A Monoclonal Antibody with a High Affinity for Ricin Isoforms D and E Provides Strong Protection against Ricin Poisoning
Abstract
1. Introduction
2. Results
2.1. Production and Selection of a New Generation of Anti-Ricin Monoclonal Antibodies (mAbs)
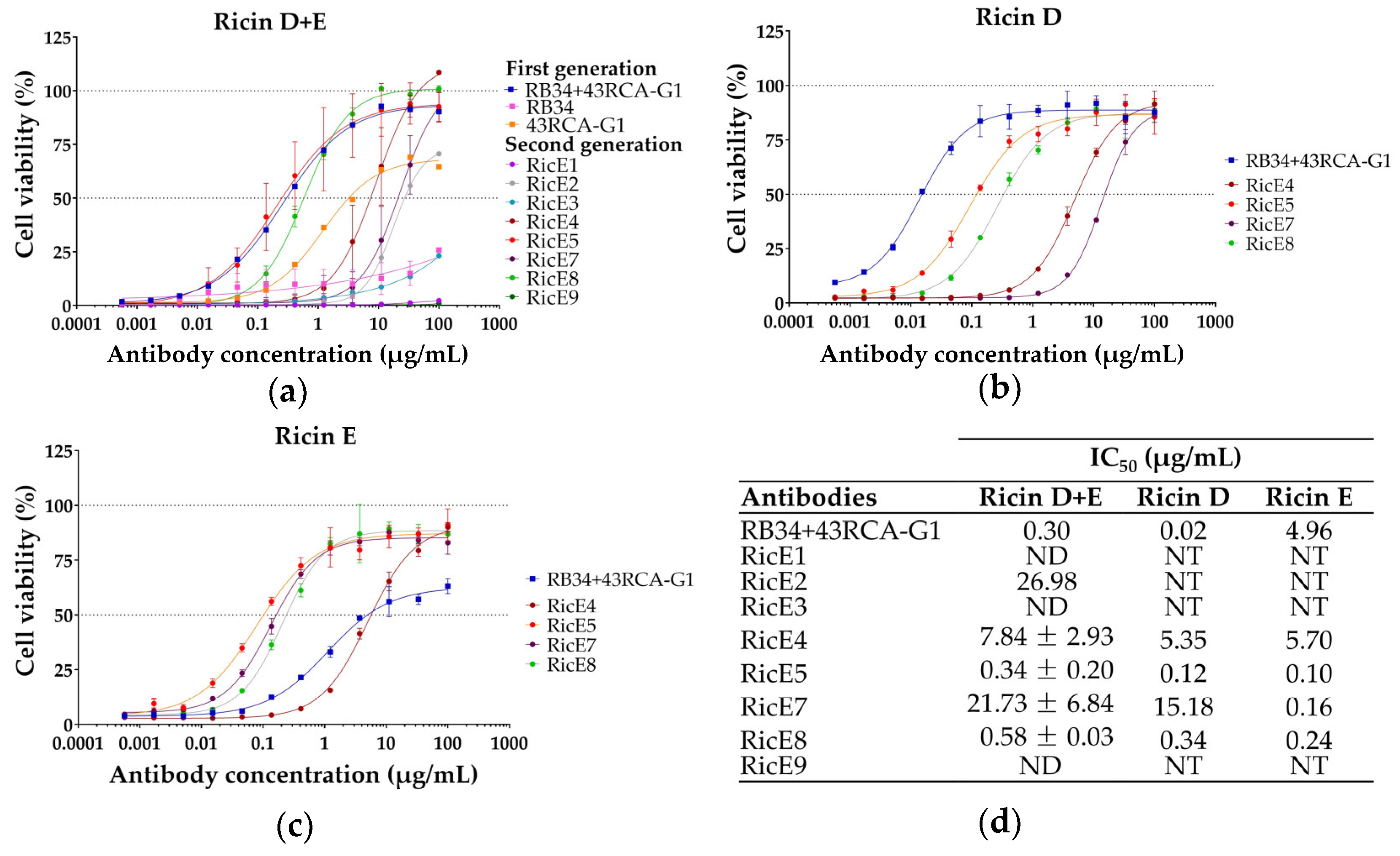
2.1.1. Screening for In Vitro Neutralizing Capacity of mAbs
2.1.2. Binding Kinetics of mAbs to Ricin Isoforms and Antibody Chain Specificity
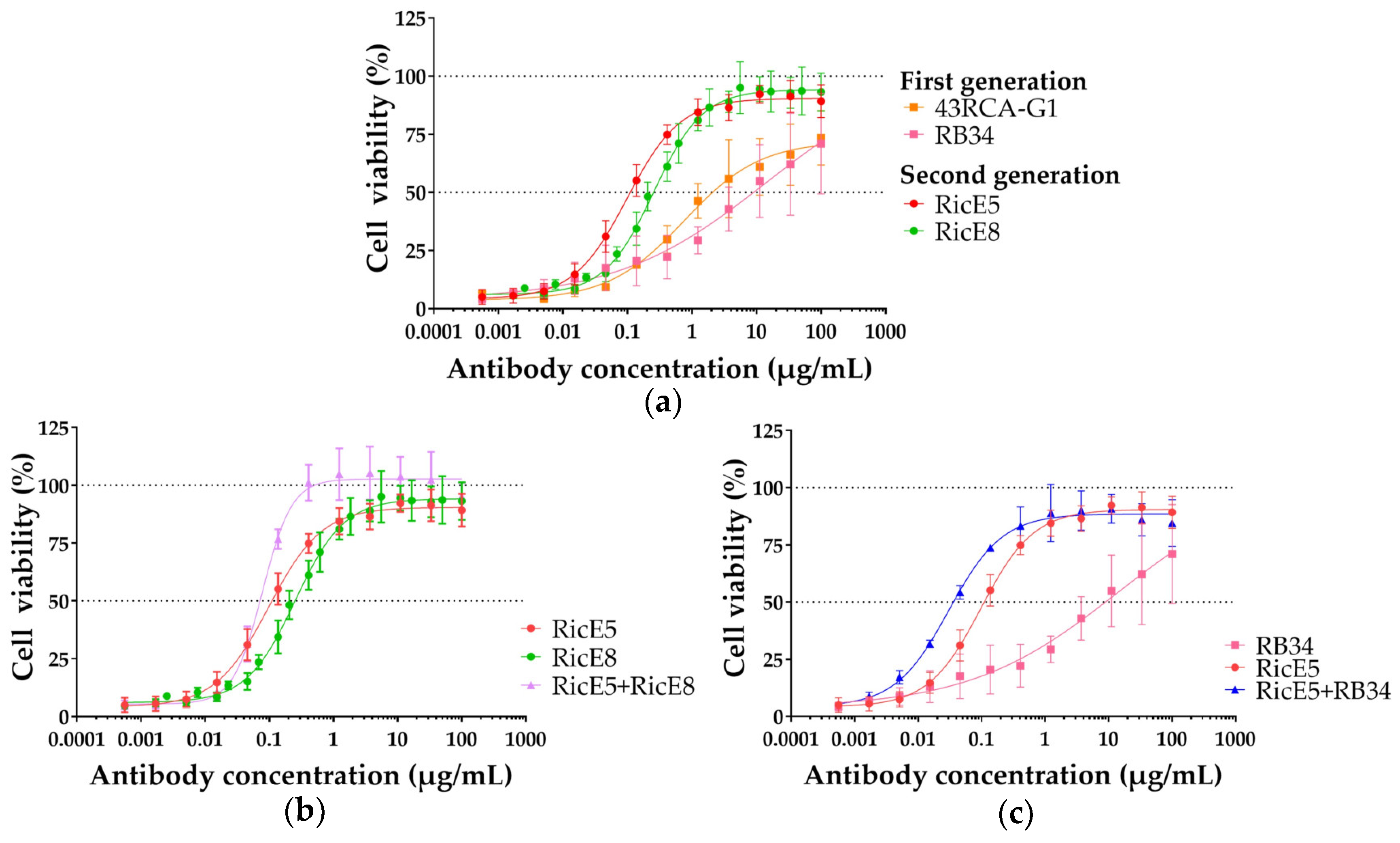
2.2. In-Depth Analysis of the In Vitro Neutralizing Capacity of the Two Selected mAbs
2.2.1. In Vitro Neutralizing Capacity of Individual mAbs RicE5 and RicE8 and Their Combinations
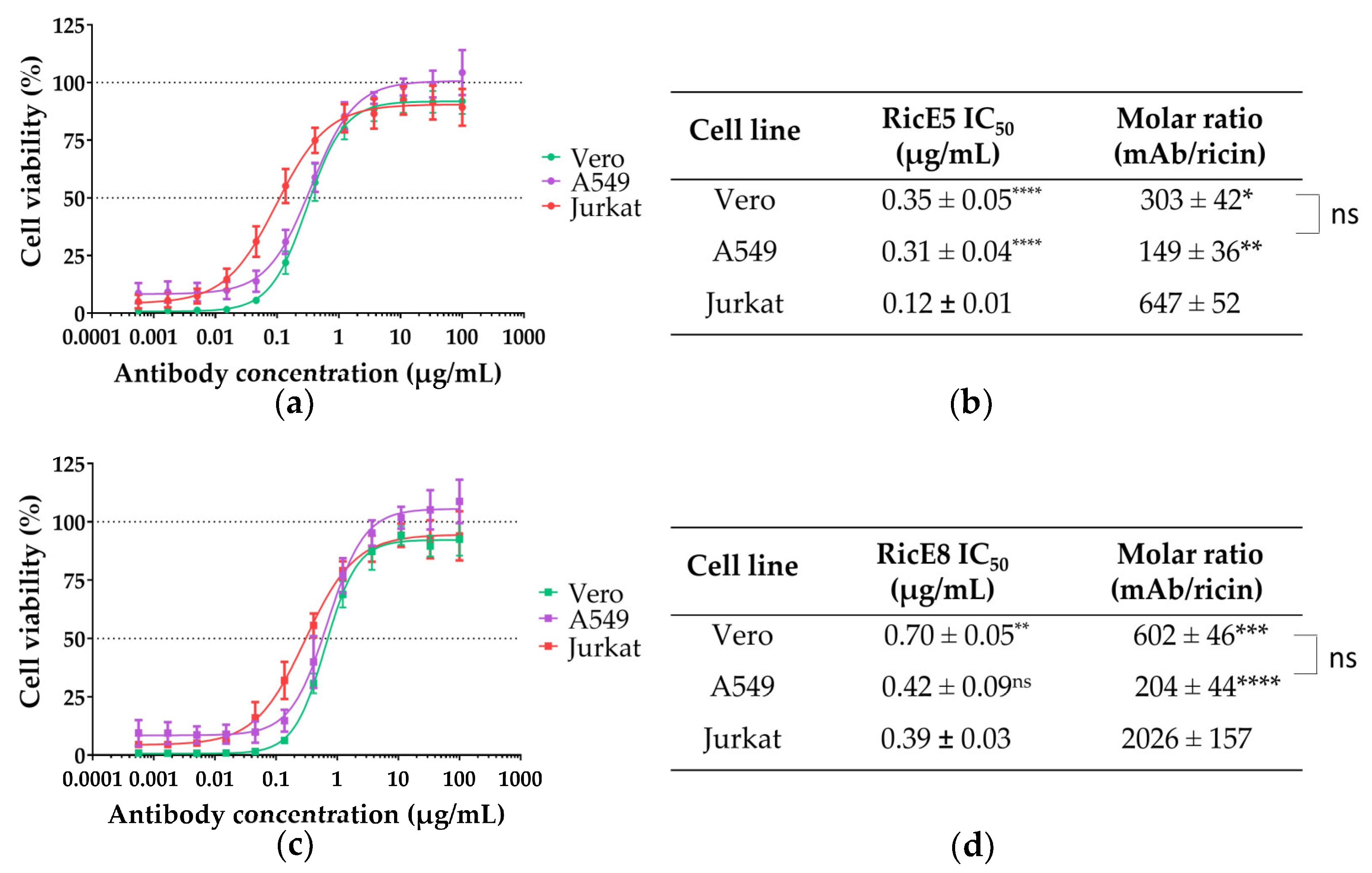
2.2.2. In Vitro Neutralizing Capacity of RicE5 and RicE8 across Various Cell Lines
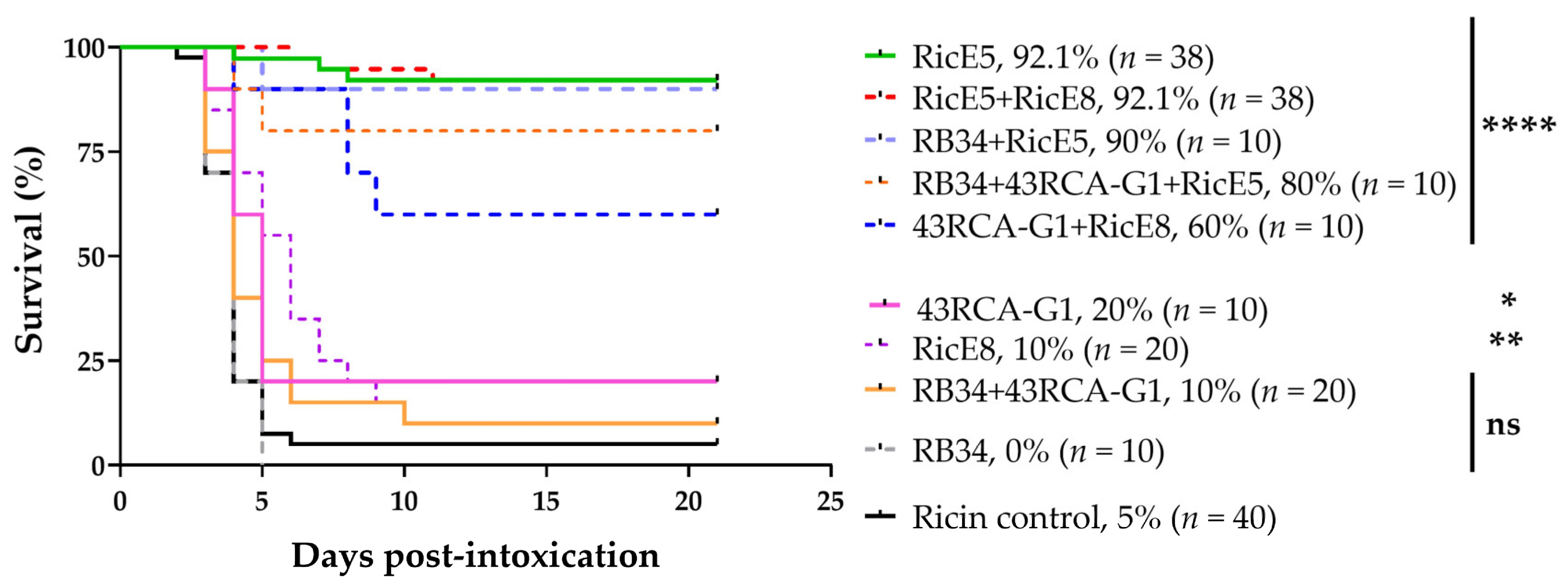
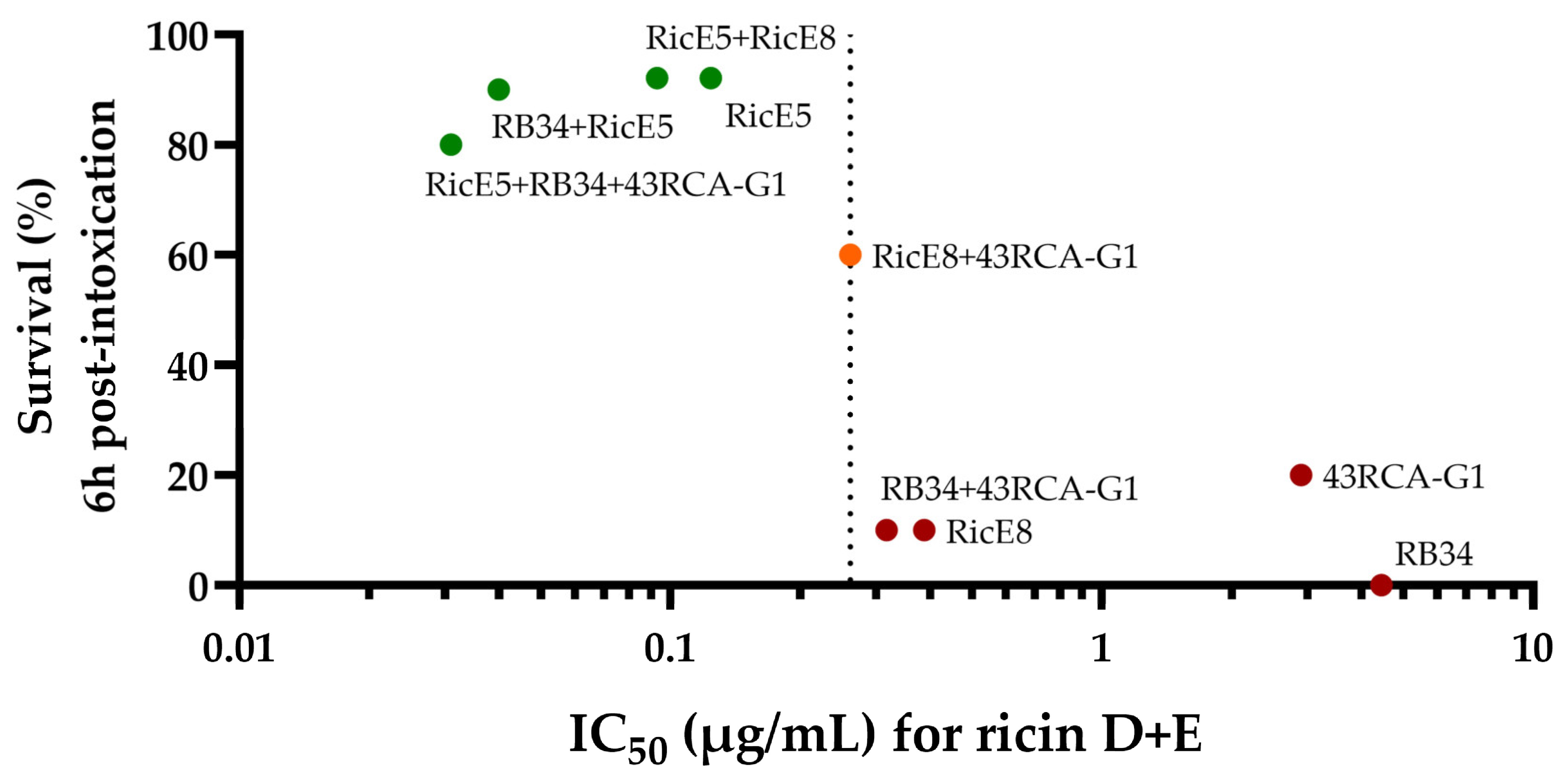
2.3. In Vivo Protective Capacity
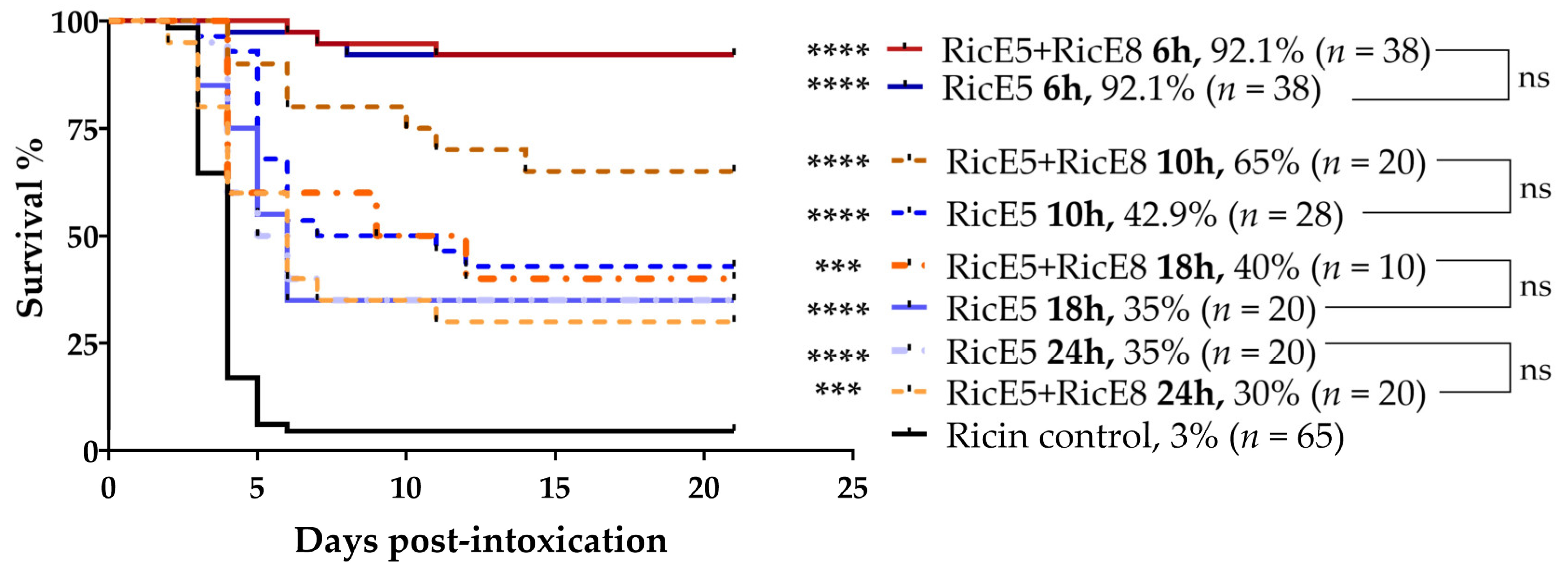
