TDP-43 and Alzheimer’s Disease Pathology in the Brain of a Harbor Porpoise Exposed to the Cyanobacterial Toxin BMAA
Abstract
:1. Introduction
2. Results
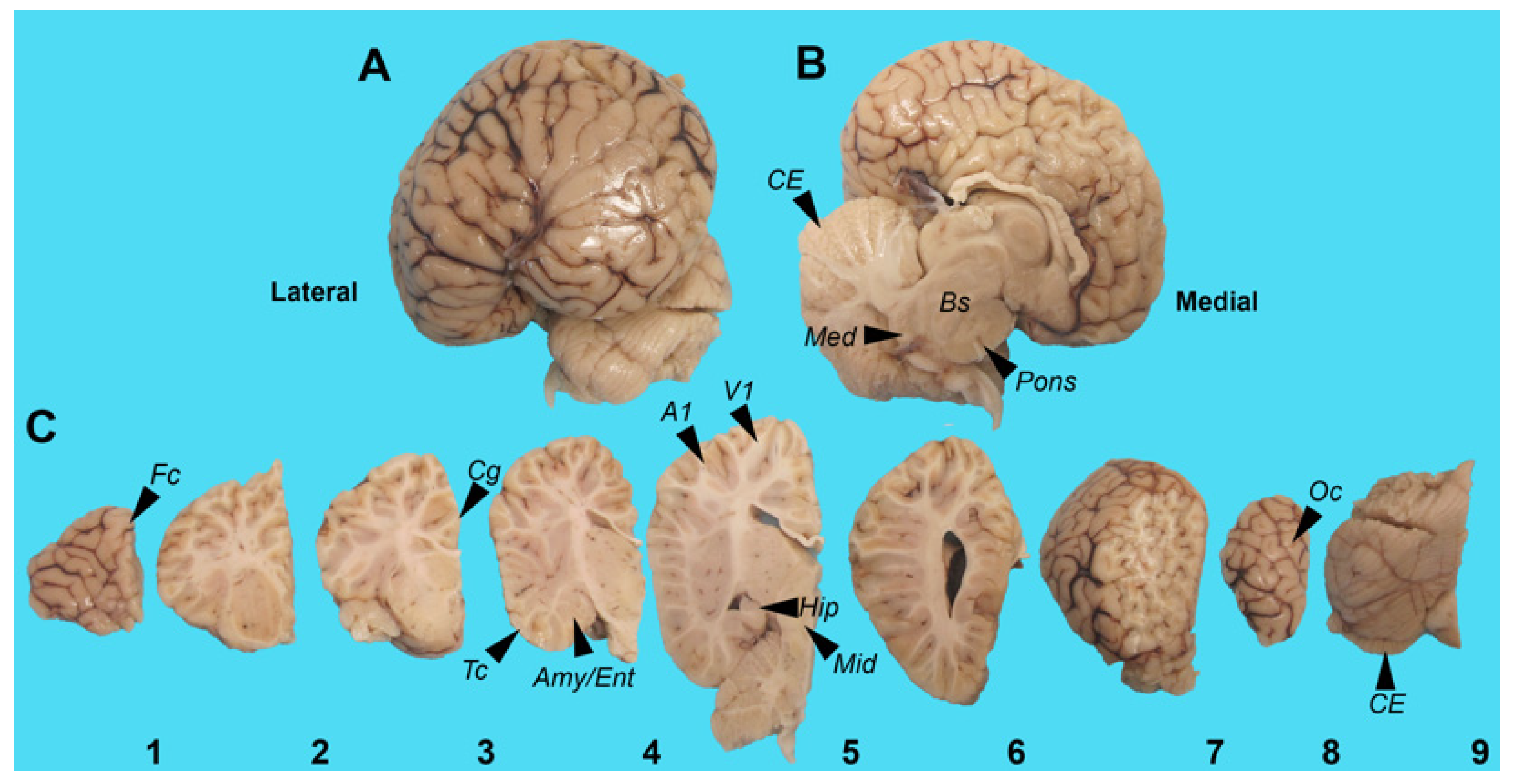
2.1. Case History
2.2. Cyanobacterial Toxin BMAA Exposure
2.3. Expression of Genes Implicated in Alzheimer’s Disease and TDP-43 Proteinopathy
| Gene Probes | Neurological Diseases | Associated Neuropathology | Harbor Porpoise (qPCR) | Human (BioGPS) | ||
|---|---|---|---|---|---|---|
| PcV1:CE | Fc:CE | Pc:CE | PreFc:CE | |||
| APP [34] | AD | Aβ+ plaques | 1.41 ± 0.34 | 2.88 ± 0.56 p < 0.0001 | 1.73 | 4.20 |
| PSEN1 [35] | AD | Aβ+ plaques | 1.23 ± 0.02 | 1.33 ± 0.03 | 7.48 | 7.56 |
| PSEN2 [36] | AD | Aβ+ plaques | 0.66 ± 0.03 | 0.79 ± 0.01 | 1.30 | 1.46 |
| GRN [37] | FTLD | progranulin loss | 1.38 ± 0.02 | 1.96 ± 0.04 | 1.24 | 1.42 |
| MAPT [38] | Tauopathies | NFTs | 1.52 ± 0.07 | 2.40 ± 0.14 p = 0.0134 | 4.08 | 7.24 |
| TARBDP [39] | TDP-43 proteinopathies | IC | 1.21 ± 0.01 | 1.95 ± 0.04 | 1.40 | 2.28 |
| C9orf72 [39] | ALS and FTD | IC | 1.22 ± 0.09 | 1.66 ± 0.09 | 1.03 | 0.96 |
2.4. Neuropathology
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Harbor Porpoise Brain
5.2. HPLC/FD
5.3. qPCR Analysis
5.4. Immunohistochemistry and Digital Pathology
5.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruck, J.N. Decades-long social memory in bottlenose dolphins. Proc. Biol. Sci. 2013, 280, 20131726. [Google Scholar] [CrossRef] [PubMed]
- Cairomicron, O. External measures of cognition. Front. Hum. Neurosci. 2011, 5, 108. [Google Scholar] [CrossRef]
- Hof, P.R.; Chanis, R.; Marino, L. Cortical complexity in cetacean brains. Anat. Rec. Discov. Mol. Cell Evol. Biol. 2005, 287, 1142–1152. [Google Scholar] [CrossRef]
- Dell, L.A.; Patzke, N.; Spocter, M.A.; Siegel, J.M.; Manger, P.R. Organization of the sleep-related neural systems in the brain of the harbour porpoise (Phocoena phocoena). J. Comp. Neurol. 2016, 524, 1999–2017. [Google Scholar] [CrossRef]
- Kruger, L. Edward Tyson’s 1680 account of the ‘porpess’ brain and its place in the history of comparative neurology. J. Hist. Neurosci. 2003, 12, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.A.; Garamszegi, S.P.; Banack, S.A.; Dooley, P.D.; Coyne, T.M.; McLean, D.W.; Rotstein, D.S.; Mash, D.C.; Cox, P.A. BMAA, Methylmercury, and Mechanisms of Neurodegeneration in Dolphins: A Natural Model of Toxin Exposure. Toxins 2021, 13, 697. [Google Scholar] [CrossRef]
