Strain
4.3. Characterization of PAN-CF@YF1
4.4. The Effect of Different Experimental Conditions on the Immobilization of Sphingopyxis sp. YF1
4.5. Optimization of the Immobilizing Conditions by RSM
4.6. Degradation of MC-LR by PAN-CF@YF1 and Its Reusability
4.7. qRT-PCR
4.8. Construction and Operation of Bioreactor for MC-LR Removal
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Gorham, T.; Jia, Y.; Shum, C.K.; Lee, J. Ten-year survey of cyanobacterial blooms in Ohio’s waterbodies using satellite remote sensing. Harmful Algae 2017, 66, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Svirčev, Z.; Lalić, D.; Bojadžija Savić, G.; Tokodi, N.; Drobac Backović, D.; Chen, L.; Meriluoto, J.; Codd, G.A. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef]
- Shahmohamadloo, R.S.; Almirall, X.O.; Simmons, D.B.; Poirier, D.G.; Bhavsar, S.P.; Sibley, P.K. Fish tissue accumulation and proteomic response to microcystins is species-dependent. Chemosphere 2022, 287 Pt 1, 132028. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Deng, S.; Tang, Y.; Liu, Y.; Yang, Y.; Xu, S.; Tang, P.; Lu, Y.; Duan, Y.; Wei, J.; et al. Microcystin-LR Combined with Cadmium Exposures and the Risk of Chronic Kidney Disease: A Case-Control Study in Central China. Environ. Sci. Technol. 2022, 56, 15818–15827. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Wang, X.; Chen, L.; Liu, W.; Cai, D.; Deng, S.; Chu, H.; Liu, Y.; Feng, X.; et al. Long-term environmental levels of microcystin-LR exposure induces colorectal chronic inflammation, fibrosis and barrier disruption via CSF1R/Rap1b signaling pathway. J. Hazard. Mater. 2022, 440, 129793. [Google Scholar] [CrossRef] [PubMed]
- Bouaïcha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural Diversity, Characterization and Toxicology of Microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef]
- Svirčev, Z.; Lujić, J.; Marinović, Z.; Drobac, D.; Tokodi, N.; Stojiljković, B.; Meriluoto, J. Toxicopathology induced by microcystins and nodularin: A histopathological review. Journal of environmental science and health. Part C Environ. Carcinog. Ecotoxicol. Rev. 2015, 33, 125–167. [Google Scholar] [CrossRef]
- Tan, J.; Liu, L.; Li, F.; Chen, Z.; Chen, G.Y.; Fang, F.; Guo, J.; He, M.; Zhou, X. Screening of Endocrine Disrupting Potential of Surface Waters via an Affinity-Based Biosensor in a Rural Community in the Yellow River Basin, China. Environ. Sci. Technol. 2022, 56, 14350–14360. [Google Scholar] [CrossRef]
- Sangolkar, L.N.; Maske, S.S.; Chakrabarti, T. Methods for determining microcystins (peptide hepatotoxins) and microcystin-producing cyanobacteria. Water Res. 2006, 40, 3485–3496. [Google Scholar] [CrossRef]
- He, H.; Wu, S.; Wahome, P.G.; Bertin, M.J.; Pedone, A.C.; Beauchesne, K.R.; Moeller, P.D.; Carter, G.T. Microcystins Containing Doubly Homologated Tyrosine Residues from a Microcystis aeruginosa Bloom: Structures and Cytotoxicity. J. Nat. Prod. 2018, 81, 1368–1375. [Google Scholar] [CrossRef]
- Pan, C.; Qin, H.; Jin, H.; Chen, W.; Guo, H.; Han, X. Environmental exposure to microcystin-LR increases the risks of urinary bladder proliferation and carcinogenesis: Evidence from case control, animal, and in vitro studies. Toxicology 2022, 480, 153326. [Google Scholar] [CrossRef] [PubMed]
- Mashile, P.P.; Mpupa, A.; Nomngongo, P.N. Adsorptive removal of microcystin-LR from surface and wastewater using tyre-based powdered activated carbon: Kinetics and isotherms. Toxicon Off. J. Int. Soc. Toxinol. 2018, 145, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.I.; Huang, J.L.; Jiao, F.U.; Wu, M.S.; Cui, C.W. Ming-song Wu and Chong-wei Cui, Degradation of microcystin-RR in water by chlorine dioxide. J. China Univ. Min. Technol. 2008, 18, 623–628. [Google Scholar]