2.3.1. Treatments 6 h after Intoxication
2.3.2. Treatments at 10 h, 18 h and 24 h after Intoxication and Addition of Anti-Inflammatory Molecules
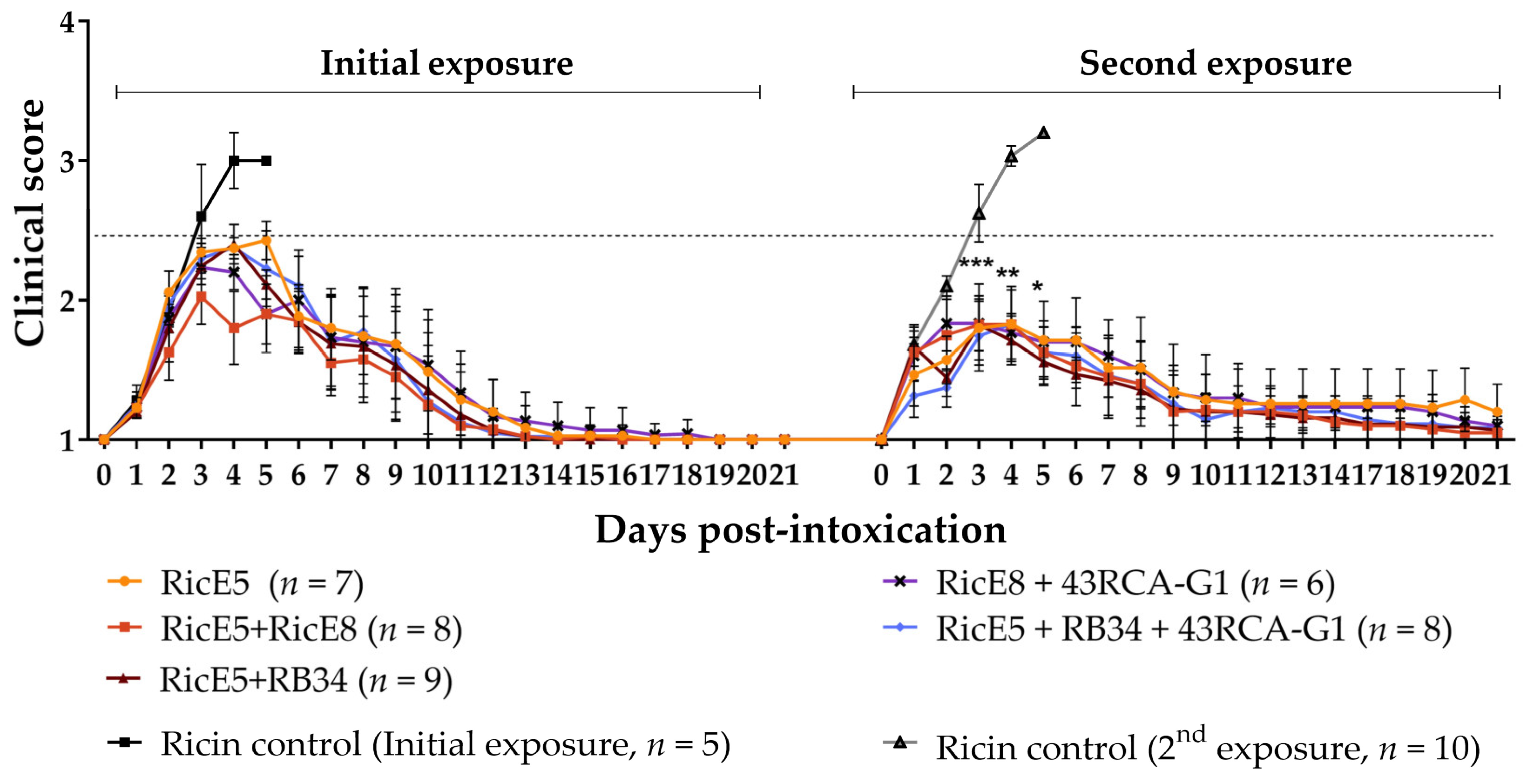
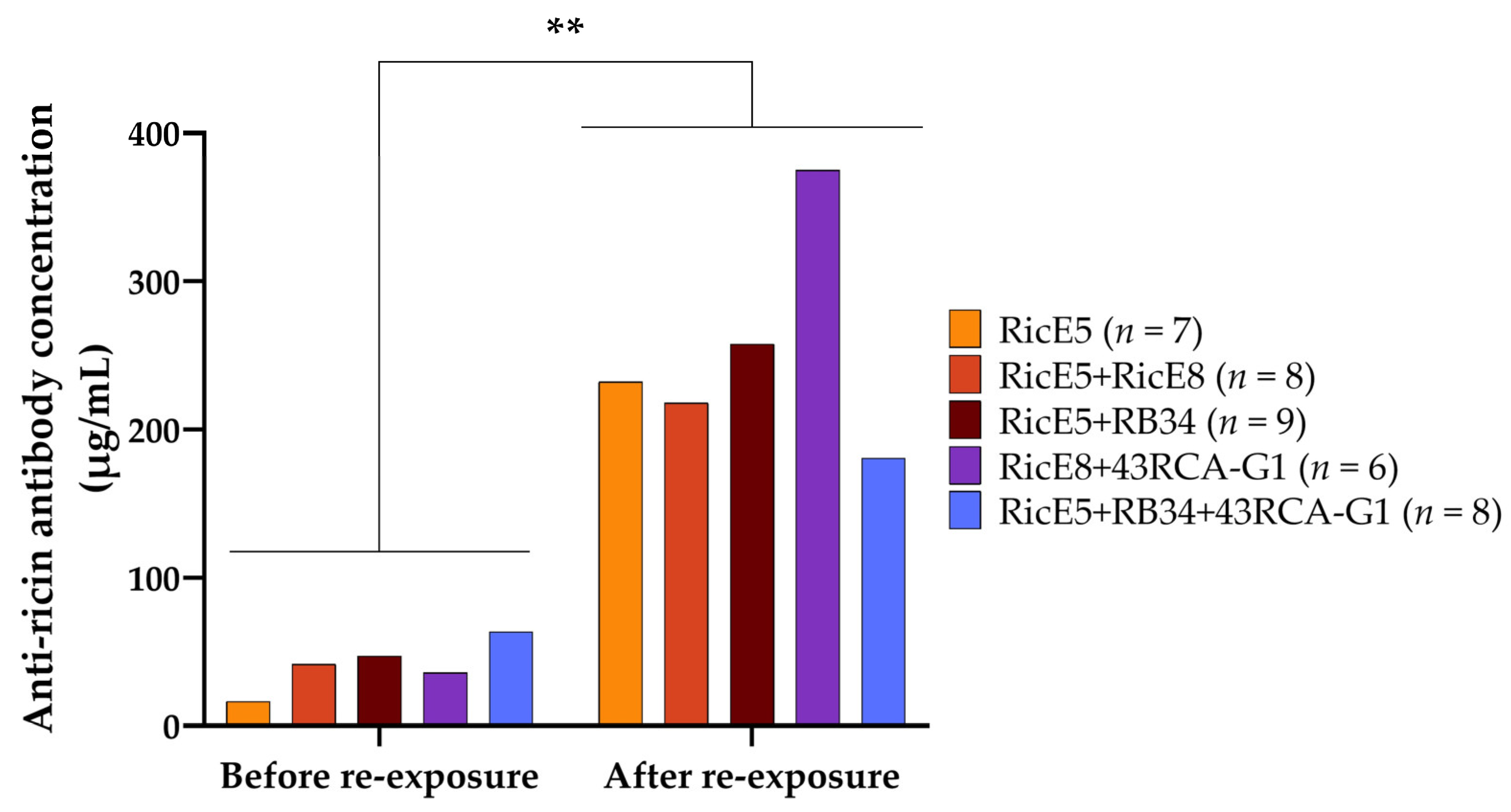
2.4. Induced Long-Term Immunity to Ricin Elicited by Passive Immunotherapy
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethics Statement
5.2. Reagents
5.2.1. Ricin Extracts
5.2.2. Preparation of Inactivated Ricin E for Mice Immunization
5.2.3. Reagents for Enzyme Immuno-Assay (EIA)
5.3. Production of Monoclonal Anti-Ricin Antibodies (mAbs)
5.3.1. Mice Immunization and Hybridoma Production
5.3.2. Evaluation of the Polyclonal Response in Mouse Plasma Following Immunization
5.3.3. mAb Purification and Recombinant Antibody Production
5.4. Cell Viability Assays
5.4.1. Cell Growth Conditions
5.4.2. Determination of the 50% Cytotoxic Dose (CD50) of Ricin in Various Cell Lines