- Davis, D.A.; Mondo, K.; Stern, E.; Annor, A.K.; Murch, S.J.; Coyne, T.M.; Brand, L.E.; Niemeyer, M.E.; Sharp, S.; Bradley, W.G.; et al. Cyanobacterial neurotoxin BMAA and brain pathology in stranded dolphins. PLoS ONE 2019, 14, e0213346. [Google Scholar] [CrossRef]
- Pintore, M.D.; Mignone, W.; Di Guardo, G.; Mazzariol, S.; Ballardini, M.; Florio, C.L.; Goria, M.; Romano, A.; Caracappa, S.; Giorda, F.; et al. Neuropathologic Findings in Cetaceans Stranded in Italy (2002–2014). J. Wildl. Dis. 2018, 54, 295–303. [Google Scholar] [CrossRef]
- Sips, G.J.; Chesik, D.; Glazenburg, L.; Wilschut, J.; De Keyser, J.; Wilczak, N. Involvement of morbilliviruses in the pathogenesis of demyelinating disease. Rev. Med. Virol. 2007, 17, 223–244. [Google Scholar] [CrossRef]
- Vacher, M.C.; Durrant, C.S.; Rose, J.; Hall, A.J.; Spires-Jones, T.L.; Gunn-Moore, F.; Dagleish, M.P. Alzheimer’s disease-like neuropathology in three species of oceanic dolphin. Eur. J. Neurosci. 2023, 57, 1161–1179. [Google Scholar] [CrossRef]
- Di Guardo, G. Alzheimer’s disease, cellular prion protein, and dolphins. Alzheimers Dement. 2018, 14, 259–260. [Google Scholar] [CrossRef]
- Reif, J.S.; Schaefer, A.M.; Bossart, G.D. Atlantic Bottlenose Dolphins (Tursiops truncatus) as A Sentinel for Exposure to Mercury in Humans: Closing the Loop. Vet. Sci. 2015, 2, 407–422. [Google Scholar] [CrossRef]
- Tilbury, K.L.; Stein, J.E.; Meador, J.P.; Krone, C.A.; Chan, S.L. Chemical contaminants in harbor porpoise (Phocoena phocoena) from the north Atlantic coast: Tissue concentrations and intra- and inter-organ distribution. Chemosphere 1997, 34, 2159–2181. [Google Scholar] [CrossRef]
- Van de Vijver, K.I.; Holsbeek, L.; Das, K.; Blust, R.; Joiris, C.; De Coen, W. Occurrence of perfluorooctane sulfonate and other perfluorinated alkylated substances in harbor porpoises from the Black Sea. Environ. Sci. Technol. 2007, 41, 315–320. [Google Scholar] [CrossRef]
- Banack, S.A.; Johnson, H.E.; Cheng, R.; Cox, P.A. Production of the neurotoxin BMAA by a marine cyanobacterium. Mar. Drugs 2007, 5, 180–196. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce beta-N-methylamino-L-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef]
- Cox, P.A.; Kostrzewa, R.M.; Guillemin, G.J. BMAA and Neurodegenerative Illness. Neurotox. Res. 2018, 33, 178–183. [Google Scholar] [CrossRef]
- Cox, P.A.; Sacks, O.W. Cycad neurotoxins, consumption of flying foxes, and ALS-PDC disease in Guam. Neurology 2002, 58, 956–959. [Google Scholar] [CrossRef]
- Banack, S.A.; Murch, S.J.; Cox, P.A. Neurotoxic flying foxes as dietary items for the Chamorro people, Marianas Islands. J. Ethnopharmacol. 2006, 106, 97–104. [Google Scholar] [CrossRef]