- Ebrahimi, A.; Jafari, N.; Ebrahimpour, K.; Karimi, M.; Rostamnia, S.; Behnami, A.; Ghanbari, R.; Mohammadi, A.; Rahimi, B.; Abdolahnejad, A. A novel ternary heterogeneous TiO2/BiVO4/NaY-Zeolite nanocomposite for photocatalytic degradation of microcystin-leucine arginine (MC-LR) under visible light. Ecotoxicol. Environ. Saf. 2021, 210, 111862. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.; Majado, M.E.; Meriluoto, J.; Acero, J.L. Oxidation of microcystins by permanganate: Reaction kinetics and implications for water treatment. Water Res. 2007, 41, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zheng, H.; Zhai, X.; Wang, Z. Characteristics and mechanisms of microcystin-LR adsorption by giant reed-derived biochars: Role of minerals, pores, and functional groups. J. Clean. Prod. 2018, 176, 463–473. [Google Scholar] [CrossRef]
- Sun, J.; Bu, L.; Chen, S.; Lu, X.; Wu, Y.; Shi, Z.; Zhou, S. Oxidation of Microcystic-LR via the solar/chlorine process: Radical mechanism, pathways and toxicity assessment. Ecotoxicol. Environ. Saf. 2019, 183, 109509. [Google Scholar] [CrossRef]
- Khadgi, N.; Upreti, A.R. Photocatalytic degradation of Microcystin-LR by visible light active and magnetic, ZnFe2O4-Ag/rGO nanocomposite and toxicity assessment of the intermediates. Chemosphere 2019, 221, 441–451. [Google Scholar] [CrossRef]
- Yi, Y.L.; Yu, X.B.; Zhang, C.; Wang, G.X. Growth inhibition and microcystin degradation effects of Acinetobacter guillouiae A2 on Microcystis aeruginosa. Res. Microbiol. 2015, 166, 93–101. [Google Scholar] [CrossRef]
- Talha, M.A.; Goswami, M.; Giri, B.S.; Sharma, A.; Rai, B.N.; Singh, R.S. Bioremediation of Congo red dye in immobilized batch and continuous packed bed bioreactor by Brevibacillus parabrevis using coconut shell bio-char. Bioresour. Technol. 2018, 252, 37–43. [Google Scholar] [CrossRef] [PubMed]
- de Lerma, N.L.; Peinado, R.A.; Puig-Pujol, A.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Influence of two yeast strains in free, bioimmobilized or immobilized with alginate forms on the aromatic profile of long aged sparkling wines. Food Chem. 2018, 250, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Velkova, Z.; Kirova, G.; Stoytcheva, M.; Kostadinova, S.; Todorova, K.; Gochev, V. Immobilized microbial biosorbents for heavy metals removal. Eng. Life Sci. 2018, 18, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Morón-López, J.; Nieto-Reyes, L.; Senán-Salinas, J.; Molina, S.; El-Shehawy, R. Recycled desalination membranes as a support material for biofilm development: A new approach for microcystin removal during water treatment. Sci. Total Environ. 2019, 647, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Corbel, S.; Mougin, C.; Bouaïcha, N. Cyanobacterial toxins: Modes of actions, fate in aquatic and soil ecosystems, phytotoxicity and bioaccumulation in agricultural crops. Chemosphere 2014, 96, 1–15. [Google Scholar] [CrossRef]
- Chen, Y.W.; Chen, C.Y.; Chiang, S.C.; Lui, M.T.; Kao, S.Y.; Yang, M.H. Predictors and impact of microsurgical complications in patients with locally advanced oral squamous cell carcinoma. Cancer Sci. 2012, 103, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Massey, I.Y.; Peng, T.; Danping, C.; Yang, F. Optimization of Biodegradation Characteristics of Sphingopyxis sp. YF1 against Crude Microcystin-LR Using Response Surface Methodology. Toxins 2022, 14, 240. [Google Scholar] [CrossRef]
