Exploring the Central Mechanisms of Botulinum Toxin in Parkinson’s Disease: A Systematic Review from Animal Models to Human Evidence
Abstract
:1. Introduction
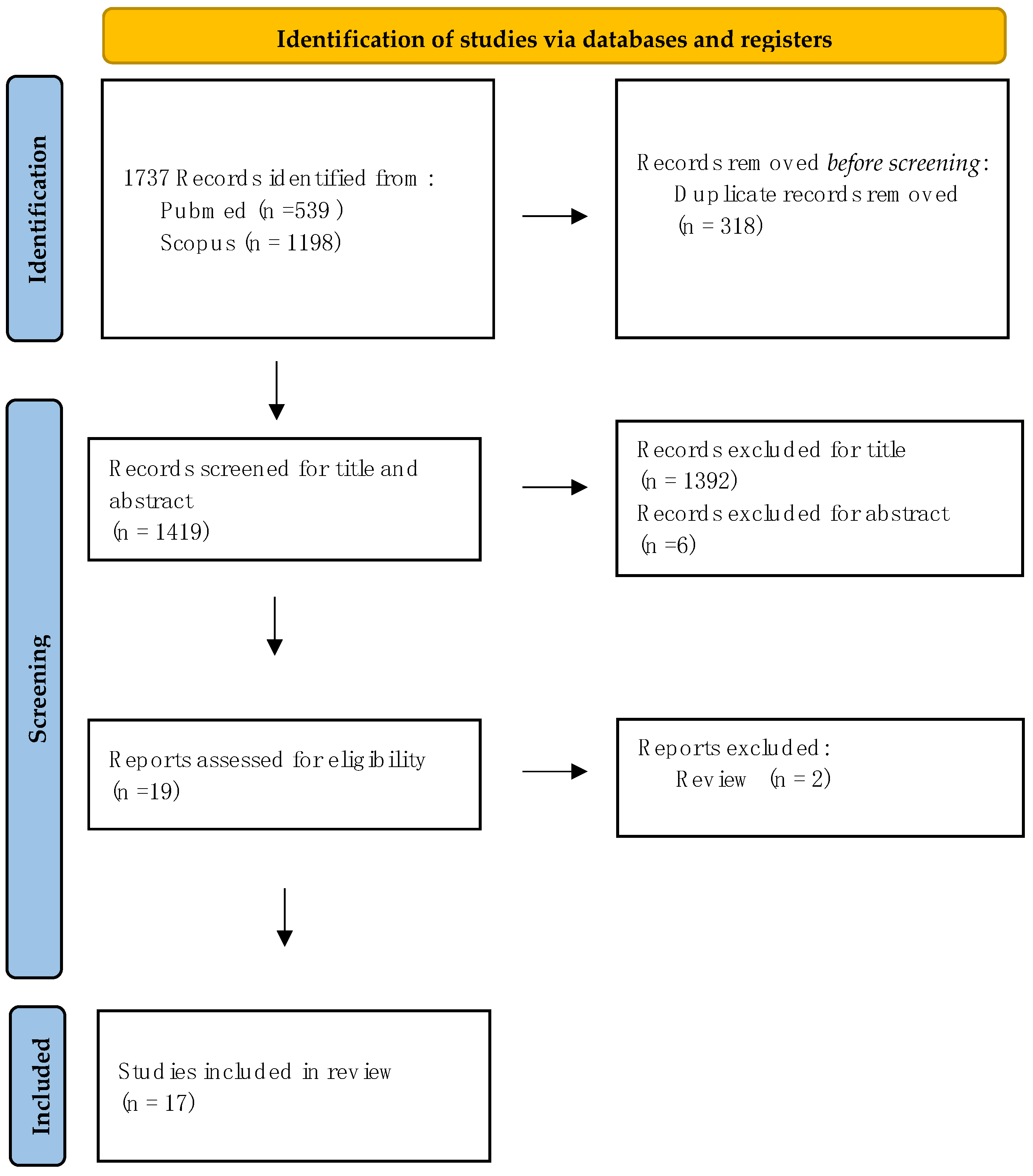
2. Methods
2.1. Search Strategy
2.2. Study Selection
- (I)
- Firstly, studies exploring PD animal models where molecular, motor behavioural, and non-motor changes after central nervous system (CNS) injection of BoNT were assessed.
- (II)
- Secondly, studies focusing on PD human subjects that evaluated the central effect of BoNT treatment through clinical, neuroradiological, and neurophysiological assessments.