- Murch, S.J.; Cox, P.A.; Banack, S.A.; Steele, J.C.; Sacks, O.W. Occurrence of beta-methylamino-l-alanine (BMAA) in ALS/PDC patients from Guam. Acta Neurol. Scand. 2004, 110, 267–269. [Google Scholar] [CrossRef]
- Mimuro, M.; Yoshida, M.; Kuzuhara, S.; Kokubo, Y. Amyotrophic lateral sclerosis and parkinsonism-dementia complex of the Hohara focus of the Kii Peninsula: A multiple proteinopathy? Neuropathology 2018, 38, 98–107. [Google Scholar] [CrossRef]
- Geser, F.; Winton, M.J.; Kwong, L.K.; Xu, Y.; Xie, S.X.; Igaz, L.M.; Garruto, R.M.; Perl, D.P.; Galasko, D.; Lee, V.M.; et al. Pathological TDP-43 in parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol. 2008, 115, 133–145. [Google Scholar] [CrossRef]
- Davis, D.A.; Cox, P.A.; Banack, S.A.; Lecusay, P.D.; Garamszegi, S.P.; Hagan, M.J.; Powell, J.T.; Metcalf, J.S.; Palmour, R.M.; Beierschmitt, A.; et al. l-Serine Reduces Spinal Cord Pathology in a Vervet Model of Preclinical ALS/MND. J. Neuropathol. Exp. Neurol. 2020, 79, 393–406. [Google Scholar] [CrossRef]
- Cox, P.A.; Davis, D.A.; Mash, D.C.; Metcalf, J.S.; Banack, S.A. Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. Proc. Biol. Sci. 2016, 283, 20152397. [Google Scholar] [CrossRef]
- Yin, H.Z.; Yu, S.; Hsu, C.I.; Liu, J.; Acab, A.; Wu, R.; Tao, A.; Chiang, B.J.; Weiss, J.H. Intrathecal infusion of BMAA induces selective motor neuron damage and astrogliosis in the ventral horn of the spinal cord. Exp. Neurol. 2014, 261, 1–9. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Davis, D.A.; Mondo, K.; Seely, M.S.; Murch, S.J.; Glover, W.B.; Divoll, T.; Evers, D.C.; Mash, D.C. Cyanobacterial Neurotoxin BMAA and Mercury in Sharks. Toxins 2016, 8, 238. [Google Scholar] [CrossRef]
- Mondo, K.; Hammerschlag, N.; Basile, M.; Pablo, J.; Banack, S.A.; Mash, D.C. Cyanobacterial neurotoxin beta-N-methylamino-L-alanine (BMAA) in shark fins. Mar. Drugs 2012, 10, 509–520. [Google Scholar] [CrossRef]
- Brand, L.E.; Pablo, J.; Compton, A.; Hammerschlag, N.; Mash, D.C. Cyanobacterial blooms and the occurrence of the neurotoxin beta-N-methylamino-L-alanine (BMAA) in South Florida aquatic food webs. Harmful Algae 2010, 9, 620–635. [Google Scholar] [CrossRef]
- Pablo, J.; Banack, S.A.; Cox, P.A.; Johnson, T.E.; Papapetropoulos, S.; Bradley, W.G.; Buck, A.; Mash, D.C. Cyanobacterial neurotoxin BMAA in ALS and Alzheimer’s disease. Acta Neurol. Scand. 2009, 120, 216–225. [Google Scholar] [CrossRef]
- Garamszegi, S.P.; Banack, S.A.; Duque, L.L.; Metcalf, J.S.; Stommel, E.W.; Cox, P.A.; Davis, D.A. Detection of beta-N-methylamino-l-alanine in postmortem olfactory bulbs of Alzheimer’s disease patients using UHPLC-MS/MS: An autopsy case-series study. Toxicol. Rep. 2023, 10, 87–96. [Google Scholar] [CrossRef]