- Wei, J.; Pengji, Z.; Zhang, J.; Peng, T.; Luo, J.; Yang, F. Biodegradation of MC-LR and its key bioactive moiety Adda by Sphingopyxis sp. YF1: Comprehensive elucidation of the mechanisms and pathways. Water Res. 2023, 229, 119397. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef]
- Li, R.; Wang, B.; Niu, A.; Cheng, N.; Chen, M.; Zhang, X.; Yu, Z.; Wang, S. Application of biochar immobilized microorganisms for pollutants removal from wastewater: A review. Sci. Total. Environ. 2022, 837, 155563. [Google Scholar] [CrossRef]
- Wu, P.; Li, G.; He, Y.; Luo, D.; Li, L.; Guo, J.; Ding, P.; Yang, F. High-efficient and sustainable biodegradation of microcystin-LR using Sphingopyxis sp. YF1 immobilized Fe(3)O(4)@chitosan. Colloids Surf. B Biointerfaces 2020, 185, 110633. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; He, X.; Wu, P.; He, Y.; Zhang, Y.; Tang, S.; Song, X.; He, Y.; Wei, Y.; Ding, P.; et al. Biodegradation of microcystin-RR and nutrient pollutants using Sphingopyxis sp. YF1 immobilized activated carbon fibers-sodium alginate. Env. Sci. Pollut. Res. Int. 2020, 27, 10811–10821. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.; Ku, B.C.; Kim, J.; Joh, H.I. Structural Evolution of Polyacrylonitrile Fibers in Stabilization and Carbonization. Adv. Chem. Eng. Sci. 2012, 2, 275–282. [Google Scholar] [CrossRef]
- Kruppke, I.; Sherif, F.; Richter, M.; Cherif, C. Development of Porous-Polyacrylonitrile-Based Fibers Using Nanocellulose Additives as Precursor for Carbon Fiber Manufacturing. Polymers 2023, 15, 565. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R. Carbon Fiber Biocompatibility for Implants; Fibers: Basel, Switzerland, 2016; Volume 4. [Google Scholar]
- Ho, L.; Meyn, T.; Keegan, A.; Hoefel, D.; Brookes, J.; Saint, C.P.; Newcombe, G. Bacterial degradation of microcystin toxins within a biologically active sand filter. Water Res. 2006, 40, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ding, P.; Lu, S.; Wu, P.; Wei, X.; Huang, R.; Kai, T. Cell density-dependent regulation of microcystin synthetase genes (mcy) expression and microcystin-LR production in Microcystis aeruginosa that mimics quorum sensing. Ecotoxicol. Environ. Saf. 2021, 220, 112330. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Pradhan, M.; Patel, R.J.; Singhvi, G.; Alexander, A. Design and optimization of curcumin loaded nano lipid carrier system using Box-Behnken design. Biomed. Pharmacother. 2021, 141, 111919. [Google Scholar] [CrossRef] [PubMed]
- Goemaere, I.; Punj, D.; Harizaj, A.; Woolston, J.; Thys, S.; Sterck, K.; De Smedt, S.C.; De Vos, W.H.; Braeckmans, K. Response Surface Methodology to Efficiently Optimize Intracellular Delivery by Photoporation. Int. J. Mol. Sci. 2023, 24, 3147. [Google Scholar] [CrossRef]
- Erge, A.; Eren, Ö. Chicken gelatin modification by caffeic acid: A response surface methodology investigation. Food Chem. 2021, 351, 129269. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Awosusi, A.A.; Yoro, K.O.; Singo, M.; Oloye, O.; Ayeni, A.O.; Bodunrin, M.; Daramola, M.O. Microbial cell immobilization in biohydrogen production: A short overview. Crit. Rev. Biotechnol. 2018, 38, 157–171. [Google Scholar] [CrossRef]
- Erratt, K.J.; Creed, I.F.; Freeman, E.C.; Trick, C.G.; Westrick, J.; Birbeck, J.A.; Watson, L.C.; Zastepa, A. Deep Cyanobacteria Layers: An Overlooked Aspect of Managing Risks of Cyanobacteria. Environ. Sci. Technol. 2022, 56, 17902–17912. [Google Scholar] [CrossRef]
