Exploring the Diversity and Function of Serine Proteases in Toxicofera Reptile Venoms: A Comprehensive Overview
Abstract
:1. Introduction
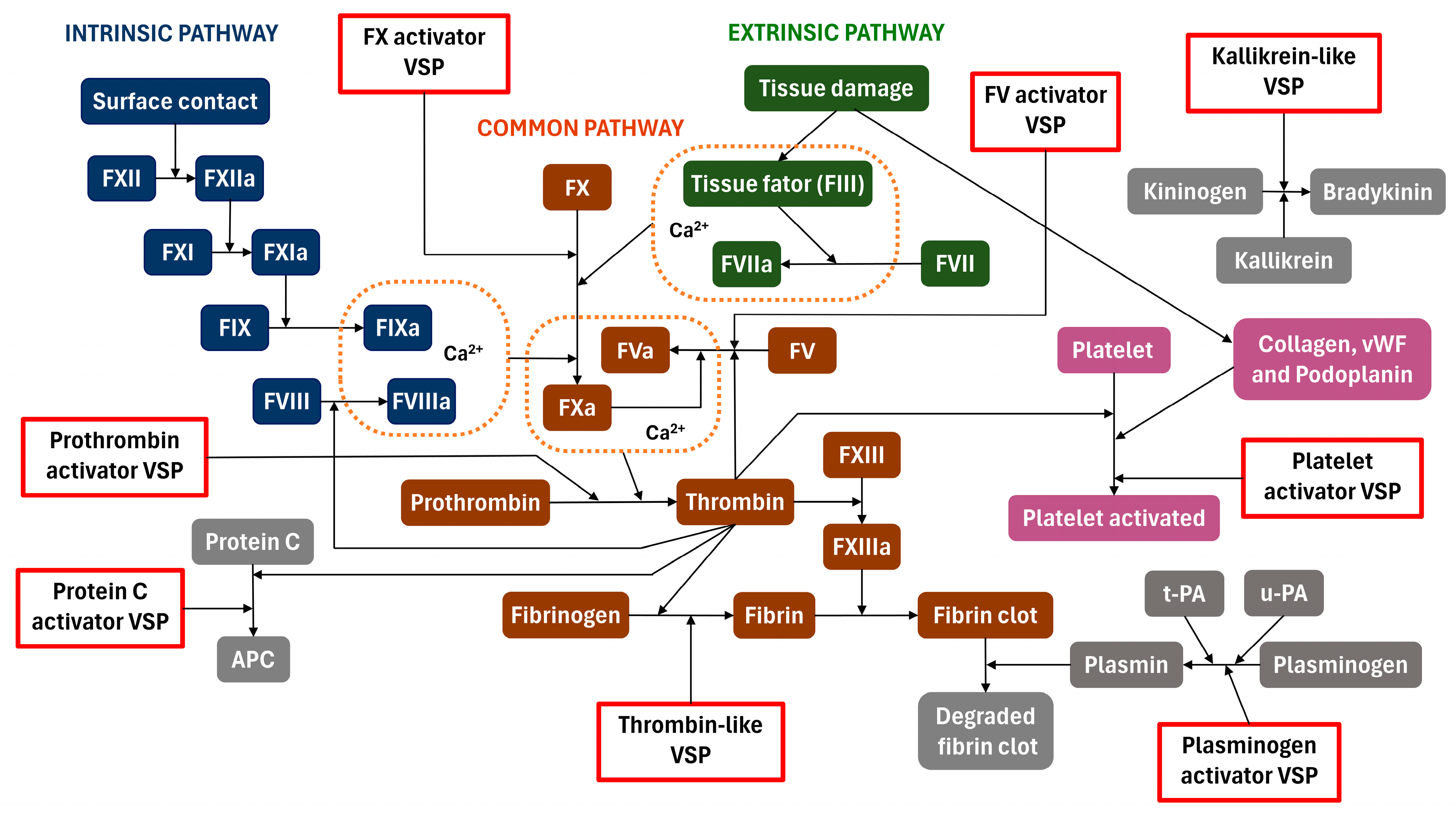
2. Biological Activities
2.1. Factor X Activators
2.2. Factor V Activators
2.3. Prothrombin Activators
2.4. Thrombin-like
2.5. Protein C Activator
2.6. Kallikrein-like
2.7. Plasminogen Activator
2.8. Platelet Activator
3. Structural Features
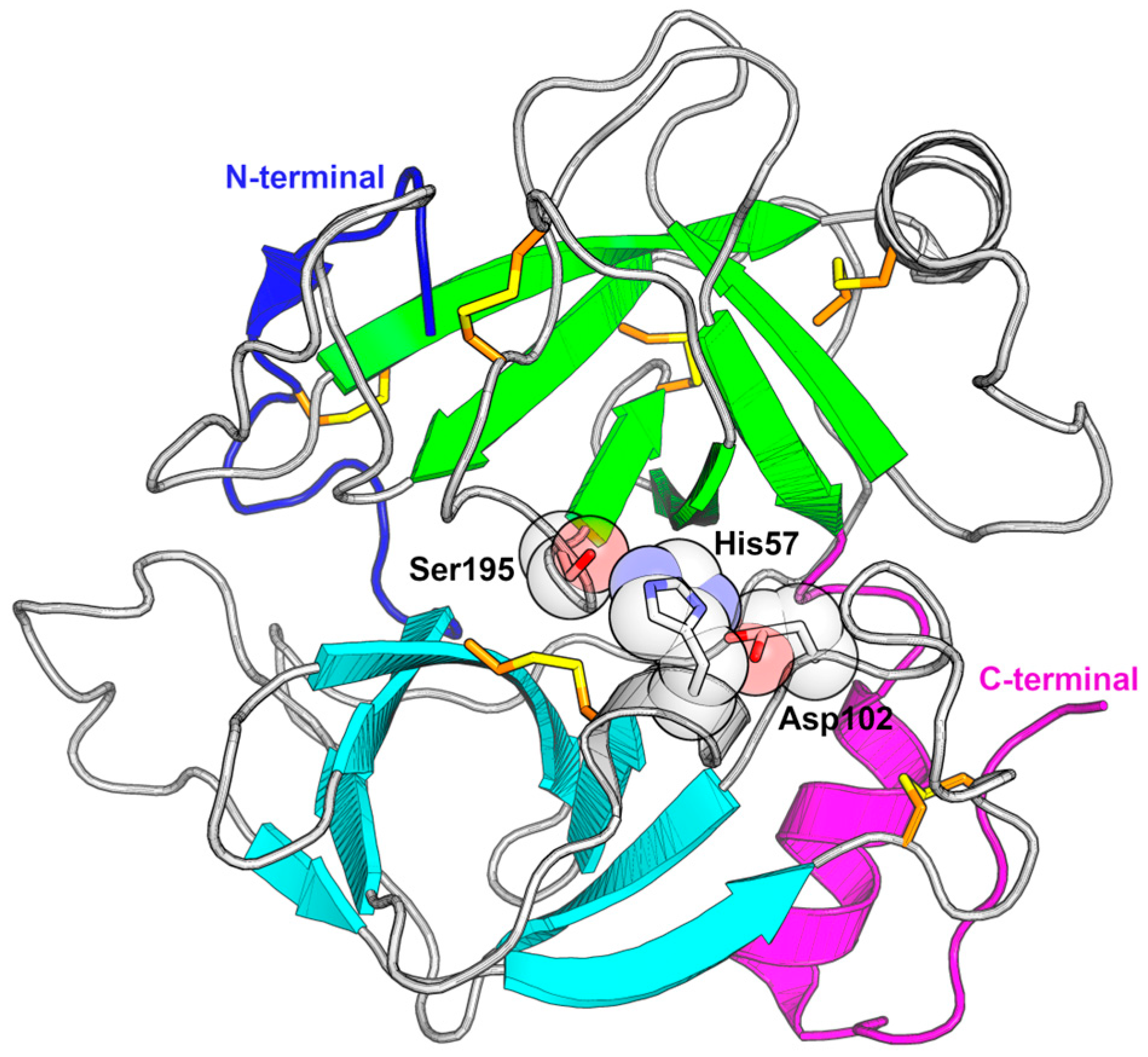
3.1. Catalytic Triad Modification
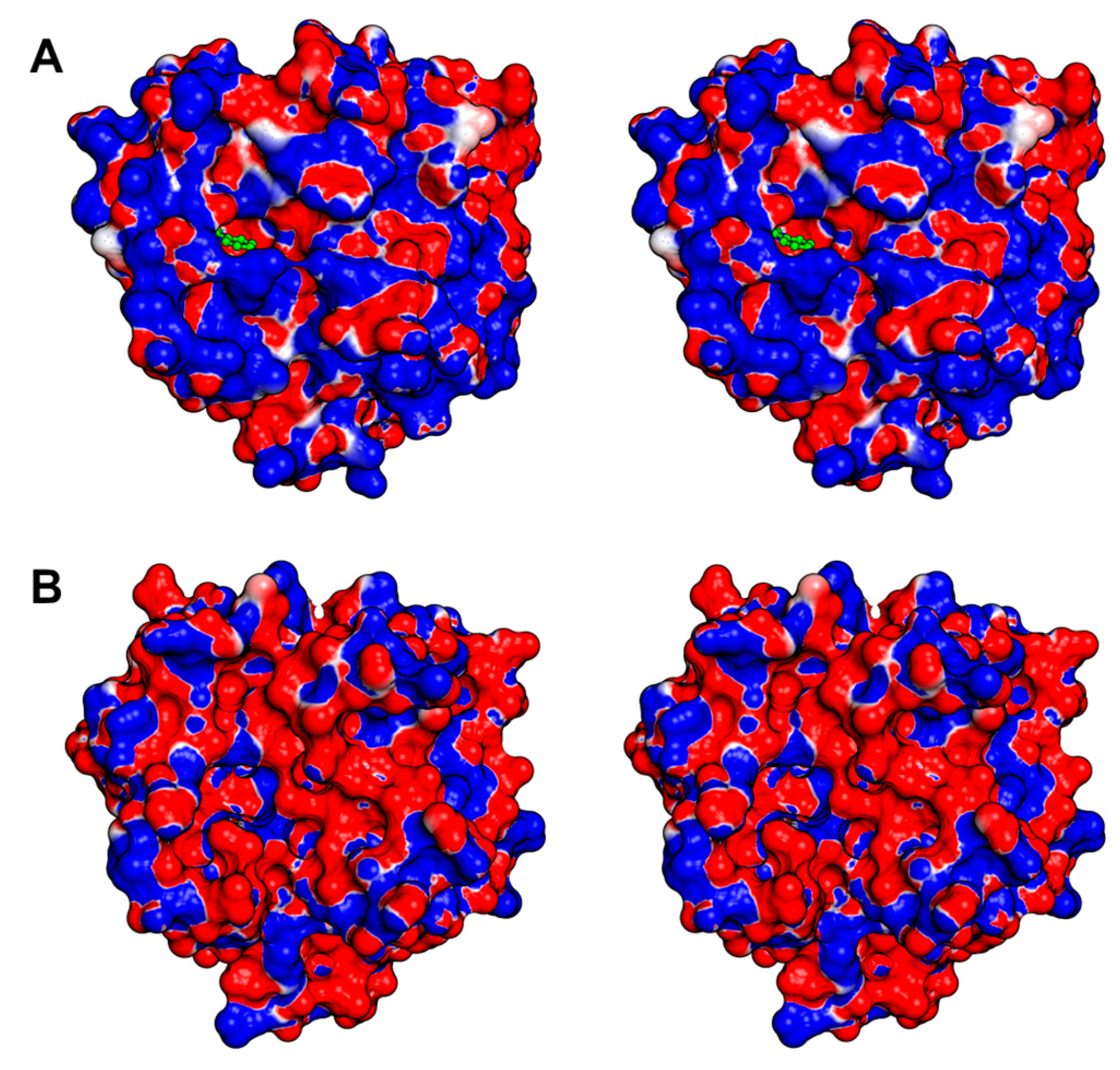
3.2. Glycosylation in SVSPs
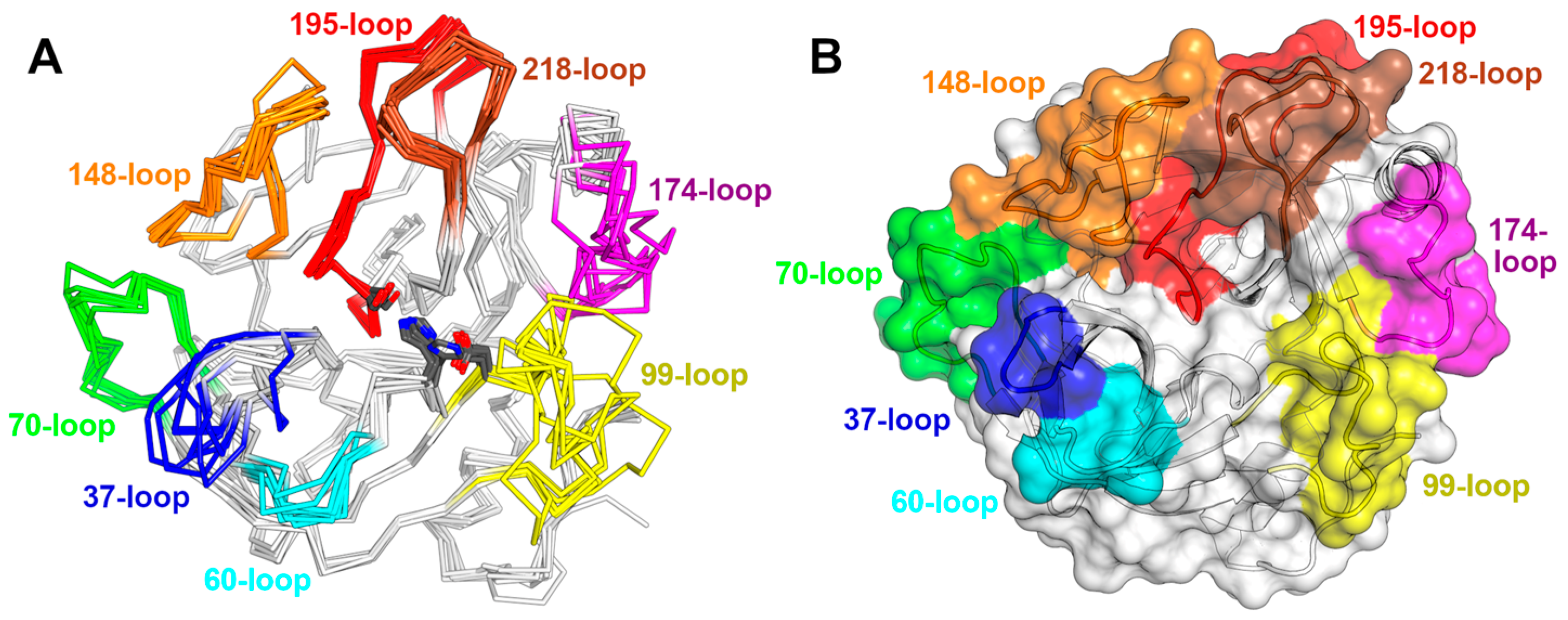
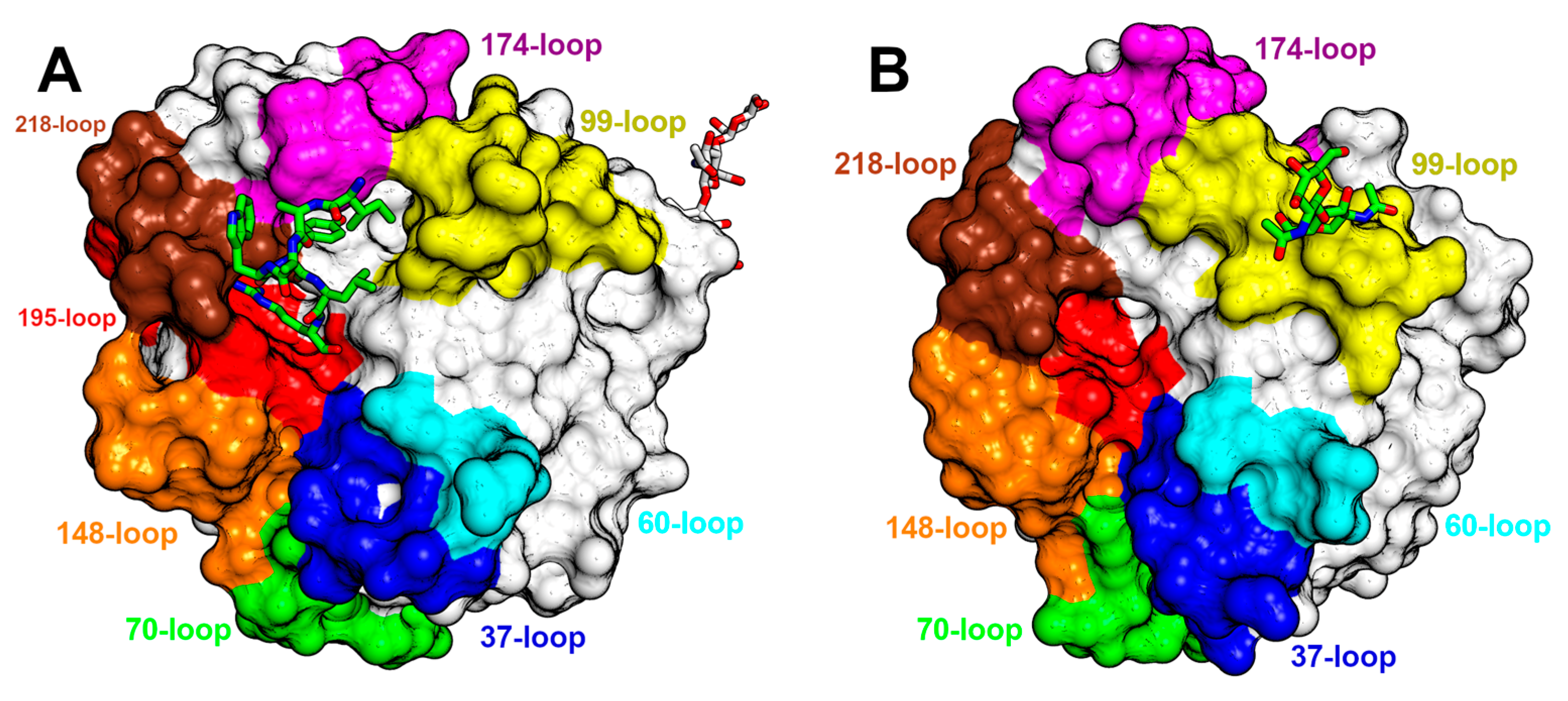
3.3. An Overview of the Crystal Structures
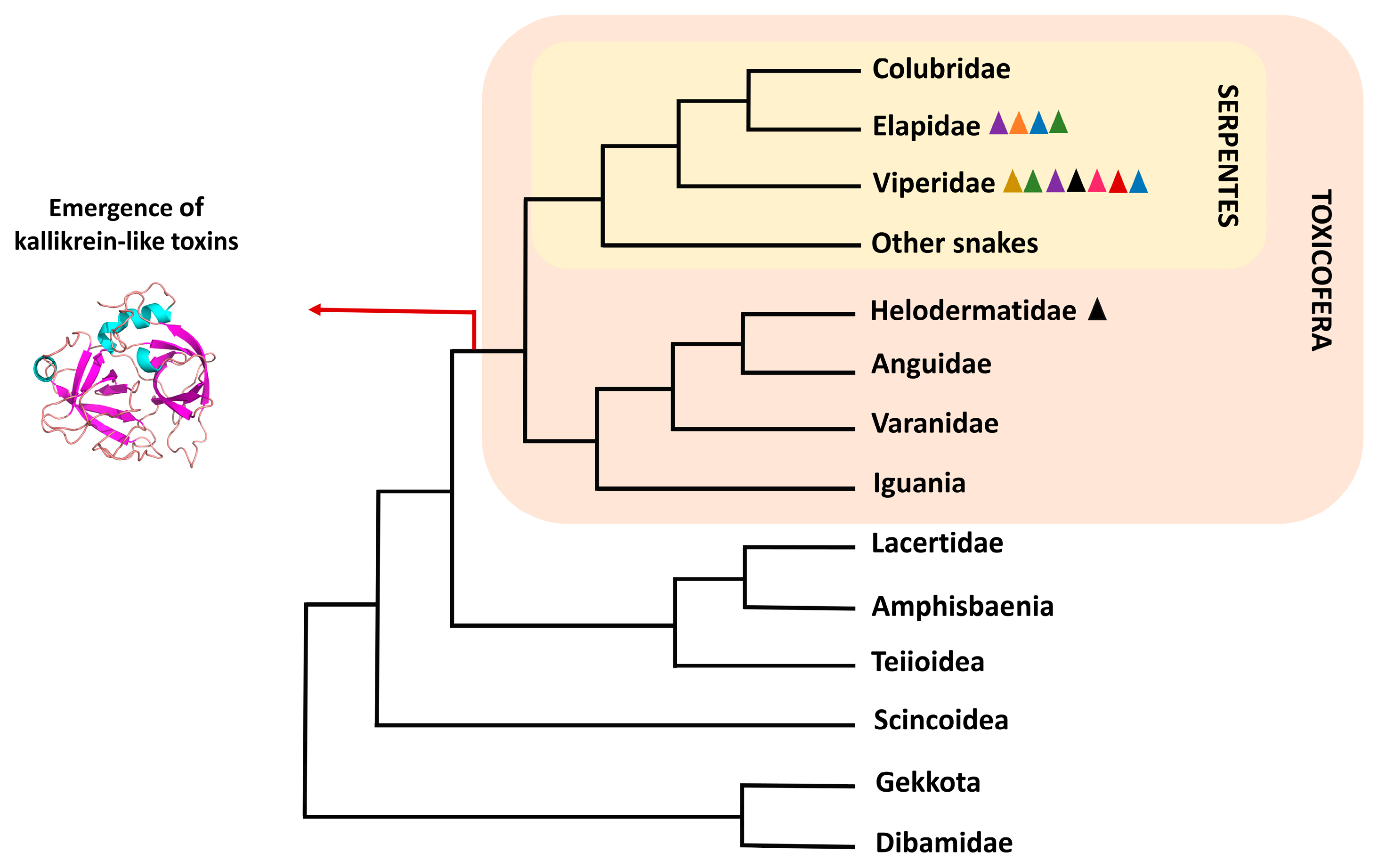
4. Emergence and Evolution
4.1. Evolution from Salivary Enzyme
4.2. Serine Proteases in Toxicoferans
4.3. Gene Evolution and Enzyme Diversification
4.4. Non-Homologous Serine Proteases
5. Therapeutic and Diagnostic Application
6. Enzyme Acquisition
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Q.; Clemetson, J.M.; Clemetson, K.J. Snake Venoms and Hemostasis. J. Thromb. Haemost. 2005, 3, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N.; Hedges, S.B. The Phylogeny of Squamate Reptiles (Lizards, Snakes, and Amphisbaenians) Inferred from Nine Nuclear Protein-Coding Genes. Comptes Rendus Biol. 2005, 328, 1000–1008. [Google Scholar] [CrossRef]
- Fry, B.G.; Vidal, N.; Norman, J.A.; Vonk, F.J.; Scheib, H.; Ramjan, S.F.R.; Kuruppu, S.; Fung, K.; Hedges, S.B.; Richardson, M.K.; et al. Early Evolution of the Venom System in Lizards and Snakes. Nature 2006, 439, 584–588. [Google Scholar] [CrossRef]
- Fry, B.G.; Wroe, S.; Teeuwisse, W.; van Osch, M.J.P.; Moreno, K.; Ingle, J.; McHenry, C.; Ferrara, T.; Clausen, P.; Scheib, H.; et al. A Central Role for Venom in Predation by Varanus komodoensis (Komodo Dragon) and the Extinct Giant Varanus (Megalania) Priscus. Proc. Natl. Acad. Sci. USA 2009, 106, 8969–8974. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M.T. The Long Road of Research on Snake Venom Serine Proteinases. Toxicon 2013, 62, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Simões, T.R.; Pyron, R.A. The Squamate Tree of Life. Bull. Mus. Comp. Zool. 2021, 163, 47–95. [Google Scholar] [CrossRef]
- Hedstrom, L. Serine Protease Mechanism and Specificity. Chem. Rev. 2002, 102, 4501–4524. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Waller, M.; Barrett, A.J.; Bateman, A. MEROPS: The Database of Proteolytic Enzymes, Their Substrates and Inhibitors. Nucleic Acids Res. 2014, 42, D503–D509. [Google Scholar] [CrossRef]