- Berntzon, L.; Ronnevi, L.O.; Bergman, B.; Eriksson, J. Detection of BMAA in the human central nervous system. Neuroscience 2015, 292, 137–147. [Google Scholar] [CrossRef]
- Meneely, J.P.; Chevallier, O.P.; Graham, S.; Greer, B.; Green, B.D.; Elliott, C.T. beta-methylamino-L-alanine (BMAA) is not found in the brains of patients with confirmed Alzheimer’s disease. Sci. Rep. 2016, 6, 36363. [Google Scholar] [CrossRef]
- Montine, T.J.; Li, K.; Perl, D.P.; Galasko, D. Lack of beta-methylamino-l-alanine in brain from controls, AD, or Chamorros with PDC. Neurology 2005, 65, 768–769. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef]
- Kelleher, R.J., 3rd; Shen, J. Presenilin-1 mutations and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 629–631. [Google Scholar] [CrossRef]
- Cai, Y.; An, S.S.; Kim, S. Mutations in presenilin 2 and its implications in Alzheimer’s disease and other dementia-associated disorders. Clin. Interv. Aging 2015, 10, 1163–1172. [Google Scholar] [CrossRef]
- Kuang, L.; Hashimoto, K.; Huang, E.J.; Gentry, M.S.; Zhu, H. Frontotemporal dementia non-sense mutation of progranulin rescued by aminoglycosides. Hum. Mol. Genet. 2020, 29, 624–634. [Google Scholar] [CrossRef]
- Strang, K.H.; Golde, T.E.; Giasson, B.I. MAPT mutations, tauopathy, and mechanisms of neurodegeneration. Lab. Investig. 2019, 99, 912–928. [Google Scholar] [CrossRef]
- Gendron, T.F.; Rademakers, R.; Petrucelli, L. TARDBP mutation analysis in TDP-43 proteinopathies and deciphering the toxicity of mutant TDP-43. J. Alzheimers Dis. 2013, 33 (Suppl. S1), S35–S45. [Google Scholar] [CrossRef]
- Mitake, S.; Ojika, K.; Hirano, A. Hirano bodies and Alzheimer’s disease. Kaohsiung J. Med. Sci. 1997, 13, 10–18. [Google Scholar]
- Yamazaki, Y.; Matsubara, T.; Takahashi, T.; Kurashige, T.; Dohi, E.; Hiji, M.; Nagano, Y.; Yamawaki, T.; Matsumoto, M. Granulovacuolar degenerations appear in relation to hippocampal phosphorylated tau accumulation in various neurodegenerative disorders. PLoS ONE 2011, 6, e26996. [Google Scholar] [CrossRef] [PubMed]
- Moloney, C.M.; Lowe, V.J.; Murray, M.E. Visualization of neurofibrillary tangle maturity in Alzheimer’s disease: A clinicopathologic perspective for biomarker research. Alzheimers Dement. 2021, 17, 1554–1574. [Google Scholar] [CrossRef]
- Gobler, C.J. Climate Change and Harmful Algal Blooms: Insights and perspective. Harmful Algae 2020, 91, 101731. [Google Scholar] [CrossRef] [PubMed]