- Du, Y.; Cheng, Q.; Qian, M.; Liu, Y.; Wang, F.; Ma, J.; Zhang, X.; Lin, H. Biodegradation of sulfametoxydiazine by Alcaligenes aquatillis FA: Performance, degradation pathways, and mechanisms. J. Hazard. Mater. 2023, 452, 131186. [Google Scholar] [CrossRef]
- Matsumoto, S.; Ohtaki, A.; Hori, K. Carbon fiber as an excellent support material for wastewater treatment biofilms. Environ. Sci. Technol. 2012, 46, 10175–10181. [Google Scholar] [CrossRef]
- Nie, H.; Nie, M.; Diwu, Z.; Wang, L.; Yan, H.; Bai, X. Immobilization of Rhodococcus qingshengii strain FF on the surface of polyethylene and its adsorption and biodegradation of mimic produced water. J. Hazard. Mater. 2021, 403, 124075. [Google Scholar] [CrossRef]
- Casciato, D.A.; Stewart, P.R.; Rosenblatt, J.E. Growth curves of anaerobic bacteria in solid media. Appl. Microbiol. 1975, 29, 610–614. [Google Scholar] [CrossRef]
- You, L.; Tong, X.; Te, S.H.; Tran, N.H.; bte Sukarji, N.H.; He, Y.; Gin, K.Y.H. Multi-class secondary metabolites in cyanobacterial blooms from a tropical water body: Distribution patterns and real-time prediction. Water Res. 2022, 212, 118129. [Google Scholar] [CrossRef]
- Nybom, S.M.K.; Dziga, D.; Heikkilä, J.E.; Kull, T.P.J.; Salminen, S.J.; Meriluoto, J.A.O. Characterization of microcystin-LR removal process in the presence of probiotic bacteria. Toxicon 2012, 59, 171–181. [Google Scholar] [CrossRef]
- He, Y.; Wei, G.; Tang, B.; Salam, M.; Shen, A.; Wei, Y.; Zhou, X.; Liu, M.; Yang, Y.; Li, H.; et al. Microplastics benefit bacteria colonization and induce microcystin degradation. J. Hazard. Mater. 2022, 431, 128524. [Google Scholar] [CrossRef]
- Wang, S.; Yin, Y.; Wang, J. Microbial degradation of triclosan by a novel strain of Dyella sp. Appl. Microbiol. Biotechnol. 2018, 102, 1997–2006. [Google Scholar] [CrossRef]
- Bourne, D.G.; Jones, G.J.; Blakeley, R.L.; Jones, A.; Negri, A.P.; Riddles, P. Enzymatic pathway for the bacterial degradation of the cyanobacterial cyclic peptide toxin microcystin LR. Appl. Environ. Microbiol. 1996, 62, 4086–4094. [Google Scholar] [CrossRef]
- Mu, D.S.; Liang, Q.Y.; Wang, X.M.; Lu, D.C.; Shi, M.J.; Chen, G.J.; Du, Z.J. Metatranscriptomic and comparative genomic insights into resuscitation mechanisms during enrichment culturing. Microbiome 2018, 6, 230. [Google Scholar] [CrossRef] [PubMed]
- e Silva, N.C.G.; de Macedo, A.C.; Pinheiro, Á.D.T.; Rocha, M.V.P. Phenol biodegradation by Candida tropicalis ATCC 750 immobilized on cashew apple bagasse. J. Environ. Chem. Eng. 2019, 7, 103076. [Google Scholar] [CrossRef]
- Tripathi, P.; Tiwari, S.; Sonwani, R.K.; Singh, R.S. A step towards enhancing the efficiency of biofilm mediated degradation of brilliant green dye in packed bed bioreactor: Statistical and toxicity analysis. Process Saf. Environ. Prot. 2023, 170, 1228–1239. [Google Scholar] [CrossRef]
- Ghodake, G.S.; Yang, J.; Shinde, S.S.; Mistry, B.M.; Kim, D.Y.; Sung, J.S.; Kadam, A.A. Paper waste extracted α-cellulose fibers super-magnetized and chitosan-functionalized for covalent laccase immobilization. Bioresour. Technol. 2018, 261, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Guo, J.; Huang, F.; Massey, I.Y.; Huang, R.; Li, Y.; Wen, C.; Ding, P.; Zeng, W.; Liang, G. Removal of Microcystin-LR by a Novel Native Effective Bacterial Community Designated as YFMCD4 Isolated from Lake Taihu. Toxins 2018, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Nybom, S.M.K.; Salminen, S.J.; Meriluoto, J.A.O. Removal of microcystin-LR by strains of metabolically active probiotic bacteria. FEMS Microbiol. Lett. 2007, 270, 27–33. [Google Scholar] [CrossRef]