5.4.3. Evaluation of Antibody Neutralizing Efficacy against Ricin
5.5. Bio-Layer Interferometry Measurements
5.6. Evaluation of Ricin-Chain Binding Specificity by Enzyme Immuno-Assay (EIA)
5.7. Animal Experiments
5.7.1. Passive Immunotherapy Protection Experiments
5.7.2. Evaluation of Active Immune Response Induction
5.8. Characterization of the Induced Long-Term Immunity against Ricin in Mice
5.8.1. Evaluation of the Polyclonal Response against Ricin in Murine Plasma by EIA
5.8.2. Pharmacokinetics of mAb RicE5 + RicE8 Combination
5.8.3. Evaluation of the Neutralizing Capacity of the Murine Plasma against Ricin
5.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schieltz, D.M.; McWilliams, L.G.; Kuklenyik, Z.; Prezioso, S.M.; Carter, A.J.; Williamson, Y.M.; McGrath, S.C.; Morse, S.A.; Barr, J.R. Quantification of Ricin, RCA and Comparison of Enzymatic Activity in 18 Ricinus communis Cultivars by Isotope Dilution Mass Spectrometry. Toxicon 2015, 95, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Audi, J.; Belson, M.; Patel, M.; Schier, J.; Osterloh, J. Ricin Poisoning: A Comprehensive Review. JAMA 2005, 294, 2342–2351. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Tsurugi, K. RNA N-Glycosidase Activity of Ricin A-Chain. Mechanism of Action of the Toxic Lectin Ricin on Eukaryotic Ribosomes. J. Biol. Chem. 1987, 262, 8128–8130. [Google Scholar] [CrossRef] [PubMed]
- Grela, P.; Szajwaj, M.; Horbowicz-Drozdzal, P.; Tchórzewski, M. How Ricin Damages the Ribosome. Toxins 2019, 11, 241. [Google Scholar] [CrossRef]
- Crompton, R.; Gall, D. Georgi Markov—Death in a Pellet. Med. Leg. J. 1980, 48, 51–62. [Google Scholar] [CrossRef]
- Henry, S. Man Charged after French Police Foil Paris Ricin Terror Plot. Available online: https://www.telegraph.co.uk/news/2018/05/18/french-police-foil-ricin-terror-plot-arrest-egyptian-brothers/ (accessed on 21 August 2024).