- Alagon, A.; Possani, L.D.; Smart, J.; Schleuning, W.D. Helodermatine, a Kallikrein-like, Hypotensive Enzyme from the Venom of Heloderma horridum horridum (Mexican Beaded Lizard). J. Exp. Med. 1986, 164, 1835–1845. [Google Scholar] [CrossRef]
- Utaisincharoen, P.; Mackessy, S.P.; Miller, R.A.; Tu, A.T. Complete Primary Structure and Biochemical Properties of Gilatoxin, a Serine Protease with Kallikrein-like and Angiotensin-Degrading Activities. J. Biol. Chem. 1993, 268, 21975–21983. [Google Scholar] [CrossRef]
- Serrano, S.M.T.; Maroun, R.C. Snake Venom Serine Proteinases: Sequence Homology vs. Substrate Specificity, a Paradox to Be Solved. Toxicon 2005, 45, 1115–1132. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.D.; Viala, V.L.; Nachtigall, P.G.; Broe, M.; Gibbs, H.L.; Serrano, S.M.d.T.; Moura-da-Silva, A.M.; Ho, P.L.; Nishiyama-Jr, M.Y.; Junqueira-de-Azevedo, I.L.M. Tracking the Recruitment and Evolution of Snake Toxins Using the Evolutionary Context Provided by the Bothrops jararaca Genome. Proc. Natl. Acad. Sci. USA 2021, 118, e2015159118. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Masood, R.; Ali, I.; Ullah, K.; Ali, H.; Akbar, H.; Betzel, C. Thrombin-like Enzymes from Snake Venom: Structural Characterization and Mechanism of Action. Int. J. Biol. Macromol. 2018, 114, 788–811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, W.; Ma, B.; Huang, K.; Song, M.; Zhang, N.; Zhang, Y.; Wang, Y.; Dai, Y.; Luo, Y. Isolation and Characterisation of a Kallikrein-like Enzyme from Agkistrodon halys pallas Snake Venom. J. Sci. Food Agric. 2012, 92, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Marrakchi, N.; Zingali, R.B.; Karoui, H.; Bon, C.; el Ayeb, M. Cerastocytin, a New Thrombin-like Platelet Activator from the Venom of the Tunisian Viper Cerastes cerastes. Biochim. Biophys. Acta 1995, 1244, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.F.; Felicori, L.F.; Chavez-Olortegui, C.; Magalhaes, H.B.P.; Hermogenes, A.L.; Diniz, M.V.; Junqueira-de-Azevedo, I.d.L.M.; Magalhaes, A.; Richardson, M. Biochemical Characterization and Molecular Cloning of a Plasminogen Activator Proteinase (LV-PA) from Bushmaster Snake Venom. Biochim. Biophys. Acta 2006, 1760, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Al-Saleh, S.S. Biochemical Characterization of a Factor X Activator Protein Purified from Walterinnesia aegyptia Venom. Blood Coagul. Fibrinolysis 2015, 26, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K. The Pro-Coagulant Fibrinogenolytic Serine Protease Isoenzymes Purified from Daboia russelii russelii Venom Coagulate the Blood through Factor V Activation: Role of Glycosylation on Enzymatic Activity. PLoS ONE 2014, 9, e86823. [Google Scholar] [CrossRef] [PubMed]