3. Results
3.1. Evidence from Animal Models
3.1.1. Molecular Evidence
BoNT Effects on Cholinergic System
BoNT Effects on Dopaminergic System
BoNT Effects on Glutamatergic and GABAergic Systems
BoNT Effects on Serotoninergic and Noradrenergic Systems
3.1.2. Evidence for BoNT Effects on Motor Behaviour
3.1.3. Evidence for BoNT Effects on Non-Motor Behaviours
3.2. Evidence in Humans
4. Discussion
4.1. How Does BoNT Interact with PD Pathways? Evidence from Animal Models
4.2. Are BoNT’s Central Mechanisms Clinically Relevant in Animal Models of PD?
4.3. Can BoNT Exert Central Effects in Patients with PD?
4.4. Limitations and Gaps of the Current Literature
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choudhury, S.; Baker, M.R.; Chatterjee, S.; Kumar, H. Botulinum Toxin: An Update on Pharmacology and Newer Products in Development. Toxins 2021, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M. How Does Botulinum Toxin Work? Ann. Neurol. 2000, 48, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Anandan, C.; Jankovic, J. Botulinum Toxin in Movement Disorders: An Update. Toxins 2021, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.Y.; Burns, M.R.; Malaty, I.A. An Update on Botulinum Toxin in Neurology. Neurol. Clin. 2021, 39, 209–229. [Google Scholar] [CrossRef] [PubMed]
- Jocson, A.; Lew, M. Use of Botulinum Toxin in Parkinson’s Disease. Parkinsonism Relat. Disord. 2019, 59, 57–64. [Google Scholar] [CrossRef]
- Jost, W.H. Use of Botulinum Neurotoxin in Parkinson’s Disease: A Critical Appraisal. Toxins 2021, 13, 87. [Google Scholar] [CrossRef]
- Giannantoni, A.; Conte, A.; Proietti, S.; Giovannozzi, S.; Rossi, A.; Fabbrini, G.; Porena, M.; Berardelli, A. Botulinum Toxin Type A in Patients With Parkinson’s Disease and Refractory Overactive Bladder. J. Urol. 2011, 186, 960–964. [Google Scholar] [CrossRef]
- Currà, A.; Trompetto, C.; Abbruzzese, G.; Berardelli, A. Central Effects of Botulinum Toxin Type A: Evidence and Supposition. Mov. Disord. 2004, 19, S60–S64. [Google Scholar] [CrossRef]
- Curra, A.; Berardelli, A. Do the Unintended Actions of Botulinum Toxin at Distant Sites Have Clinical Implications? Neurology 2009, 72, 1095–1099. [Google Scholar] [CrossRef]
- Eleopra, R.; Tugnoli, V.; Rossetto, O.; De Grandis, D.; Montecucco, C. Different Time Courses of Recovery after Poisoning with Botulinum Neurotoxin Serotypes A and E in Humans. Neurosci. Lett. 1998, 256, 135–138. [Google Scholar] [CrossRef]
- Gilio, F.; Currà, A.; Lorenzano, C.; Modugno, N.; Manfredi, M.; Berardelli, A. Effects of Botulinum Toxin Type A on Intracortical Inhibition in Patients with Dystonia. Ann. Neurol. 2000, 48, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, M.L.; Mastaglia, F.L.; Walters, S.E.; Archer, S.-A.R.; Thickbroom, G.W. Primary Writing Tremor: Motor Cortex Reorganisation and Disinhibition. J. Clin. Neurosci. 2005, 12, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Thickbroom, G.W.; Byrnes, M.L.; Stell, R.; Mastaglia, F.L. Reversible Reorganisation of the Motor Cortical Representation of the Hand in Cervical Dystonia. Mov. Disord. 2003, 18, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.; Hutchinson, M. Molding the Sensory Cortex: Spatial Acuity Improves after Botulinum Toxin Treatment for Cervical Dystonia. Mov. Disord. 2007, 22, 2443–2446. [Google Scholar] [CrossRef] [PubMed]
- Blood, A.J.; Kuster, J.K.; Waugh, J.L.; Levenstein, J.M.; Multhaupt-Buell, T.J.; Sudarsky, L.R.; Breiter, H.C.; Sharma, N. White Matter Changes in Cervical Dystonia Relate to Clinical Effectiveness of Botulinum Toxin Treatment. Front. Neurol. 2019, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Weise, D.; Weise, C.M.; Naumann, M. Central Effects of Botulinum Neurotoxin—Evidence from Human Studies. Toxins 2019, 11, 21. [Google Scholar] [CrossRef]
- Dresel, C.; Haslinger, B.; Castrop, F.; Wohlschlaeger, A.M.; Ceballos-Baumann, A.O. Silent Event-Related fMRI Reveals Deficient Motor and Enhanced Somatosensory Activation in Orofacial Dystonia. Brain 2006, 129, 36–46. [Google Scholar] [CrossRef]
- Opavský, R.; Hluštík, P.; Otruba, P.; Kaňovský, P. Sensorimotor Network in Cervical Dystonia and the Effect of Botulinum Toxin Treatment: A Functional MRI Study. J. Neurol. Sci. 2011, 306, 71–75. [Google Scholar] [CrossRef]
- Opavský, R.; Hluštík, P.; Otruba, P.; Kaňovský, P. Somatosensory Cortical Activation in Cervical Dystonia and Its Modulation With Botulinum Toxin: An fMRI Study. Int. J. Neurosci. 2012, 122, 45–52. [Google Scholar] [CrossRef]