- Unknown Ricin Threat: Cologne Anti-Terror Police Search Flats. Available online: https://www.bbc.com/news/world-europe-44494010 (accessed on 21 August 2024).
- Sehgal, P.; Kumar, O.; Kameswararao, M.; Ravindran, J.; Khan, M.; Sharma, S.; Vijayaraghavan, R.; Prasad, G.B.K.S. Differential Toxicity Profile of Ricin Isoforms Correlates with Their Glycosylation Levels. Toxicology 2011, 282, 56–67. [Google Scholar] [CrossRef]
- Lin, T.T.S.; Li, S.S.L. Purification and Physicochemical Properties of Ricins and Agglutinins from Ricinus communis. Eur. J. Biochem. 1980, 105, 453–459. [Google Scholar] [CrossRef]
- Araki, T.; Funatsu, G. The Complete Amino Acid Sequence of the B-Chain of Ricin E Isolated from Small-Grain Castor Bean Seeds. Ricin E Is a Gene Recombination Product of Ricin D and Ricinus communis Agglutinin. Biochim. Biophys. Acta (BBA)—Protein Struct. Mol. Enzymol. 1987, 911, 191–200. [Google Scholar] [CrossRef]
- Mise, T.; Funatsu, G.; Ishiguro, M. Isolation and Characterization of Ricin E from Castor Beans. Agric. Biol. Chem. 1977, 41, 2041–2046. [Google Scholar] [CrossRef]
- Griffiths, G.D.; Rice, P.; Allenby, A.C.; Bailey, S.C.; Upshall, D.G. Inhalation Toxicology and Histopathology of Ricin and Abrin Toxins. Inhal. Toxicol. 1995, 7, 269–288. [Google Scholar] [CrossRef]
- Fredriksson, S.Å.; Hulst, A.G.; Artursson, E.; De Jong, A.L.; Nilsson, C.; Van Baar, B.L.M. Forensic Identification of Neat Ricin and of Ricin from Crude Castor Bean Extracts by Mass Spectrometry. Anal. Chem. 2005, 77, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Despeyroux, D.; Walker, N.; Pearce, M.; Fisher, M.; McDonnell, M.; Bailey, S.C.; Griffiths, G.D.; Watts, P. Characterization of Ricin Heterogeneity by Electrospray Mass Spectrometry, Capillary Electrophoresis, and Resonant Mirror. Anal. Biochem. 2000, 279, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Worbs, S.; Köhler, K.; Pauly, D.; Avondet, M.A.; Schaer, M.; Dorner, M.B.; Dorner, B.G. Ricinus communis Intoxications in Human and Veterinary Medicine-a Summary of Real Cases. Toxins 2011, 3, 1332–1372. [Google Scholar] [CrossRef] [PubMed]
- Angalakurthi, S.K.; Vance, D.J.; Rong, Y.; Nguyen, C.M.T.; Rudolph, M.J.; Volkin, D.; Russell Middaugh, C.; Weis, D.D.; Mantis, N.J. A Collection of Single-Domain Antibodies That Crowd Ricin Toxin’s Active Site. Antibodies 2018, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Stechmann, B.; Bai, S.K.; Gobbo, E.; Lopez, R.; Merer, G.; Pinchard, S.; Panigai, L.; Tenza, D.; Raposo, G.; Beaumelle, B.; et al. Inhibition of Retrograde Transport Protects Mice from Lethal Ricin Challenge. Cell 2010, 141, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Vance, D.J.; Tremblay, J.M.; Rong, Y.; Angalakurthi, S.K.; Volkin, D.B.; Middaugh, C.R.; Weis, D.D.; Shoemaker, C.B.; Mantis, N.J. High-Resolution Epitope Positioning of a Large Collection of Neutralizing and Non-Neutralizing Single-Domain on the Enzymatic and Binding Subunits of Ricin Toxin. Clin. Vaccine Immunol. 2017, 24, e00236-17. [Google Scholar] [CrossRef]
- Neal, L.M.; O’Hara, J.; Brey, R.N.; Mantis, N.J. A Monoclonal Immunoglobulin G Antibody Directed against an Immunodominant Linear Epitope on the Ricin A Chain Confers Systemic and Mucosal Immunity to Ricin. Infect. Immun. 2010, 78, 552–561. [Google Scholar] [CrossRef]
- Respaud, R.; Marchand, D.; Pelat, T.; Tchou-Wong, K.M.; Roy, C.J.; Parent, C.; Cabrera, M.; Guillemain, J.; Mac Loughlin, R.; Levacher, E.; et al. Development of a Drug Delivery System for Efficient Alveolar Delivery of a Neutralizing Monoclonal Antibody to Treat Pulmonary Intoxication to Ricin. J. Control. Release 2016, 234, 21–32. [Google Scholar] [CrossRef]
- Pelat, T.; Hust, M.; Hale, M.; Lefranc, M.P.; Dübel, S.; Thullier, P. Isolation of a Human-like Antibody Fragment (ScFv) That Neutralizes Ricin Biological Activity. BMC Biotechnol. 2009, 9, 60. [Google Scholar] [CrossRef]
- Tolman, L.E.; Yates, J.L.; Rong, Y.; Reynolds-Peterson, C.; Ehrbar, D.; Torres-Velez, F.J.; Mantis, N.J. Durable Immunity to Ricin Toxin Elicited by Intranasally Administered Monoclonal Antibody–Based Immune Complexes. Immunohorizons 2022, 6, 324–333. [Google Scholar] [CrossRef]
- Rong, Y.; Pauly, M.; Guthals, A.; Pham, H.; Ehrbar, D.; Zeitlin, L.; Mantis, N.J. A Humanized Monoclonal Antibody Cocktail to Prevent Pulmonary Ricin Intoxication. Toxins 2020, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Torres-Velez, F.J.; Ehrbar, D.; Doering, J.; Song, R.; Mantis, N.J. An Intranasally Administered Monoclonal Antibody Cocktail Abrogates Ricin Toxin-Induced Pulmonary Tissue Damage and Inflammation. Hum. Vaccines Immunother. 2020, 16, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Falach, R.; Sapoznikov, A.; Alcalay, R.; Aftalion, M.; Ehrlich, S.; Makovitzki, A.; Agami, A.; Mimran, A.; Rosner, A.; Sabo, T.; et al. Generation of Highly Efficient Equine-Derived Antibodies for Post-Exposure Treatment of Ricin Intoxications by Vaccination with Monomerized Ricin. Toxins 2018, 10, 466. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.C.; Yin, J.; Chau, D.; Cherwonogrodzky, J.W.; Hu, W.-G. Active Immunity Induced by Passive IgG Post-Exposure Protection against Ricin. Toxins 2014, 6, 380–393. [Google Scholar] [CrossRef]
- Orsini Delgado, M.L.; Avril, A.; Prigent, J.; Dano, J.; Rouaix, A.; Worbs, S.; Dorner, B.G.; Rougeaux, C.; Becher, F.; Fenaille, F.; et al. Ricin Antibodies’ Neutralizing Capacity against Different Ricin Isoforms and Cultivars. Toxins 2021, 13, 100. [Google Scholar] [CrossRef]