- Heisler, J.; Glibert, P.; Burkholder, J.; Anderson, D.; Cochlan, W.; Dennison, W.; Gobler, C.; Dortch, Q.; Heil, C.; Humphries, E.; et al. Eutrophication and Harmful Algal Blooms: A Scientific Consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Hallegraeff, G.M.; Anderson, D.M.; Belin, C.; Bottein, M.Y.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Commun. Earth Environ. 2021, 2, 117. [Google Scholar] [CrossRef]
- Van den Heuvel-Greve, M.J.; van den Brink, A.M.; Kotterman, M.J.J.; Kwadijk, C.; Geelhoed, S.C.V.; Murphy, S.; van den Broek, J.; Heesterbeek, H.; Grone, A.; LL, I.J. Polluted porpoises: Generational transfer of organic contaminants in harbour porpoises from the southern North Sea. Sci. Total Environ. 2021, 796, 148936. [Google Scholar] [CrossRef] [PubMed]
- Fenton, H.; Daoust, P.Y.; Forzan, M.J.; Vanderstichel, R.V.; Ford, J.K.; Spaven, L.; Lair, S.; Raverty, S. Causes of mortality of harbor porpoises Phocoena phocoena along the Atlantic and Pacific coasts of Canada. Dis. Aquat. Organ. 2017, 122, 171–183. [Google Scholar] [CrossRef]
- Schneider, T.; Simpson, C.; Desai, P.; Tucker, M.; Lobner, D. Neurotoxicity of isomers of the environmental toxin L-BMAA. Toxicon 2020, 184, 175–179. [Google Scholar] [CrossRef]
- Martin, R.M.; Stallrich, J.; Bereman, M.S. Mixture designs to investigate adverse effects upon co-exposure to environmental cyanotoxins. Toxicology 2019, 421, 74–83. [Google Scholar] [CrossRef]
- Rush, T.; Liu, X.; Lobner, D. Synergistic toxicity of the environmental neurotoxins methylmercury and beta-N-methylamino-L-alanine. Neuroreport 2012, 23, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Newell, M.E.; Adhikari, S.; Halden, R.U. Systematic and state-of the science review of the role of environmental factors in Amyotrophic Lateral Sclerosis (ALS) or Lou Gehrig’s Disease. Sci. Total Environ. 2022, 817, 152504. [Google Scholar] [CrossRef]
- Meneses, A.; Koga, S.; O’Leary, J.; Dickson, D.W.; Bu, G.; Zhao, N. TDP-43 Pathology in Alzheimer’s Disease. Mol. Neurodegener. 2021, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.C.; Dugger, B.N.; Dickson, D.W.; Wang, D.S. TDP-43 in aging and Alzheimer’s disease—A review. Int. J. Clin. Exp. Pathol. 2011, 4, 147–155. [Google Scholar] [PubMed]
- Nelson, P.T.; Dickson, D.W.; Trojanowski, J.Q.; Jack, C.R.; Boyle, P.A.; Arfanakis, K.; Rademakers, R.; Alafuzoff, I.; Attems, J.; Brayne, C.; et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain 2019, 142, 1503–1527. [Google Scholar] [CrossRef]