- Priori, A.; Berardelli, A.; Mercuri, B.; Manfredi, M. Physiological Effects Produced by Botulinum Toxin: Changes in Reciprocal Inhibition between Forearm Muscles. Brain 1995, 118, 801–807. [Google Scholar] [CrossRef]
- Costanzo, M.; Belvisi, D.; Berardelli, I.; Maraone, A.; Baione, V.; Ferrazzano, G.; Cutrona, C.; Leodori, G.; Pasquini, M.; Conte, A.; et al. Effect of Botulinum Toxin on Non-Motor Symptoms in Cervical Dystonia. Toxins 2021, 13, 647. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.; Cattai, L.; Teive, H. Pain Relief in Cervical Dystonia with Botulinum Toxin Treatment. Toxins 2015, 7, 2321–2335. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, H.; Harada, N.; Nishiyama, S.; Ohba, H.; Kakiuchi, T. Cholinergic Neuronal Modulation Alters Dopamine D 2 Receptor Availability In Vivo by Regulating Receptor Affinity Induced by Facilitated Synaptic Dopamine Turnover: Positron Emission Tomography Studies with Microdialysis in the Conscious Monkey Brain. J. Neurosci. 2000, 20, 7067–7073. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ham, H.J.; Yeo, I.J.; Jeon, S.H.; Lim, J.H.; Yoo, S.S.; Son, D.J.; Jang, S.-S.; Lee, H.; Shin, S.-J.; Han, S.B.; et al. Botulinum Toxin A Ameliorates Neuroinflammation in the MPTP and 6-OHDA-Induced Parkinson’s Disease Models. Biomol. Ther. 2022, 30, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Wree, A.; Mix, E.; Hawlitschka, A.; Antipova, V.; Witt, M.; Schmitt, O.; Benecke, R. Intrastriatal Botulinum Toxin Abolishes Pathologic Rotational Behaviour and Induces Axonal Varicosities in the 6-OHDA Rat Model of Parkinson’s Disease. Neurobiol. Dis. 2011, 41, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Itakura, M.; Kohda, T.; Kubo, T.; Semi, Y.; Azuma, Y.-T.; Nakajima, H.; Kozaki, S.; Takeuchi, T. Botulinum Neurotoxin A Subtype 2 Reduces Pathological Behaviors More Effectively than Subtype 1 in a Rat Parkinson’s Disease Model. Biochem. Biophys. Res. Commun. 2014, 447, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Hawlitschka, A.; Holzmann, C.; Witt, S.; Spiewok, J.; Neumann, A.-M.; Schmitt, O.; Wree, A.; Antipova, V. Intrastriatally Injected Botulinum Neurotoxin-A Differently Effects Cholinergic and Dopaminergic Fibers in C57BL/6 Mice. Brain Res. 2017, 1676, 46–56. [Google Scholar] [CrossRef]
- Mann, T.; Zilles, K.; Klawitter, F.; Cremer, M.; Hawlitschka, A.; Palomero-Gallagher, N.; Schmitt, O.; Wree, A. Acetylcholine Neurotransmitter Receptor Densities in the Striatum of Hemiparkinsonian Rats Following Botulinum Neurotoxin-A Injection. Front. Neuroanat. 2018, 12, 65. [Google Scholar] [CrossRef]
- Hawlitschka, A.; Berg, C.; Schmitt, O.; Holzmann, C.; Wree, A.; Antipova, V. Repeated Intrastriatal Application of Botulinum Neurotoxin-A Did Not Influence Choline Acetyltransferase-Immunoreactive Interneurons in Hemiparkinsonian Rat Brain – A Histological, Stereological and Correlational Analysis. Brain Res. 2020, 1742, 146877. [Google Scholar] [CrossRef]
- Wedekind, F.; Oskamp, A.; Lang, M.; Hawlitschka, A.; Zilles, K.; Wree, A.; Bauer, A. Intrastriatal Administration of Botulinum Neurotoxin A Normalizes Striatal D 2 R Binding and Reduces Striatal D 1 R Binding in Male Hemiparkinsonian Rats. J. Neurosci. Res. 2018, 96, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Zilles, K.; Dikow, H.; Hellfritsch, A.; Cremer, M.; Piel, M.; Rösch, F.; Hawlitschka, A.; Schmitt, O.; Wree, A. Dopamine, Noradrenaline and Serotonin Receptor Densities in the Striatum of Hemiparkinsonian Rats Following Botulinum Neurotoxin-A Injection. Neuroscience 2018, 374, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Kurth, J.; Hawlitschka, A.; Stenzel, J.; Lindner, T.; Polei, S.; Hohn, A.; Krause, B.; Wree, A. [18F]Fallypride-PET/CT Analysis of the Dopamine D2/D3 Receptor in the Hemiparkinsonian Rat Brain Following Intrastriatal Botulinum Neurotoxin A Injection. Molecules 2018, 23, 587. [Google Scholar] [CrossRef] [PubMed]
- Alberts, T.; Antipova, V.; Holzmann, C.; Hawlitschka, A.; Schmitt, O.; Kurth, J.; Stenzel, J.; Lindner, T.; Krause, B.J.; Wree, A.; et al. Olfactory Bulb D2/D3 Receptor Availability after Intrastriatal Botulinum Neurotoxin-A Injection in a Unilateral 6-OHDA Rat Model of Parkinson’s Disease. Toxins 2022, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Tsang, A.R.; Rajakumar, N.; Jog, M.S. Intrapallidal Injection of Botulinum Toxin A Recovers Gait Deficits in a Parkinsonian Rodent Model. Acta Physiol. 2019, 226, e13230. [Google Scholar] [CrossRef]