- Kitano, E.S.; Garcia, T.C.; Menezes, M.C.; Tashima, A.K.; Zelanis, A.; Serrano, S.M.T. Cotiarinase Is a Novel Prothrombin Activator from the Venom of Bothrops Cotiara. Biochimie 2013, 95, 1655–1659. [Google Scholar] [CrossRef]
- Murakami, M.T.; Arni, R.K. Crystallization and Preliminary X-Ray Crystallographic Studies of Protac, a Commercial Protein C Activator Isolated from Agkistrodon contortrix contortrix Venom. Biochim. Biophys. Acta 2005, 1752, 202–204. [Google Scholar] [CrossRef]
- Markland, F.S.; Kettner, C.; Schiffman, S.; Shaw, E.; Bajwa, S.S.; Reddy, K.N.; Kirakossian, H.; Patkos, G.B.; Theodor, I.; Pirkle, H. Kallikrein-like Activity of Crotalase, a Snake Venom Enzyme That Clots Fibrinogen. Proc. Natl. Acad. Sci. USA 1982, 79, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Fujimura, Y.; Titani, K. Snake Venom Proteases Affecting Hemostasis and Thrombosis. Biochim. Biophys. Acta 2000, 1477, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Higgins, D.G. Clustal Omega for Making Accurate Alignments of Many Protein Sequences. Protein Sci. 2018, 27, 135–145. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering Key Features in Protein Structures with the New ENDscript Server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Stenberg, L.M.; Stenflo, J. Chapter 642—Coagulation Factor Xa. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 2908–2915. ISBN 978-0-12-382219-2. [Google Scholar]
- Hertzberg, M. Biochemistry of Factor X. Blood Rev. 1994, 8, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Grundy, J.E.; Lavigne, N.; Hirama, T.; MacKenzie, C.R.; Pryzdial, E.L. Binding of Plasminogen and Tissue Plasminogen Activator to Plasmin-Modulated Factor X and Factor Xa. Biochemistry 2001, 40, 6293–6302. [Google Scholar] [CrossRef] [PubMed]
- Tans, G.; Rosing, J. Snake Venom Activators of Factor X: An Overview. Haemostasis 2001, 31, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Farid, T.; Nasser, H.; Zaki, K.; el-Asmar, M.F. Low Molecular Weight Factor X Activator from Cerastes Vipera (Sahara Sand Viper) Venom. Toxicon 1993, 31, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Latinović, Z.; Leonardi, A.; Koh, C.Y.; Kini, R.M.; Trampuš Bakija, A.; Pungerčar, J.; Križaj, I. The Procoagulant Snake Venom Serine Protease Potentially Having a Dual, Blood Coagulation Factor V and X-Activating Activity. Toxins 2020, 12, 358. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Y.L.; Bon, C. An Activator of Blood Coagulation Factor X from the Venom of Bungarus fasciatus. Toxicon 1995, 33, 1277–1288. [Google Scholar] [CrossRef]
- Lee, W.H.; Zhang, Y.; Wang, W.Y.; Xiong, Y.L.; Gao, R. Isolation and Properties of a Blood Coagulation Factor X Activator from the Venom of King Cobra (Ophiophagus hannah). Toxicon 1995, 33, 1263–1276. [Google Scholar] [CrossRef]