- De Boer, E.M.J.; Orie, V.K.; Williams, T.; Baker, M.R.; De Oliveira, H.M.; Polvikoski, T.; Silsby, M.; Menon, P.; van den Bos, M.; Halliday, G.M.; et al. TDP-43 proteinopathies: A new wave of neurodegenerative diseases. J. Neurol. Neurosurg. Psychiatry 2020, 92, 86–95. [Google Scholar] [CrossRef]
- Hasegawa, M.; Arai, T.; Akiyama, H.; Nonaka, T.; Mori, H.; Hashimoto, T.; Yamazaki, M.; Oyanagi, K. TDP-43 is deposited in the Guam parkinsonism-dementia complex brains. Brain 2007, 130, 1386–1394. [Google Scholar] [CrossRef]
- Kuusisto, E.; Salminen, A.; Alafuzoff, I. Early accumulation of p62 in neurofibrillary tangles in Alzheimer’s disease: Possible role in tangle formation. Neuropathol. Appl. Neurobiol. 2002, 28, 228–237. [Google Scholar] [CrossRef]
- Fecto, F.; Yan, J.; Vemula, S.P.; Liu, E.; Yang, Y.; Chen, W.; Zheng, J.G.; Shi, Y.; Siddique, N.; Arrat, H.; et al. SQSTM1 mutations in familial and sporadic amyotrophic lateral sclerosis. Arch. Neurol. 2011, 68, 1440–1446. [Google Scholar] [CrossRef]
- Chevaleyre, V.; Piskorowski, R.A. Hippocampal Area CA2: An Overlooked but Promising Therapeutic Target. Trends Mol. Med. 2016, 22, 645–655. [Google Scholar] [CrossRef]
- Meira, T.; Leroy, F.; Buss, E.W.; Oliva, A.; Park, J.; Siegelbaum, S.A. A hippocampal circuit linking dorsal CA2 to ventral CA1 critical for social memory dynamics. Nat. Commun. 2018, 9, 4163. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.S.; Tischbein, M.; Cox, P.A.; Stommel, E.W. Cyanotoxins and the Nervous System. Toxins 2021, 13, 660. [Google Scholar] [CrossRef]
- Al-Sammak, M.A.; Hoagland, K.D.; Cassada, D.; Snow, D.D. Co-occurrence of the cyanotoxins BMAA, DABA and anatoxin-a in Nebraska reservoirs, fish, and aquatic plants. Toxins 2014, 6, 488–508. [Google Scholar] [CrossRef]
- Main, B.J.; Rodgers, K.J. Assessing the Combined Toxicity of BMAA and Its Isomers 2,4-DAB and AEG In Vitro Using Human Neuroblastoma Cells. Neurotox. Res. 2018, 33, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Violi, J.P.; Pu, L.; Pravadali-Cekic, S.; Bishop, D.P.; Phillips, C.R.; Rodgers, K.J. ffects of the Toxic Non-Protein Amino Acid β-Methylamino-L-Alanine (BMAA) on Intracellular Amino Acid Levels in Neuroblastoma Cells. Toxins 2023, 15, 647. [Google Scholar] [CrossRef] [PubMed]
- Banack, S.A.; Caller, T.; Henegan, P.; Haney, J.; Murby, A.; Metcalf, J.S.; Powell, J.; Cox, P.A.; Stommel, E. Detection of cyanotoxins, beta-N-methylamino-L-alanine and microcystins, from a lake surrounded by cases of amyotrophic lateral sclerosis. Toxins 2015, 7, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.L.; Peccia, J.; Arnold, W. Preliminary Report on Air Sampling of Particle-Associated Microcystins and BMAA Pilot Study in Lee County, Florida: Fall 2018–Winter 2019; Florida Gulf Coast University: Fort Myers, FL, USA, 2019. [Google Scholar]