- Antipova, V.; Hawlitschka, A.; Mix, E.; Schmitt, O.; Dräger, D.; Benecke, R.; Wree, A. Behavioral and Structural Effects of Unilateral Intrastriatal Injections of Botulinum Neurotoxin a in the Rat Model of Parkinson’s Disease: Intrastriatal BoNT in Hemiparkinsonian Rat. J. Neurosci. Res. 2013, 91, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Antipova, V.A.; Holzmann, C.; Schmitt, O.; Wree, A.; Hawlitschka, A. Botulinum Neurotoxin A Injected Ipsilaterally or Contralaterally into the Striatum in the Rat 6-OHDA Model of Unilateral Parkinson’s Disease Differently Affects Behavior. Front. Behav. Neurosci. 2017, 11, 119. [Google Scholar] [CrossRef]
- Hawlitschka, A.; Holzmann, C.; Wree, A.; Antipova, V. Repeated Intrastriatal Botulinum Neurotoxin-A Injection in Hemiparkinsonian Rats Increased the Beneficial Effect on Rotational Behavior. Toxins 2018, 10, 368. [Google Scholar] [CrossRef]
- Antipova, V.; Holzmann, C.; Hawlitschka, A.; Wree, A. Botulinum Neurotoxin-A Injected Intrastriatally into Hemiparkinsonian Rats Improves the Initiation Time for Left and Right Forelimbs in Both Forehand and Backhand Directions. Int. J. Mol. Sci. 2019, 20, 992. [Google Scholar] [CrossRef]
- Tsang, A.R.; Rajakumar, N.; Jog, M.S. Botulinum Toxin A Injection into the Entopeduncular Nucleus Improves Dynamic Locomotory Parameters in Hemiparkinsonian Rats. PLOS ONE 2019, 14, e0223450. [Google Scholar] [CrossRef]
- Antipova, V.; Holzmann, C.; Hawlitschka, A.; Witt, M.; Wree, A. Antidepressant-Like Properties of Intrastriatal Botulinum Neurotoxin-A Injection in a Unilateral 6-OHDA Rat Model of Parkinson’s Disease. Toxins 2021, 13, 505. [Google Scholar] [CrossRef] [PubMed]
- Samotus, O.; Chen, R.; Jog, M. Changes in Cortical Excitability and Parkinson Tremor After Botulinum Toxin Therapy. Neurology 2021, 97, e1413–e1424. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.J.; Tan, E.-K.; Chao, Y.-X. Historical Perspective: Models of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 2464. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, C.; Schiavo, G. Mechanism of Action of Tetanus and Botulinum Neurotoxins. Mol. Microbiol. 1994, 13, 1–8. [Google Scholar] [CrossRef]
- Simpson, L.L. Identification of the Major Steps in Botulinum Toxin Action. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, C.; Dräger, D.; Mix, E.; Hawlitschka, A.; Antipova, V.; Benecke, R.; Wree, A. Effects of Intrastriatal Botulinum Neurotoxin A on the Behavior of Wistar Rats. Behav. Brain Res. 2012, 234, 107–116. [Google Scholar] [CrossRef]
- Ungerstedt, U.; Butcher, L.L.; Butcher, S.G.; Ande´n, N.-E.; Fuxe, K. Direct Chemical Stimulation of Dopaminergic Mechanisms in the Neostriatum of the Rat. Brain Res. 1969, 14, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Ungerstedt, U.; Arbuthnott, G.W. Quantitative Recording of Rotational Behavior in Rats after 6-Hydroxy-Dopamine Lesions of the Nigrostriatal Dopamine System. Brain Res. 1970, 24, 485–493. [Google Scholar] [CrossRef]
- Walsh, R.N.; Cummins, R.A. The Open-Field Test: A Critical Review. Psychol. Bull. 1976, 83, 482–504. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Schallert, T.; Fleming, S.M.; Leasure, J.L.; Tillerson, J.L.; Bland, S.T. CNS Plasticity and Assessment of Forelimb Sensorimotor Outcome in Unilateral Rat Models of Stroke, Cortical Ablation, Parkinsonism and Spinal Cord Injury. Neuropharmacology 2000, 39, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.J.; Roberts, D.J. A Rotarod Suitable for Quantitative Measurements of Motor Incoordination in Naive Mice. Naunyn-Schmiedebergs Arch. Für Pharmakol. Und Exp. Pathol. 1968, 259, 211. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.J.; Roberts, D.J. The Quantitative Measurement of Motor Inco-Ordination in Naive Mice Using an Accelerating Rotarod. J. Pharm. Pharmacol. 2011, 20, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Döbrössy, M.D.; Dunnett, S.B. The Corridor Task: Striatal Lesion Effects and Graft-Mediated Recovery in a Model of Huntington’s Disease. Behav. Brain Res. 2007, 179, 326–330. [Google Scholar] [CrossRef]
- Dowd, E.; Monville, C.; Torres, E.M.; Dunnett, S.B. The Corridor Task: A Simple Test of Lateralised Response Selection Sensitive to Unilateral Dopamine Deafferentation and Graft-Derived Dopamine Replacement in the Striatum. Brain Res. Bull. 2005, 68, 24–30. [Google Scholar] [CrossRef]