- Prigent, J.; Panigai, L.; Lamourette, P.; Sauvaire, D.; Devilliers, K.; Plaisance, M.; Volland, H.; Créminon, C.; Simon, S. Neutralising Antibodies against Ricin Toxin. PLoS ONE 2011, 6, e20166. [Google Scholar] [CrossRef]
- Sully, E.K.; Whaley, K.J.; Bohorova, N.; Bohorov, O.; Goodman, C.; Kim, D.H.; Pauly, M.H.; Velasco, J.; Hiatt, E.; Morton, J.; et al. Chimeric Plantibody Passively Protects Mice against Aerosolized Ricin Challenge. Clin. Vaccine Immunol. 2014, 21, 777–782. [Google Scholar] [CrossRef]
- Czajka, T.F.; Vance, D.J.; Davis, S.; Rudolph, M.J.; Mantis, N.J. Single-Domain Antibodies Neutralize Ricin Toxin Intracellularly by Blocking Access to Ribosomal P-Stalk Proteins. J. Biol. Chem. 2022, 298, 101742. [Google Scholar] [CrossRef]
- Vance, D.J.; Tremblay, J.M.; Mantis, N.J.; Shoemaker, C.B. Stepwise Engineering of Heterodimeric Single Domain Camelid VHH Antibodies That Passively Protect Mice from Ricin Toxin. J. Biol. Chem. 2013, 288, 36538–36547. [Google Scholar] [CrossRef]
- Herrera, C.; Klokk, T.I.; Cole, R.; Sandvig, K.; Mantis, N.J. A Bispecific Antibody Promotes Aggregation of Ricin Toxin on Cell Surfaces and Alters Dynamics of Toxin Internalization and Trafficking. PLoS ONE 2016, 11, e0156893. [Google Scholar] [CrossRef]
- Rasetti-Escargueil, C.; Avril, A. Medical Countermeasures against Ricin Intoxication. Toxins 2023, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Mize, R.R.; Marrero, L.; Corti, M.; Kirk, J.M.; Pincus, S.H. Antibody to Ricin A Chain Hinders Intracellular Routing of Toxin and Protects Cells Even after Toxin Has Been Internalized. PLoS ONE 2013, 8, e62417. [Google Scholar] [CrossRef] [PubMed]
- Yermakova, A.; Klokk, T.I.; O’Hara, J.M.; Cole, R.; Sandvig, K.; Mantis, N.J. Neutralizing Monoclonal Antibodies against Disparate Epitopes on Ricin Toxin’s Enzymatic Subunit Interfere with Intracellular Toxin Transport. Sci. Rep. 2016, 6, 22721. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, M.J.; Czajka, T.F.; Davis, S.A.; Thi Nguyen, C.M.; Li, X.-p.; Tumer, N.E.; Vance, D.J.; Mantis, N.J. Intracellular Neutralization of Ricin Toxin by Single-Domain Antibodies Targeting the Active Site. J. Mol. Biol. 2020, 432, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Vance, D.J.; Rudolph, M.J.; Davis, S.A.; Mantis, N.J. Structural Basis of Antibody-Mediated Inhibition of Ricin Toxin Attachment to Host Cells. Biochemistry 2023, 62, 3181–3187. [Google Scholar] [CrossRef]
- Noy-Porat, T.; Alcalay, R.; Epstein, E.; Sabo, T.; Kronman, C.; Mazor, O. Extended Therapeutic Window for Post-Exposure Treatment of Ricin Intoxication Conferred by the Use of High-Affinity Antibodies. Toxicon 2017, 127, 100–105. [Google Scholar] [CrossRef]
- Noy-Porat, T.; Rosenfeld, R.; Ariel, N.; Epstein, E.; Alcalay, R.; Zvi, A.; Kronman, C.; Ordentlich, A.; Mazor, O. Isolation of Anti-Ricin Protective Antibodies Exhibiting High Affinity from Immunized-Human Primates. Toxins 2016, 8, 64. [Google Scholar] [CrossRef]
- Roy, C.J.; Van Slyke, G.; Ehrbar, D.; Bornholdt, Z.A.; Brennan, M.B.; Campbell, L.; Chen, M.; Kim, D.; Mlakar, N.; Whaley, K.J.; et al. Passive Immunization with an Extended Half-Life Monoclonal Antibody Protects Rhesus Macaques against Aerosolized Ricin Toxin. NPJ Vaccines 2020, 5, 13. [Google Scholar] [CrossRef]
- Roy, C.J.; Ehrbar, D.J.; Bohorova, N.; Bohorov, O.; Kim, D.; Pauly, M.; Whaley, K.; Rong, Y.; Torres-Velez, F.J.; Vitetta, E.S.; et al. Rescue of Rhesus Macaques from the Lethality of Aerosolized Ricin Toxin. JCI Insight 2019, 4, e124771. [Google Scholar] [CrossRef]
- Rudolph, M.J.; Vance, D.J.; Cheung, J.; Franklin, M.C.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Herrera, C.; Shoemaker, C.B.; Mantis, N.J. Crystal Structures of Ricin Toxin’s Enzymatic Subunit (RTA) in Complex with Neutralizing and Non-Neutralizing Single-Chain Antibodies. J. Mol. Biol. 2014, 426, 3057–3068. [Google Scholar] [CrossRef]
- Rong, Y.; Van Slyke, G.; Vance, D.J.; Westfall, J.; Ehrbar, D.; Mantis, N.J. Spatial Location of Neutralizing and Non-Neutralizing B Cell Epitopes on Domain 1 of Ricin Toxin’s Binding Subunit. PLoS ONE 2017, 12, e0180999. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, S.; Xu, N.; Liu, W. Ricin Toxin and Its Neutralizing Antibodies: A Review. Toxicon 2022, 214, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.; Tremblay, J.M.; Shoemaker, C.B.; Mantis, N.J. Mechanisms of Ricin Toxin Neutralization Revealed through Engineered Homodimeric and Heterodimeric Camelid Antibodies. J. Biol. Chem. 2015, 290, 27880–27889. [Google Scholar] [CrossRef] [PubMed]
- Gal, Y.; Sapoznikov, A.; Falach, R.; Ehrlich, S.; Aftalion, M.; Sabo, T.; Kronman, C. Potent Antiedematous and Protective Effects of Ciprofloxacin in Pulmonary Ricinosis. Antimicrob. Agents Chemother. 2016, 60, 7153–7158. [Google Scholar] [CrossRef] [PubMed]
- Gal, Y.; Mazor, O.; Alcalay, R.; Seliger, N.; Aftalion, M.; Sapoznikov, A.; Falach, R.; Kronman, C.; Sabo, T. Antibody/Doxycycline Combined Therapy for Pulmonary Ricinosis: Attenuation of Inflammation Improves Survival of Ricin-Intoxicated Mice. Toxicol. Rep. 2014, 1, 496–504. [Google Scholar] [CrossRef]
- Lord, J.M.; Roberts, L.M.; Robertus, J.D. Ricin: Structure, Mode of Action, and Some Current Applications. FASEB J. 1994, 8, 201–208. [Google Scholar] [CrossRef]
- Worbs, S.; Skiba, M.; Söderström, M.; Rapinoja, M.L.; Zeleny, R.; Russmann, H.; Schimmel, H.; Vanninen, P.; Fredriksson, S.Å.; Dorner, B.G. Characterization of Ricin and R. communis Agglutinin Reference Materials. Toxins 2015, 7, 4906–4934. [Google Scholar] [CrossRef]