- Desforges, J.P.; Mikkelsen, B.; Dam, M.; Riget, F.; Sveegaard, S.; Sonne, C.; Dietz, R.; Basu, N. Mercury and neurochemical biomarkers in multiple brain regions of five Arctic marine mammals. Neurotoxicology 2021, 84, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Joiris, C.R.; Holsbeek, L.; Bolbat, D.; Gascard, C.; Stanev, T.; Komakhidze, A.; Baumgartner, W.; Birkun, A. Total and organic mercury in the Black Sea harbour porpoise Phocoena phocoena relicta. Mar. Pollut. Bull. 2001, 42, 905–911. [Google Scholar] [CrossRef]
- Van Elk, C.; van de Bildt, M.; van Run, P.; de Jong, A.; Getu, S.; Verjans, G.; Osterhaus, A.; Kuiken, T. Central nervous system disease and genital disease in harbor porpoises (Phocoena phocoena) are associated with different herpesviruses. Vet. Res. 2016, 47, 28. [Google Scholar] [CrossRef]
- Rubio-Guerri, C.; Melero, M.; Esperon, F.; Belliere, E.N.; Arbelo, M.; Crespo, J.L.; Sierra, E.; Garcia-Parraga, D.; Sanchez-Vizcaino, J.M. Unusual striped dolphin mass mortality episode related to cetacean morbillivirus in the Spanish Mediterranean sea. BMC Vet. Res. 2013, 9, 106. [Google Scholar] [CrossRef]
- Guzman-Verri, C.; Gonzalez-Barrientos, R.; Hernandez-Mora, G.; Morales, J.A.; Baquero-Calvo, E.; Chaves-Olarte, E.; Moreno, E. Brucella ceti and brucellosis in cetaceans. Front. Cell Infect. Microbiol. 2012, 2, 3. [Google Scholar] [CrossRef]
- Leblanc, P.; Vorberg, I.M. Viruses in neurodegenerative diseases: More than just suspects in crimes. PLoS Pathog. 2022, 18, e1010670. [Google Scholar] [CrossRef]
- Geraci, J.R.; Lounsbury, V.L.; Yates, N. Marine Mammals Ashore, A Field Guide for Strandings, 2nd ed.; National Aquarium in Baltimore, Inc.: Baltimore, MD, USA, 2005; pp. 198–199. [Google Scholar]
- Breathnach, A.S.; Goldby, F. The amygdaloid nuclei, hippocampus and other parts of the rhinencephalon in the porpoise (Phocaena phocaena). J. Anat. 1954, 88, 267–291. [Google Scholar]
- Marino, L.; Sudheimer, K.; Sarko, D.; Sirpenski, G.; Johnson, J.I. Neuroanatomy of the harbor porpoise (Phocoena phocoena) from magnetic resonance images. J. Morphol. 2003, 257, 308–347. [Google Scholar] [CrossRef] [PubMed]
- LeDuc, R.G.; Perrin, W.F.; Dizon, A.E. Phylogenetic Relationships Among the Delphinid Cetaceans Based on Full Cytochrome B Sequences. Mar. Mammal Sci. 1999, 15, 619–648. [Google Scholar] [CrossRef]