- Olsson, M.; Nikkhah, G.; Bentlage, C.; Bjorklund, A. Forelimb Akinesia in the Rat Parkinson Model: Differential Effects of Dopamine Agonists and Nigral Transplants as Assessed by a New Stepping Test. J. Neurosci. 1995, 15, 3863–3875. [Google Scholar] [CrossRef]
- Hwang, C.J.; Kim, Y.E.; Son, D.J.; Park, M.H.; Choi, D.-Y.; Park, P.-H.; Hellström, M.; Han, S.-B.; Oh, K.-W.; Park, E.K.; et al. Parkin Deficiency Exacerbate Ethanol-Induced Dopaminergic Neurodegeneration by P38 Pathway Dependent Inhibition of Autophagy and Mitochondrial Function. Redox Biol. 2017, 11, 456–468. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of Open: Closed Arm Entries in an Elevated plus-Maze as a Measure of Anxiety in the Rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural Despair in Rats: A New Model Sensitive to Antidepressant Treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef]
- Chermat, R.; Thierry, B.; Mico, J.A.; Steru, L.; Simon, P. Adaptation of the Tail Suspension Test to the Rat. J. Pharmacol. 1986, 17, 348–350. [Google Scholar]
- Lehmkuhl, A.M.; Dirr, E.R.; Fleming, S.M. Olfactory Assays for Mouse Models of Neurodegenerative Disease. J. Vis. Exp. 2014, 51804. [Google Scholar] [CrossRef]
- Björklund, A.; Dunnett, S.B. The Amphetamine Induced Rotation Test: A Re-Assessment of Its Use as a Tool to Monitor Motor Impairment and Functional Recovery in Rodent Models of Parkinson’s Disease. J. Park. Dis. 2019, 9, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-Invasive Electrical and Magnetic Stimulation of the Brain, Spinal Cord, Roots and Peripheral Nerves: Basic Principles and Procedures for Routine Clinical and Research Application. An Updated Report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef]
- Aosaki, T.; Miura, M.; Suzuki, T.; Nishimura, K.; Masuda, M. Acetylcholine–Dopamine Balance Hypothesis in the Striatum: An Update. Geriatr. Gerontol. Int. 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Zhai, S.; Surmeier, D.J. Striatal Synaptic Adaptations in Parkinson’s Disease. Neurobiol. Dis. 2022, 167, 105686. [Google Scholar] [CrossRef] [PubMed]
- Spehlmann, R.; Stahl, S. Dopamine acetylcholine imbalance in parkinson’s disease. Lancet 1976, 307, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, N.I.; Yarnall, A.J.; Weil, R.S.; Moro, E.; Moehle, M.S.; Borghammer, P.; Bedard, M.-A.; Albin, R.L. Cholinergic System Changes in Parkinson’s Disease: Emerging Therapeutic Approaches. Lancet Neurol. 2022, 21, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Obeso, I.; Wilkinson, L.; Casabona, E.; Speekenbrink, M.; Luisa Bringas, M.; Álvarez, M.; Álvarez, L.; Pavón, N.; Rodríguez-Oroz, M.C.; Macías, R.; et al. The Subthalamic Nucleus and Inhibitory Control: Impact of Subthalamotomy in Parkinson’s Disease. Brain 2014, 137, 1470–1480. [Google Scholar] [CrossRef]
- Aron, A.R.; Poldrack, R.A. Cortical and Subcortical Contributions to Stop Signal Response Inhibition: Role of the Subthalamic Nucleus. J. Neurosci. 2006, 26, 2424–2433. [Google Scholar] [CrossRef]
- Nauta, H.J.W.; Cole, M. Efferent Projections of the Subthalamic Nucleus: An Autoradiographic Study in Monkey and Cat. J. Comp. Neurol. 1978, 180, 1–16. [Google Scholar] [CrossRef]
- Hariz, M.; Blomstedt, P. Deep Brain Stimulation for Parkinson’s Disease. J. Intern. Med. 2022, 292, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.B.; Sørensen, J.C.H.; Ettrup, K.S.; Orlowski, D.; Bjarkam, C.R. Pirouetting Pigs: A Large Non-Primate Animal Model Based on Unilateral 6-Hydroxydopamine Lesioning of the Nigrostriatal Pathway. Brain Res. Bull. 2018, 139, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-Motor Features of Parkinson Disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, F.; Rossi, C.; Gianfranceschi, L.; Rossetto, O.; Caleo, M. Long-Distance Retrograde Effects of Botulinum Neurotoxin A. J. Neurosci. 2008, 28, 3689–3696. [Google Scholar] [CrossRef] [PubMed]
- Restani, L.; Antonucci, F.; Gianfranceschi, L.; Rossi, C.; Rossetto, O.; Caleo, M. Evidence for Anterograde Transport and Transcytosis of Botulinum Neurotoxin A (BoNT/A). J. Neurosci. 2011, 31, 15650–15659. [Google Scholar] [CrossRef] [PubMed]
- Sailer, A.; Cunic, D.I.; Paradiso, G.O.; Gunraj, C.A.; Wagle-Shukla, A.; Moro, E.; Lozano, A.M.; Lang, A.E.; Chen, R. Subthalamic Nucleus Stimulation Modulates Afferent Inhibition in Parkinson Disease. Neurology 2007, 68, 356–363. [Google Scholar] [CrossRef]