- Somdee, T.; Thunders, M.; Ruck, J.; Lys, I.; Allison, M.; Page, R. Degradation of [Dha(7)]MC-LR by a Microcystin Degrading Bacterium Isolated from Lake Rotoiti, New Zealand. ISRN Microbiol. 2013, 2013, 596429. [Google Scholar] [CrossRef]
- Kumar, P.; Hegde, K.; Brar, S.K.; Cledon, M.; Kermanshahi-Pour, A.; Roy-Lachapelle, A.; Galvez-Cloutier, R. Biodegradation of microcystin-LR using acclimatized bacteria isolated from different units of the drinking water treatment plant. Environ. Pollut. 2018, 242 Pt A, 407–416. [Google Scholar] [CrossRef]
- Dziga, D.; Sworzen, M.; Wladyka, B.; Wasylewski, M. Genetically engineered bacteria immobilized in alginate as an option of cyanotoxins removal. Int. J. Environ. Sci. Dev. 2013, 4, 360. [Google Scholar] [CrossRef]
- Phujomjai, Y.; Somdee, A.; Somdee, T. Biodegradation of microcystin [Dha(7)]MC-LR by a novel microcystin-degrading bacterium in an internal airlift loop bioreactor. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2016, 73, 267–274. [Google Scholar] [CrossRef]



| Sum of Squares | df | Mean Square | F Value | p-Value | ||
|---|---|---|---|---|---|---|
| Model | 375.41 | 9 | 41.71 | 44.22 | <0.0001 | significant |
| A | 59.59 | 1 | 59.59 | 63.17 | <0.0001 | |
| B | 27.71 | 1 | 27.71 | 29.38 | 0.001 | |
| C | 81.99 | 1 | 81.99 | 86.93 | <0.0001 | |
| AB | 4 | 1 | 4 | 4.24 | 0.0784 | |
| AC | 1.56 | 1 | 1.56 | 1.66 | 0.239 | |
| BC | 29.64 | 1 | 29.64 | 31.43 | 0.0008 | |
| A2 | 28.25 | 1 | 28.25 | 29.95 | 0.0009 | |
| B2 | 72.86 | 1 | 72.86 | 77.24 | <0.0001 | |
| C2 | 52.61 | 1 | 52.61 | 55.77 | 0.0001 | |
| Residual | 6.6 | 7 | 0.94 | |||
| Lack of Fit | 1.6 | 3 | 0.53 | 0.43 | 0.7446 | not significant |
| Pure Error | 5 | 4 | 1.25 | |||
| Cor Total | 382.01 | 16 |
| Factor | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| pH (A) | 6 | 7 | 8 |
| Supporting materials (B) | 0.03 | 0.04 | 0.05 |
| Time (C) | 36 | 48 | 60 |
| Std. | A | B (g/100 mL) | C (h) | Immobilized Cells (mg/g) |
|---|---|---|---|---|
| 1 | 1 | 0 | −1 | 15.00 |
| 2 | −1 | 0 | 1 | 15.00 |
| 3 | −1 | −1 | 0 | 13.33 |
| 4 | 0 | −1 | −1 | 15.56 |
| 5 | 1 | −1 | 0 | 20.00 |
| 6 | 1 | 1 | 0 | 14.67 |
| 7 | −1 | 0 | −1 | 10.00 |
| 8 | 0 | 1 | 1 | 18.00 |
| 9 | 1 | 0 | 1 | 22.50 |
| 10 | 0 | −1 | 1 | 16.67 |
| 11 | 0 | 1 | −1 | 6.00 |
| 12 | −1 | 1 | 0 | 12.00 |
| 13 | 0 | 0 | 0 | 22.50 |
| 14 | 0 | 0 | 0 | 22.50 |
| 15 | 0 | 0 | 0 | 22.50 |
| 16 | 0 | 0 | 0 | 21.25 |
| 17 | 0 | 0 | 0 | 20.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Zhang, J.; Yang, L.; Zhang, S.; Long, X.; Zeng, Q.; Li, Z.; Ren, X.; Yang, F. Reusable and Practical Biocomposite Based on Sphingopyxis sp. YF1 and Polyacrylonitrile-Based Carbon Fiber for the Efficient Bioremediation of Microcystin-LR-Contaminated Water. Toxins 2024, 16, 20. https://doi.org/10.3390/toxins16010020
Ma T, Zhang J, Yang L, Zhang S, Long X, Zeng Q, Li Z, Ren X, Yang F. Reusable and Practical Biocomposite Based on Sphingopyxis sp. YF1 and Polyacrylonitrile-Based Carbon Fiber for the Efficient Bioremediation of Microcystin-LR-Contaminated Water. Toxins. 2024; 16(1):20. https://doi.org/10.3390/toxins16010020
Chicago/Turabian StyleMa, Tian, Jiajia Zhang, Lili Yang, Shengyu Zhang, Xizi Long, Qingyi Zeng, Zhongyu Li, Xiaoya Ren, and Fei Yang. 2024. "Reusable and Practical Biocomposite Based on Sphingopyxis sp. YF1 and Polyacrylonitrile-Based Carbon Fiber for the Efficient Bioremediation of Microcystin-LR-Contaminated Water" Toxins 16, no. 1: 20. https://doi.org/10.3390/toxins16010020