- Amiconi, G.; Amoresano, A.; Boumis, G.; Brancaccio, A.; De Cristofaro, R.; De Pascalis, A.; Di Girolamo, S.; Maras, B.; Scaloni, A. A Novel Venombin B from Agkistrodon contortrix contortrix: Evidence for Recognition Properties in the Surface around the Primary Specificity Pocket Different from Thrombin. Biochemistry 2000, 39, 10294–10308. [Google Scholar] [CrossRef] [PubMed]
- Gerads, I.; Tans, G.; Yukelson, L.; Zwaal, R.F.; Rosing, J. Activation of Bovine Factor V by an Activator Purified from the Venom of Naja naja oxiana. Toxicon 1992, 30, 1065–1079. [Google Scholar] [CrossRef]
- Nakayama, D.; Ben Ammar, Y.; Miyata, T.; Takeda, S. Structural Basis of Coagulation Factor V Recognition for Cleavage by RVV-V. FEBS Lett. 2011, 585, 3020–3025. [Google Scholar] [CrossRef]
- Marsh, N.; Williams, V. Practical Applications of Snake Venom Toxins in Haemostasis. Toxicon 2005, 45, 1171–1181. [Google Scholar] [CrossRef]
- Tokunaga, F.; Nagasawa, K.; Tamura, S.; Miyata, T.; Iwanaga, S.; Kisiel, W. The Factor V-Activating Enzyme (RVV-V) from Russell’s Viper Venom. Identification of Isoproteins RVV-V Alpha, -V Beta, and -V Gamma and Their Complete Amino Acid Sequences. J. Biol. Chem. 1988, 263, 17471–17481. [Google Scholar] [CrossRef] [PubMed]
- Rosing, J.; Govers-Riemslag, J.W.; Yukelson, L.; Tans, G. Factor V Activation and Inactivation by Venom Proteases. Haemostasis 2001, 31, 241–246. [Google Scholar] [CrossRef]
- Siigur, E.; Samel, M.; Tõnismägi, K.; Subbi, J.; Reintamm, T.; Siigur, J. Isolation, Properties and N-Terminal Amino Acid Sequence of a Factor V Activator from Vipera lebetina (Levantine Viper) Snake Venom. Biochim. Biophys. Acta 1998, 1429, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Joseph, J.S.; Kini, R.M. Group D Prothrombin Activators from Snake Venom Are Structural Homologues of Mammalian Blood Coagulation Factor Xa. Biochem. J. 2003, 369, 635–642. [Google Scholar] [CrossRef]
- Speijer, H.; Govers-Riemslag, J.W.; Zwaal, R.F.; Rosing, J. Prothrombin Activation by an Activator from the Venom of Oxyuranus scutellatus (Taipan Snake). J. Biol. Chem. 1986, 261, 13258–13267. [Google Scholar] [CrossRef]
- Stocker, K.; Hauer, H.; Müller, C.; Triplett, D.A. Isolation and Characterization of Textarin, a Prothrombin Activator from Eastern Brown Snake (Pseudonaja textilis) Venom. Toxicon 1994, 32, 1227–1236. [Google Scholar] [CrossRef]
- Rao, V.S.; Kini, R.M. Pseutarin C, a Prothrombin Activator from Pseudonaja textilis Venom: Its Structural and Functional Similarity to Mammalian Coagulation Factor Xa-Va Complex. Thromb. Haemost. 2002, 88, 611–619. [Google Scholar] [CrossRef]