- Wagle Shukla, A.; Moro, E.; Gunraj, C.; Lozano, A.; Hodaie, M.; Lang, A.; Chen, R. Long-Term Subthalamic Nucleus Stimulation Improves Sensorimotor Integration and Proprioception. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1020–1028. [Google Scholar] [CrossRef]
- Belvisi, D.; Leodori, G.; Costanzo, M.; Conte, A.; Berardelli, A. How Does Botulinum Toxin Really Work? In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2023; Volume 169, pp. 441–479. ISBN 978-0-323-99026-4. [Google Scholar]
- Habermann, E.; Erdmann, G. Pharmacokinetic and Histoautoradiographic Evidence for the Intraaxonal Movement of Toxin in the Pathogenesis of Tetanus. Toxicon 1978, 16, 611–623. [Google Scholar] [CrossRef]
| Animal Models | ||||
|---|---|---|---|---|
| 1. Evidence for Molecular and Structural Changes | ||||
| 1.1 BoNT Effects on Cholinergic System | ||||
| Author, Year | Subjects | Design | Methods | Main results |
| Wree et al., 2011 [26] | 6-OHDA rats | Longitudinal (1 month) | Immunohistochemistry |
|
| Itakura et al., 2014 [27] | 6-OHDA rats | Longitudinal (23 days) | Immunofluorescence analysis | BoNT-A2 increases cleaved SNAP-25 compared with that of the control group and the BoNT-A1 group. |
| Hawlitschka et al., 2017 [28] | C57BL/6 mice | Longitudinal (9 months) | Immunohistochemistry |
|
| Mann et al., 2018 [29] | 6-OHDA rats | Longitudinal (9 months) | Autoradiography | BoNT reduces interhemispheric differences for mAchRs and nAchRs in hemi-PD rats. |
| Hawlitschka et al., 2020 [30] | 6-OHDA rats | Longitudinal (19 months) | Immunohistochemistry Stereological analysis |
|
| 1.2 BoNT Effects on Dopaminergic System | ||||
| Wedekind et al., 2018 [31] | 6-OHDA rats | Longitudinal (5 months) | Histology Receptor autoradiography [11C]raclopride-PET/CT scans |
|
| Mann et al., 2018 [32] | 6-OHDA rats | Longitudinal (9 months) | Receptor autoradiography |
|
| Mann et al., 2018 [33] | 6-OHDA rats | Longitudinal (6 months) | [18F]fallypride-PET/CT scans |
|
| Ham et al., 2022 [25] | MPTP mice 6-OHDA mice | Longitudinal | Immunohistochemistry Western blot analysis ELISA (Dopamine; Ach) RT-PCR (TNF-α, IL-1β, IL-6) | BoNT-A:
|
| Alberts et al., 2022 [34] | 6-OHDA rats | Longitudinal (6 months) | [18 F]fallypride-PET/CT Immunohistochemistry analysis fMRI |
|
| 1.3 BoNT Effects on Glutamatergic and GABAergic Systems | ||||
| Tsang et al., 2018 [35] | 6-OHDA rats | Longitudinal (3 months) | Immunofluorescence | BoNT-A reduces synaptophysin and vGluT2 labelling. |
| 1.4 BoNT Effects on Serotoninergic and Noradrenergic Systems | ||||
| Mann et al., 2018 [32] | 6-OHDA rats | Longitudinal (9 months) | Receptor autoradiography | BoNT does not affect reduced alfa1, alfa2 and 5HT2a receptor density. |
| 2. Evidence for BoNT Effects On Motor Behaviour | ||||
| Wree et al., 2011 [26] | 6-OHDA rats | Longitudinal (12 months) | Drug-induced rotation test | BoNT-A abolishes apomorphine-induced rotations up to 6 months. |
| Antipova et al., 2013 [36] | 6-OHDA rats | Longitudinal (12 months) | Drug-induced rotation test Forced motor test (accelerod test) Spontaneous motor test |
|
| Itakura et al., 2014 [27] | 6-OHDA rats | Longitudinal (23 days) | Drug-induced rotation test | BoNT-A2 ameliorates pathogenic rotation behaviour at a lower dosage than BoNT-A1. |
| Antipova et al., 2017 [37] | 6-OHDA rats | Longitudinal (12 months) | Drug-induced rotation test Spontaneous motor test |
|
| Wedekind et al., 2018 [31] | 6-OHDA rats | Longitudinal (5 months) | Drug-induced rotation test Spontaneous motor test |
|
| Mann et al., 2018 [33] | 6-OHDA rats | Longitudinal (6 months) | Drug-induced rotation test | BoNT-A reduces apomorphine-induced rotations. |
| Hawlitschka et al., 2018 [38] | 6-OHDA rats | Longitudinal (12 months) | Drug-induced rotation test Spontaneous motor test |
|
| Tsang et al., 2018 [35] | 6-OHDA rats | Longitudinal (3 months) | Gait analysis (CatWalk) Drug-induced rotation test | BoNT-A improves the rotational asymmetry and gait abnormalities of hemi-PD rats. |
| Antipova et al., 2019 [39] | 6-OHDA rats | Longitudinal (12 months) | Drug-induced rotation test Spontaneous motor test | BoNT reduces initiation time in 6-OHDA rats. |
| Tsang et al., 2019 [40] | 6-OHDA rats | Longitudinal (3 months) | Gait analysis (CatWalk) |
|
| Ham et al., 2022 [25] | MPTP mice 6-OHDA mice | Longitudinal (3 weeks) | Motor behaviour testing (rotarod, pole test, and gait test) | BoNT induces improvement of motor behaviour test:
|
| 3. Evidence for BoNT Effects on Non-Motor Behaviours | ||||