- Chen, I.H.; Chou, L.S.; Chou, S.J.; Wang, J.H.; Stott, J.; Blanchard, M.; Jen, I.F.; Yang, W.C. Selection of suitable reference genes for normalization of quantitative RT-PCR in peripheral blood samples of bottlenose dolphins (Tursiops truncatus). Sci. Rep. 2015, 5, 15425. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Su, A.I.; Cooke, M.P.; Ching, K.A.; Hakak, Y.; Walker, J.R.; Wiltshire, T.; Orth, A.P.; Vega, R.G.; Sapinoso, L.M.; Moqrich, A.; et al. Large-scale analysis of the human and mouse transcriptomes. Proc. Natl. Acad. Sci. USA 2002, 99, 4465–4470. [Google Scholar] [CrossRef]
- Mirra, S.S.; Hart, M.N.; Terry, R.D. Making the diagnosis of Alzheimer’s disease. A primer for practicing pathologists. Arch. Pathol. Lab. Med. 1993, 117, 132–144. [Google Scholar] [PubMed]


| Brain Regions | BMAA (μg/g) | AEG (μg/g) | 2,4-DAB (μg/g) |
|---|---|---|---|
| PcA1 (n = 4) | 93.6 ± 9.1 ns | 36.5 ± 7.7 p = 0.001 | 282.6 ± 21.2 ns |
| PcV1 (n = 5) | 93.1 ± 7.5 | ND | 245.0 ± 15.4 |
| Mean + S.E. | 93.3 ± 5.4 | 16.2 ± 7.2 | 261.7 ± 13.6 |
| Brain Regions | TDP-43 pSer 409/410 | β-Amyloid 1–16 (clone 6E10) | P62/SQSTM1 | SM: Argyrophilic Neurons and Plaques |
|---|---|---|---|---|
| Amy | + | + | + | + |
| CE | (−)/+ | + | ++ | + |
| Cg | + | + | (−) | + |
| Ent | + | + | + | + |
| Fc | + | + | −/+ | + |
| Hipp | + | + | + | + |
| Med | + | + | (−) | + |
| Mid | + | + | (−) | + |
| Oc | (−) | + | (−) | + |
| PcA1 | + | + | (−) | + |
| Pons | + | + | (−) | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garamszegi, S.P.; Brzostowicki, D.J.; Coyne, T.M.; Vontell, R.T.; Davis, D.A. TDP-43 and Alzheimer’s Disease Pathology in the Brain of a Harbor Porpoise Exposed to the Cyanobacterial Toxin BMAA. Toxins 2024, 16, 42. https://doi.org/10.3390/toxins16010042
Garamszegi SP, Brzostowicki DJ, Coyne TM, Vontell RT, Davis DA. TDP-43 and Alzheimer’s Disease Pathology in the Brain of a Harbor Porpoise Exposed to the Cyanobacterial Toxin BMAA. Toxins. 2024; 16(1):42. https://doi.org/10.3390/toxins16010042
Chicago/Turabian StyleGaramszegi, Susanna P., Daniel J. Brzostowicki, Thomas M. Coyne, Regina T. Vontell, and David A. Davis. 2024. "TDP-43 and Alzheimer’s Disease Pathology in the Brain of a Harbor Porpoise Exposed to the Cyanobacterial Toxin BMAA" Toxins 16, no. 1: 42. https://doi.org/10.3390/toxins16010042
APA StyleGaramszegi, S. P., Brzostowicki, D. J., Coyne, T. M., Vontell, R. T., & Davis, D. A. (2024). TDP-43 and Alzheimer’s Disease Pathology in the Brain of a Harbor Porpoise Exposed to the Cyanobacterial Toxin BMAA. Toxins, 16(1), 42. https://doi.org/10.3390/toxins16010042