- Joseph, J.S.; Chung, M.C.; Jeyaseelan, K.; Kini, R.M. Amino Acid Sequence of Trocarin, a Prothrombin Activator from Tropidechis carinatus Venom: Its Structural Similarity to Coagulation Factor Xa. Blood 1999, 94, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Du, X.; Yang, G.; Zhou, Y.; Wu, X. cDNA Cloning and Expression of Acutin. Biochem. Biophys. Res. Commun. 1999, 255, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Wang, S.R.; Tsai, I.H. Serine Protease Isoforms of Deinagkistrodon acutus Venom: Cloning, Sequencing and Phylogenetic Analysis. Biochem. J. 2001, 354, 161–168. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Teng, M.K.; Niu, L.W. Purification and Characterization of Two Fibrinogen-Clotting Enzymes from Five-Pace Snake (Agkistrodon acutus) Venom. Toxicon 1999, 37, 999–1013. [Google Scholar] [CrossRef]
- Tang, S.-S.; Wang, X.-H.; Zhang, J.-H.; Tang, B.-S.; Qian, L.; Li, P.-Y.; Luo, L.-W. Biochemical Properties and Comparative Pharmacology of a Coagulant from Deinagkistrodon acutus Snake Venom. Eur. J. Pharm. Sci. 2013, 49, 90–98. [Google Scholar] [CrossRef]
- Castro, H.C.; Zingali, R.B.; Albuquerque, M.G.; Pujol-Luz, M.; Rodrigues, C.R. Snake Venom Thrombin-like Enzymes: From Reptilase to Now. Cell Mol. Life Sci. 2004, 61, 843–856. [Google Scholar] [CrossRef]
- Nolan, C.; Hall, L.S.; Barlow, G.H. Ancrod, the Coagulating Enzyme from Malayan Pit Viper (Agkistrodon rhodostoma) Venom. Methods Enzymol. 1976, 45, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, W.; Smith, G.F.; Su, J.L.; Parikh, I.; LeVine, H. Amino Acid Sequence Determination of Ancrod, the Thrombin-like Alpha-Fibrinogenase from the Venom of Akistrodon rhodostoma. FEBS Lett. 1992, 297, 297–301. [Google Scholar] [CrossRef]
- Stocker, K.; Barlow, G.H. The Coagulant Enzyme from Bothrops Atrox Venom (Batroxobin). Methods Enzymol. 1976, 45, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.; Stafford, A.R.; Leslie, B.A.; Kim, P.Y.; Fredenburgh, J.C.; Weitz, J.I. Batroxobin Binds Fibrin with Higher Affinity and Promotes Clot Expansion to a Greater Extent than Thrombin. J. Biol. Chem. 2013, 288, 16862–16871. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.d.O.; Fonseca, K.C.; Mamede, C.C.N.; Beletti, M.E.; Santos-Filho, N.A.; Soares, A.M.; Arantes, E.C.; Hirayama, S.N.S.; Selistre-de-Araújo, H.S.; Fonseca, F.; et al. Bhalternin: Functional and Structural Characterization of a New Thrombin-like Enzyme from Bothrops Alternatus Snake Venom. Toxicon 2010, 55, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Torres-Huaco, F.D.; Werneck, C.C.; Vicente, C.P.; Vassequi-Silva, T.; Nery-Diez, A.C.C.; Mendes, C.B.; Antunes, E.; Marangoni, S.; Damico, D.C.S. Rapid Purification and Procoagulant and Platelet Aggregating Activities of Rhombeobin: A Thrombin-like/Gyroxin-like Enzyme from Lachesis muta rhombeata Snake Venom. Biomed. Res. Int. 2013, 2013, 903292. [Google Scholar] [CrossRef] [PubMed]