| Antipova et al., 2021 [41] | 6-OHDA rats | Longitudinal (1 months) | Drug-induced rotation test Spontaneous motor tests |
|
| Alberts et al., 2022 [34] | 6-OHDA rats | Longitudinal (6 months) | Orienting odour identification test | Intrastriatal BoNT-A induces improvement of olfactory performance. |
| Human subjects | ||||
| Samotus et al., 2021 [42] | 12 PD (de novo) 7 PD (L-Dopa) with tremor | Longitudinal (4 time points 6 weeks after BoNT injection) | Clinical scales Sensor-based tremor assessment Kinematics TMS (SICI, ICF, LICI, SAI, LAI) |
|
| Test | Function Explored | Paradigm | Reference(s) |
|---|---|---|---|
| Amphetamine-induced rotation | Drug-induced rotational behaviour to explore motor impairment after formation of 6-OHDA-induced lesion | Animals are injected with d-amphetamine sulphate (2.5 mg/kg, s.c.) and monitored for 60 min. Rotations are assessed using an automated, self-constructed rotometer system and defined as the number of complete 360° turns and registered as net differences between the two directions per minute. | Ungerstedt U et al., 1969 [47] |
| Apomorphine-induced rotation | Drug-induced rotational behaviour to explore motor impairment after formation of 6-OHDA-induced lesion | Animals are injected with apomorphine (0.25 mg/kg, s.c.), followed by registration of rotation for 40 min. Rotations are assessed using an automated, self-constructed rotometer system and defined as the number of complete 360° turns and registered as net differences between the two directions per minute. | Ungerstedt U et al., 1970 [48] |
| Open field test | Spontaneous locomotor activity | Rats are placed into a square arena (50 × 50 cm) with 50 cm high walls located inside of an isolation box. The running distance of the animals within 10 min is registered as a measurement of spontaneous locomotor activity. | Walsh et al., 1976 [49]; Basso DM et al., 1995 [50]; |
| Cylinder test | Forelimb usage/preference | The use of the left and right forepaws during vertical exploration in a glass cylinder with a diameter of 20 cm is documented and analysed with a video camera system to count the initial contacts of the right or left paw and calculating the ratio of left to right forepaw use. | Schallert T et al., 2000 [51] |
| Rotarod/accelerod test | Forced motor activity | The kinematic analysis of forced motor activity is performed by computerised rotating rods starting at 4 rounds per minute (rpm) and accelerating to 40 rpm over a period of 5 min. The time spent on the rod before falling off and the maximum speed level reached are recorded. | Jones BJ et al., 1968a [52]; Jones BJ et al., 2011 [53]; |
| Corridor test | Lateralised sensory–motor integration | Rats are placed for 5 minutes in a testing corridor in which the researcher have positioned bowls containing pellets in the right and the left side. The number of right side and left side retrievals are counted and the data are expressed as the percentage of left or right side retrievals over the total number of retrievals. | Döbrössy et al., 2007 [54]; Dowd et al., 2005 [55]; |
| Stepping test | Forelimb akinesia | The rat is held by the investigator with one hand blocking both its hind limbs and the unrestrained forepaw touching the table. The rat is moved slowly sideways across the table and the number of adjusting steps of the respective unrestrained left or right forepaw are counted while moving in the forehand and backhand directions. Finally, the means of forehand and backhand steps of the left and right paws are calculated. | Olsson et al., 1995 [56] |
| Pole test | Bradykinesia | For the pole test, the time to turn and total time to place four paws on the base were measured after placing the mice at a fixed distance from the top of a metal rod (the pole). | Hwang et al., 2017 [57] |
| Elevated plus maze test | Anxiety-like behaviour | The rat is positioned in an elevated mace apparatus consistent of a central platform, an open arm, and a closed arm. During a 5 min test, the following are evaluated: time on the open arms, presence on open arms (% open time), and walking speed. All these parameters are inversely connected to anxiety. | Pellow et al., 1985 [58] |
| Forced swim test | Depressive-like behaviour | The rat is positioned in a forced swimming tank for 10 min and video recorded. During the task, the time spent struggling, time spent swimming, and time spent immobile are evaluated. | Porsolt et al., 1977 [59] |