- Kohler, G.; Milstein, C. Continuous Cultures of Fused Cells Secreting Antibody of Predefined Specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Randhawa, M.A. Calculation of LD50 Values from the Method of Miller and Tainter, 1944. J. Ayub Med. Coll. Abbottabad 2009, 21, 184–185. [Google Scholar]
- Lemley, P.V.; Wright, D.C. Mice Are Actively Immunized after Passive Monoclonal Antibody Prophylaxis and Ricin Toxin Challenge. Immunology 1992, 76, 511. [Google Scholar]
- Stoll, A.; Shenton, D.P.; Green, A.C.; Holley, J.L. Comparative Aspects of Ricin Toxicity by Inhalation. Toxins 2023, 15, 281. [Google Scholar] [CrossRef] [PubMed]
- Falach, R.; Sapoznikov, A.; Gal, Y.; Israeli, O.; Leitner, M.; Seliger, N.; Ehrlich, S.; Kronman, C.; Sabo, T. Quantitative Profiling of the In Vivo Enzymatic Activity of Ricin Reveals Disparate Depurination of Different Pulmonary Cell Types. Toxicol. Lett. 2016, 258, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Sapoznikov, A.; Falach, R.; Mazor, O.; Alcalay, R.; Gal, Y.; Seliger, N.; Sabo, T.; Kronman, C. Diverse Profiles of Ricin-Cell Interactions in the Lung Following Intranasal Exposure to Ricin. Toxins 2015, 7, 4817–4831. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, T.; Ohba, H.; Yamasaki, N.; Funatsu, G. Binding of Saccharides to Ricin e Isolated from Small Castor Beans. J. Biochem. 1989, 105, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Komatsu, N.; Muramatsu, T. Cell Lysis Induced by Ricin D and Ricin E in Various Cell Lines. Biosci. Biotechnol. Biochem. 1997, 61, 291–297. [Google Scholar] [CrossRef]
- Barbieri, L.; Battelli, M.G.; Stirpe, F. Ribosome-Inactivating Proteins from Plants. Biochim. Biophys. Acta (BBA)—Rev. Biomembr. 1993, 1154, 237–282. [Google Scholar] [CrossRef]
- Stirpe, F.; Sandvig, K.; Olsnes, S.; Pihl, A. Action of Viscumin, a Toxic Lectin from Mistletoe, on Cells in Culture. J. Biol. Chem. 1982, 257, 13271–13277. [Google Scholar] [CrossRef]
- Olsnes, S.; Sandvig, K.; Eiklid, K.; Pihl, A. Properties and Action Mechanism of the Toxic Lectin Modeccin: Interaction with Cell Lines Resistant to Modeccin, Abrin, and Ricin. J. Supramol. Struct. 1978, 9, 15–25. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Blaustein, J. The Interaction of Ricinus communis Agglutinin with Normal and Tumor Cell Surfaces. Biochim. Biophys. Acta (BBA)—Biomembr. 1972, 266, 543–547. [Google Scholar] [CrossRef]
- Baenziger, J.U.; Fiete, D. Structural Determinants of Ricinus communis Agglutinin and Toxin Specificity for Oligosaccharides. J. Biol. Chem. 1979, 254, 9795–9799. [Google Scholar] [CrossRef]
- Simmons, B.M.; Stahl, P.D.; Russell, J.H. Mannose Receptor-Mediated Uptake of Ricin Toxin and Ricin A Chain by Macrophages. Multiple Intracellular Pathways for a Chain Translocation. J. Biol. Chem. 1986, 261, 7912–7920. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, S.; Berg, T.; Turpin, E.; Frenoy, J.P. Interactions of Ricin with Sinusoidal Endothelial Rat Liver Cells. Different Involvement of Two Distinct Carbohydrate-Specific Mechanisms in Surface Binding and Internalization. Biochem. J. 1991, 277, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Sandvig, K.; Grimmer, S.; Iversen, T.G.; Rodal, K.; Torgersen, M.L.; Nicoziani, P.; Van Deurs, B. Ricin Transport into Cells: Studies of Endocytosis and Intracellular Transport. Int. J. Med. Microbiol. 2000, 290, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Sowa-Rogozí Nska, N.; Sominka, H.; Nowakowska-Gołacka, J.; Sandvig, K.; Słomí Nska-Wojewódzka, M. Intracellular Transport and Cytotoxicity of the Protein Toxin Ricin. Toxins 2019, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, J.M.; Mantis, N.J. Neutralizing Monoclonal Antibodies against Ricin’s Enzymatic Subunit Interfere with Protein Disulfide Isomerase-Mediated Reduction of Ricin Holotoxin in Vitro. J. Immunol. Methods 2013, 395, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Czajka, T.F.; Vance, D.J.; Song, R.; Mantis, N.J. A Biparatopic Intrabody Renders Vero Cells Impervious to Ricin Intoxication. bioRxiv 2024. [Google Scholar] [CrossRef]
- Legler, P.M.; Compton, J.R.; Hale, M.L.; Anderson, G.P.; Olson, M.A.; Millard, C.B.; Goldman, E.R. Stability of Isolated Antibody-Antigen Complexes as a Predictive Tool for Selecting Toxin Neutralizing Antibodies. MAbs 2017, 9, 43–57. [Google Scholar] [CrossRef]
- Rudolph, M.J.; Poon, A.Y.; Kavaliauskiene, S.; Myrann, A.G.; Reynolds-Peterson, C.; Davis, S.A.; Sandvig, K.; Vance, D.J.; Mantis, N.J. Structural Analysis of Toxin-Neutralizing, Single-Domain Antibodies That Bridge Ricin’s A-B Subunit Interface. J. Mol. Biol. 2021, 433, 167086. [Google Scholar] [CrossRef]
- Rosenfeld, R.; Alcalay, R.; Mechaly, A.; Lapidoth, G.; Epstein, E.; Kronman, C.; Fleishman, S.J.; Mazor, O. Improved Antibody-Based Ricin Neutralization by Affinity Maturation Is Correlated with Slower off-Rate Values. Protein Eng. Des. Sel. 2017, 30, 611–617. [Google Scholar] [CrossRef]
- Yermakova, A.; Vance, D.J.; Mantis, N.J. Sub-Domains of Ricin’s B Subunit as Targets of Toxin Neutralizing and Non-Neutralizing Monoclonal Antibodies. PLoS ONE 2012, 7, e44317. [Google Scholar] [CrossRef]
- Wilhelmsen, C.L.; Pitt, M.L.M. Lesions of Acute Inhaled Lethal Ricin Intoxication in Rhesus Monkeys12. Vet. Pathol. 1996, 33, 257–367. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, G.D. Understanding Ricin from a Defensive Viewpoint. Toxins 2011, 3, 1373–1392. [Google Scholar] [CrossRef] [PubMed]