- Vivas-Ruiz, D.E.; Sandoval, G.A.; Mendoza, J.; Inga, R.R.; Gontijo, S.; Richardson, M.; Eble, J.A.; Yarleque, A.; Sanchez, E.F. Coagulant Thrombin-like Enzyme (Barnettobin) from Bothrops barnetti Venom: Molecular Sequence Analysis of Its cDNA and Biochemical Properties. Biochimie 2013, 95, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Nishida, S.; Fujimura, Y.; Miura, S.; Ozaki, Y.; Usami, Y.; Suzuki, M.; Titani, K.; Yoshida, E.; Sugimoto, M.; Yoshioka, A. Purification and Characterization of Bothrombin, a Fibrinogen-Clotting Serine Protease from the Venom of Bothrops jararaca. Biochemistry 1994, 33, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.L.S.; Rodrigues, R.S.; Izidoro, L.F.M.; Menaldo, D.L.; Hamaguchi, A.; Homsi-Brandeburgo, M.I.; Fuly, A.L.; Soares, S.G.; Selistre-de-Araújo, H.S.; Barraviera, B.; et al. Biochemical and Functional Properties of a Thrombin-like Enzyme Isolated from Bothrops pauloensis Snake Venom. Toxicon 2009, 54, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Hahn, B.S.; Yang, K.Y.; Park, E.M.; Chang, I.M.; Kim, Y.S. Purification and Molecular Cloning of Calobin, a Thrombin-like Enzyme from Agkistrodon caliginosus (Korean Viper). J. Biochem. 1996, 119, 835–843. [Google Scholar] [CrossRef]
- Patiño, A.C.; Pereañez, J.A.; Gutiérrez, J.M.; Rucavado, A. Biochemical and Biological Characterization of Two Serine Proteinases from Colombian Crotalus durissus cumanensis Snake Venom. Toxicon 2013, 63, 32–43. [Google Scholar] [CrossRef]
- Marrakchi, N.; Barbouche, R.; Guermazi, S.; Karoui, H.; Bon, C.; El Ayeb, M. Cerastotin, a Serine Protease from Cerastes cerastes Venom, with Platelet-Aggregating and Agglutinating Properties. Eur. J. Biochem. 1997, 247, 121–128. [Google Scholar] [CrossRef]
- Henschen-Edman, A.H.; Theodor, I.; Edwards, B.F.; Pirkle, H. Crotalase, a Fibrinogen-Clotting Snake Venom Enzyme: Primary Structure and Evidence for a Fibrinogen Recognition Exosite Different from Thrombin. Thromb. Haemost. 1999, 81, 81–86. [Google Scholar] [CrossRef]
- Amorim, F.G.; Menaldo, D.L.; Carone, S.E.I.; Silva, T.A.; Sartim, M.A.; De Pauw, E.; Quinton, L.; Sampaio, S.V. New Insights on Moojase, a Thrombin-Like Serine Protease from Bothrops moojeni Snake Venom. Toxins 2018, 10, 500. [Google Scholar] [CrossRef]
- Oyama, E.; Takahashi, H. Purification and Characterization of a Thrombin like Enzyme, Elegaxobin II, with Lys-Bradykinin Releasing Activity from the Venom of Trimeresurus elegans (Sakishima-Habu). Toxicon 2003, 41, 559–568. [Google Scholar] [CrossRef]