| Tail suspension test | Depressive-like behaviour | Rats are slowly lifted grasping the base of the tail for a total of 60 s. The time (s) the rat spent immobile is considered a correlate of depression-like behaviour. | Chermat et al., 1986 [60] |
| Buried pellet test | Olfactory performance | The rat is put in a cage with 3 cm of clean bedding and one pellet buried 0.5 cm below in one corner of the cage or in the surface of the bedding. The rat is placed in the centre of the test cage and the latency time is measured until the rat uncovers the pellet and begins eating it. | Lehmkuhl et al., 2014 [61] |
| Wree 2011 [26] | Antipova 2013 [36] | Itakura 2014 [27] | Antipova 2017 [37] | Wedekind 2018 [31] | Hawlitschka 2020 [30] | Mann 2018 [33] | Antipova 2019 [39] | Tsang 2018 [35] | Tsang 2019 [40] | Ham 2022 [25] | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal model | 6-OHDA PD rats | 6-OHDA PD rats | 6-OHDA PD rats | 6-OHDA PD rats | 6-OHDA PD rats | 6-OHDA PD rats | 6-OHDA PD rats | 6-OHDA PD rats | 6-OHDA PD rats | 6-OHDA PD rats | 6-OHDA and MPTp PD mice | |
| Injection site | CPu | CPu | CPu | CPu | CPu | CPu | CPu | CPu | EPN | EPN | CPu | |
| Injection side | ipsi | ipsi | ipsi | ipsi | contra | ipsi | ipsi | ipsi | ipsi | ipsi | ipsi | ipsi |
| Apomorphine-induced rotations | Abolished (3 months) | Abolished (4 weeks) | BoNTA2 reduced rotations with a lower dose than BontA1 | Decreased (3 months) | Unchanged (2 weeks) | Reduced | Decreased (6 months) (more pronounced if repeated) | Decreased | Decreased | Abolished (1 week–1 month) | n.p. | n.p. |
| Amphetamine-induced rotations | n.p. | 1 ng: No changes 2 ng: Enhanced (3 months) | n.p. | Increased (6 months) | Unchanged (2 weeks) | n.p. | n.p. | Increased | n.p. | n.p. | n.p. | n.p. |
| Gait tests | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. | Increased stride and stance length |
| CatWalk apparatus (gait impairment) | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. | Improvement in gait (1 week–1 month) | Improvement in dynamic gait parameters (1 week–1 month) | n.p. |
| Stepping test (forelimb akinesia) | n.p. | n.p. | n.p. | Unchanged | Improvement (up to 9 months) | n.p. | No improvement | n.p. | Initiation time (until 6 months) | n.p. | n.p. | n.p. |
| Corridor test (lateralised sensori-motor integration/neglect contralateral to 6-OHDA-induced lesion) | n.p. | n.p. | n.p. | Unchanged (6 months) | Improvement (up to 9 months) | n.p. | No improvement (6 months) | n.p. | n.p. | n.p. | n.p. | n.p. |
| Rotarod/accelerod test (forced motor activity) | n.p. | Unchanged | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. | Increased latency to fall |
| Cylinder test (forelimb usage/preference) | n.p. | Equalisation of left and right forepaw | n.p. | Unchanged | Improvement (2 weeks) | Reduction in forelimb use asymmetry | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. |
| Open field test (spontaneous locomotor activity) | n.p. | Unchanged | n.p. | Unchanged | Unchanged | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutrona, C.; Marchet, F.; Costanzo, M.; De Bartolo, M.I.; Leodori, G.; Ferrazzano, G.; Conte, A.; Fabbrini, G.; Berardelli, A.; Belvisi, D. Exploring the Central Mechanisms of Botulinum Toxin in Parkinson’s Disease: A Systematic Review from Animal Models to Human Evidence. Toxins 2024, 16, 9. https://doi.org/10.3390/toxins16010009
Cutrona C, Marchet F, Costanzo M, De Bartolo MI, Leodori G, Ferrazzano G, Conte A, Fabbrini G, Berardelli A, Belvisi D. Exploring the Central Mechanisms of Botulinum Toxin in Parkinson’s Disease: A Systematic Review from Animal Models to Human Evidence. Toxins. 2024; 16(1):9. https://doi.org/10.3390/toxins16010009
Chicago/Turabian StyleCutrona, Carolina, Francesco Marchet, Matteo Costanzo, Maria Ilenia De Bartolo, Giorgio Leodori, Gina Ferrazzano, Antonella Conte, Giovanni Fabbrini, Alfredo Berardelli, and Daniele Belvisi. 2024. "Exploring the Central Mechanisms of Botulinum Toxin in Parkinson’s Disease: A Systematic Review from Animal Models to Human Evidence" Toxins 16, no. 1: 9. https://doi.org/10.3390/toxins16010009
APA StyleCutrona, C., Marchet, F., Costanzo, M., De Bartolo, M. I., Leodori, G., Ferrazzano, G., Conte, A., Fabbrini, G., Berardelli, A., & Belvisi, D. (2024). Exploring the Central Mechanisms of Botulinum Toxin in Parkinson’s Disease: A Systematic Review from Animal Models to Human Evidence. Toxins, 16(1), 9. https://doi.org/10.3390/toxins16010009