- Doebler, J.A.; Wiltshire, N.D.; Mayer, T.W.; Estep, J.E.; Moeller, R.B.; Traub, R.K.; Broomfield, C.A.; Calamaio, C.A.; Thompson, W.L.; Pitt, M.L. The Distribution of [125I]Ricin in Mice Following Aerosol Inhalation Exposure. Toxicology 1995, 98, 137–149. [Google Scholar] [CrossRef]
- Cook, D.L.; David, J.; Griffiths, G.D. Retrospective Identification of Ricin in Animal Tissues Following Administration by Pulmonary and Oral Routes. Toxicology 2006, 223, 61–70. [Google Scholar] [CrossRef]
- Power, C.A.; Bates, A. David vs. Goliath: The Structure, Function, and Clinical Prospects of Antibody Fragments. Antibodies 2019, 8, 28. [Google Scholar] [CrossRef]
- Vogel, P.; Rivera, V.R.; Louise, M.; Pitt, M.; Poli, M.A. Comparison of the Pulmonary Distribution and Efficacy of Antibodies given to Mice by Intratracheal Instillation or Aerosol Inhalation. Lab. Anim. Sci. 1996, 46, 516–523. [Google Scholar]
- Piquet, P.; Saadi, J.; Fenaille, F.; Kalb, S.R.; Becher, F. Rapid Detection of Ricin at Trace Levels in Complex Matrices by Asialofetuin-Coated Beads and Bottom-up Proteomics Using High-Resolution Mass Spectrometry. Anal. Bioanal. Chem. 2024, 416, 5145–5153. [Google Scholar] [CrossRef]
- Kalb, S.R.; Schieltz, D.M.; Becher, F.; Astot, C.; Fredriksson, S.Å.; Barr, J.R. Recommended Mass Spectrometry-Based Strategies to Identify Ricin-Containing Samples. Toxins 2015, 7, 4881–4894. [Google Scholar] [CrossRef]
- Grassi, J.; Frobert, Y.; Lamourette, P.; Lagoutte, B. Screening of Monoclonal Antibodies Using Antigens Labeled with Acetylcholinesterase: Application to the Peripheral Proteins of Photosystem 1. Anal. Biochem. 1988, 168, 436–450. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]


| Generation | Antibody | Ricin Isoform | KD (M) × 10−11 # | kon (M−1s−1) × 105 # | koff (s−1) × 10−5 # |
|---|---|---|---|---|---|
| 1st generation | RB34 † | Ricin D | 1.0 ± <0.1 | 5.61 ± <0.01 | 0.07 ± 0.01 |
| Ricin E | 542.0 ± 44.3 | 17.00 ± 0.31 | 330.00 ± 1.36 | ||
| 43RCA-G1 † | Ricin D | 4.8 ± <0.1 | 4.23 ± <0.01 | 2.00 ± <0.01 | |
| Ricin E | 9.6 ± <0.1 | 4.69 ± <0.01 | 4.52 ± <0.01 | ||
| 2nd generation | RicE4 | Ricin D | 6.7 ± <0.1 | 7.70 ± 0.01 | 5.18 ± <0.01 |
| Ricin E | 13.0 ± <0.1 | 4.98 ± 0.01 | 6.45 ± <0.01 | ||
| RicE5 | Ricin D | <0.1 | 4.72 ± <0.01 | <0.01 | |
| Ricin E | <0.1 | 3.35 ± <0.01 | <0.01 | ||
| RicE7 | Ricin D | 11.8 ± <0.1 | 13.67 ± 0.03 | 16.10 ± 0.04 | |
| Ricin E | <0.1 | 8.22 ± 0.01 | <0.01 | ||
| RicE8 | Ricin D | <0.1 ± <0.1 | 5.04 ± <0.01 | 0.03 ± <0.01 | |
| Ricin E | <0.1 | 3.17 ± <0.01 | <0.01 |
| IC50 (µg/mL) | |||
|---|---|---|---|
| Antibodies | Ricin D | Ricin E | Ricin D + E |
| RB34 | 0.03 ± 0.06 ns | 16.80 ± 1.52 **** | 4.45 ± 0.53 **** |
| 43RCA-G1 | 2.38 ± 0.46 ** | 2.47 ± 0.40 **** | 2.90 ± 0.37 **** |
| RicE5 | 0.14 ± 0.01 | 0.10 ± 0.01 | 0.12 ± 0.01 |
| RicE8 | 0.46 ± 0.04 ns | 0.25 ± 0.02 ns | 0.39 ± 0.03 ns |
| RicE5 + RicE8 | 0.10 ± 0.01 ns | 0.09 ± <0.01 ns | 0.09 ± 0.01 ns |
| RicE5 + RB34 | 0.03 ± 0.02 ns | 0.18 ± 0.08 ns | 0.04 ± <0.01 ns |
| RicE8 + 43RCA-G1 | 0.21 ± 0.02 ns | 0.19 ± 0.01 ns | 0.26 ± 0.05 ns |
| RB34 + 43RCA-G1 | 0.02 ± <0.01 ns | 4.78 ± 1.64 **** | 0.32 ± 0.04 ns |
| RicE5 + RicE8 + RB34 | 0.11 | 0.17 | 0.06 ± 0.01 ns |
| RicE5 + RB34 + 43RCA-G1 | 0.02 ± <0.01 ns | 0.12 ± 0.01 ns | 0.03 ± <0.01 ns |
| RicE8 + RB34 + 43RCA-G1 | 0.09 ± 0.04 ns | 0.31 ± 0.03 ns | 0.09 ± 0.03 ns |
| CD50 (pg/mL) | |||
|---|---|---|---|
| Cell Type | Ricin D | Ricin E | Ricin D + E |
| Vero | 24.9 ± 2.1 **** | 157.8 ± 15.0 **** | 47.3 ± 3.9 **** |
| A549 | 82.4 ± 4.7 **** | 126.8 ± 11.5 **** | 79.0 ± 7.0 **** |
| Jurkat | 5.6 ± 0.4 | 17.7 ± 1.6 | 9.7 ± 0.6 |
| Dilution Factor for 50% of Viability | ||
|---|---|---|
| Treatments after Initial Exposure | Before Re-Exposure | After Re-Exposure |
| RicE5 | 672 ± 85 | 2329 ± 256 * |
| RicE5 + RicE8 | 2616 ± 378 | 2710 ± 172 ns |
| RicE5 + RB34 | 1265 ± 49 | 2692 ± 337 ns |
| RicE8 + 43RCA-G1 | 1907 ± 136 | 11,142 ± 1441 **** |
| RicE5 + RB34 + 43RCA-G1 | 1790 ± 158 | 2784 ± 93 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lequesne, L.; Dano, J.; Rouaix, A.; Kropp, C.; Plaisance, M.; Gelhaye, S.; Lequesne, M.-L.; Piquet, P.; Avril, A.; Becher, F.; et al. A Monoclonal Antibody with a High Affinity for Ricin Isoforms D and E Provides Strong Protection against Ricin Poisoning. Toxins 2024, 16, 412. https://doi.org/10.3390/toxins16100412
Lequesne L, Dano J, Rouaix A, Kropp C, Plaisance M, Gelhaye S, Lequesne M-L, Piquet P, Avril A, Becher F, et al. A Monoclonal Antibody with a High Affinity for Ricin Isoforms D and E Provides Strong Protection against Ricin Poisoning. Toxins. 2024; 16(10):412. https://doi.org/10.3390/toxins16100412
Chicago/Turabian StyleLequesne, Loïs, Julie Dano, Audrey Rouaix, Camille Kropp, Marc Plaisance, Stéphanie Gelhaye, Marie-Lou Lequesne, Paloma Piquet, Arnaud Avril, François Becher, and et al. 2024. "A Monoclonal Antibody with a High Affinity for Ricin Isoforms D and E Provides Strong Protection against Ricin Poisoning" Toxins 16, no. 10: 412. https://doi.org/10.3390/toxins16100412
APA StyleLequesne, L., Dano, J., Rouaix, A., Kropp, C., Plaisance, M., Gelhaye, S., Lequesne, M.-L., Piquet, P., Avril, A., Becher, F., Orsini Delgado, M. L., & Simon, S. (2024). A Monoclonal Antibody with a High Affinity for Ricin Isoforms D and E Provides Strong Protection against Ricin Poisoning. Toxins, 16(10), 412. https://doi.org/10.3390/toxins16100412