- Yamamoto, C.; Tsuru, D.; Oda-Ueda, N.; Ohno, M.; Hattori, S.; Kim, S.-T. Flavoxobin, a Serine Protease from Trimeresurus flavoviridis (Habu Snake) Venom, Independently Cleaves Arg726-Ser727 of Human C3 and Acts as a Novel, Heterologous C3 Convertase. Immunology 2002, 107, 111–117. [Google Scholar] [CrossRef]
- Alexander, G.; Grothusen, J.; Zepeda, H.; Schwartzman, R.J. Gyroxin, a Toxin from the Venom of Crotalus durissus terrificus, Is a Thrombin-like Enzyme. Toxicon 1988, 26, 953–960. [Google Scholar] [CrossRef]
- Lu, Q.M.; Jin, Y.; Li, D.S.; Wang, W.Y.; Xiong, Y.L. Characterization of a Thrombin-like Enzyme from the Venom of Trimeresurus jerdonii. Toxicon 2000, 38, 1225–1236. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, Q.-M.; Chen, R.-Q.; Wu, J.-B.; Xiong, Y.-L. Molecular Characterization of a Weak Fibrinogen-Clotting Enzyme from Trimeresurus jerdonii Venom. Toxicon 2005, 45, 353–360. [Google Scholar] [CrossRef]
- Serrano, S.M.; Hagiwara, Y.; Murayama, N.; Higuchi, S.; Mentele, R.; Sampaio, C.A.; Camargo, A.C.; Fink, E. Purification and Characterization of a Kinin-Releasing and Fibrinogen-Clotting Serine Proteinase (KN-BJ) from the Venom of Bothrops jararaca, and Molecular Cloning and Sequence Analysis of Its cDNA. Eur. J. Biochem. 1998, 251, 845–853. [Google Scholar] [CrossRef]
- Magalhães, A.; Magalhães, H.P.B.; Richardson, M.; Gontijo, S.; Ferreira, R.N.; Almeida, A.P.; Sanchez, E.F. Purification and Properties of a Coagulant Thrombin-like Enzyme from the Venom of Bothrops leucurus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 146, 565–575. [Google Scholar] [CrossRef]
- Magalhaes, A.; Da Fonseca, B.C.; Diniz, C.R.; Gilroy, J.; Richardson, M. The Complete Amino Acid Sequence of a Thrombin-like Enzyme/Gyroxin Analogue from Venom of the Bushmaster Snake (Lachesis muta muta). FEBS Lett. 1993, 329, 116–120. [Google Scholar] [CrossRef]
- Niewiarowski, S.; Kirby, E.P.; Brudzynski, T.M.; Stocker, K. Thrombocytin, a Serine Protease from Bothrops Atrox Venom. 2. Interaction with Platelets and Plasma-Clotting Factors. Biochemistry 1979, 18, 3570–3577. [Google Scholar] [CrossRef]
- Kirby, E.P.; Niewiarowski, S.; Stocker, K.; Kettner, C.; Shaw, E.; Brudzynski, T.M. Thrombocytin, a Serine Protease from Bothrops Atrox Venom. 1. Purification and Characterization of the Enzyme. Biochemistry 1979, 18, 3564–3570. [Google Scholar] [CrossRef] [PubMed]
- Laraba-Djebari, F.; Martin-Eauclaire, M.F.; Marchot, P. A Fibrinogen-Clotting Serine Proteinase from Cerastes cerastes (Horned Viper) Venom with Arginine-Esterase and Amidase Activities. Purification, Characterization and Kinetic Parameter Determination. Toxicon 1992, 30, 1399–1410. [Google Scholar] [CrossRef]
- Matsui, T.; Sakurai, Y.; Fujimura, Y.; Hayashi, I.; Oh-Ishi, S.; Suzuki, M.; Hamako, J.; Yamamoto, Y.; Yamazaki, J.; Kinoshita, M.; et al. Purification and Amino Acid Sequence of Halystase from Snake Venom of Agkistrodon halys Blomhoffii, a Serine Protease That Cleaves Specifically Fibrinogen and Kininogen. Eur. J. Biochem. 1998, 252, 569–575. [Google Scholar] [CrossRef]
- Guo, Y.W.; Chang, T.Y.; Lin, K.T.; Liu, H.W.; Shih, K.C.; Cheng, S.H. Cloning and Functional Expression of the Mucrosobin Protein, a Beta-Fibrinogenase of Trimeresurus mucrosquamatus (Taiwan Habu). Protein Expr. Purif. 2001, 23, 483–490. [Google Scholar] [CrossRef]
- Menaldo, D.L.; Bernardes, C.P.; Santos-Filho, N.A.; Moura, L.d.A.; Fuly, A.L.; Arantes, E.C.; Sampaio, S.V. Biochemical Characterization and Comparative Analysis of Two Distinct Serine Proteases from Bothrops pirajai Snake Venom. Biochimie 2012, 94, 2545–2558. [Google Scholar] [CrossRef]
- Silva-Junior, F.P.; Guedes, H.L.M.; Garvey, L.C.; Aguiar, A.S.; Bourguignon, S.C.; Di Cera, E.; Giovanni-De-Simone, S. BJ-48, a Novel Thrombin-like Enzyme from the Bothrops jararacussu Venom with High Selectivity for Arg over Lys in P1: Role of N-Glycosylation in Thermostability and Active Site Accessibility. Toxicon 2007, 50, 18–31. [Google Scholar] [CrossRef]
- Guedes, H.L.M.; Silva, F.P.; Netto, C.C.; de Salles, C.M.C.; Alexandre, G.; Oliveira, C.L.P.; Torriani, I.; De Simone, S.G. Structural Characterization and Low-Resolution Model of BJ-48, a Thrombin-like Enzyme from Bothrops jararacussu Venom. Biophys. Chem. 2008, 132, 159–164. [Google Scholar] [CrossRef]
- Lee, J.W.; Seu, J.H.; Rhee, I.K.; Jin, I.; Kawamura, Y.; Park, W. Purification and Characterization of Brevinase, a Heterogeneous Two-Chain Fibrinolytic Enzyme from the Venom of Korean Snake, Agkistrodon blomhoffii brevicaudus. Biochem. Biophys. Res. Commun. 1999, 260, 665–670. [Google Scholar] [CrossRef]
- Fan, C.Y.; Qian, Y.C.; Yang, S.L.; Gong, Y. Cloning, Sequence Analysis and Expression in E. Coli of the cDNA of the Thrombin-like Enzyme (Pallabin) from the Venom of Agkistrodon halys pallas. Biochem. Mol. Biol. Int. 1999, 47, 217–225. [Google Scholar] [CrossRef]
- Laraba-Djebari, F.; Martin-Eauclaire, M.F.; Mauco, G.; Marchot, P. Afaâcytin, an Alpha Beta-Fibrinogenase from Cerastes cerastes (Horned Viper) Venom, Activates Purified Factor X and Induces Serotonin Release from Human Blood Platelets. Eur. J. Biochem. 1995, 233, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Huang, M.; Hu, Q.; Sun, K.; Wu, H.; Shu, W.; Li, X.; Fang, L. Agkihpin, a Novel SVTLE from Gloydius halys pallas, Promotes Platelet Aggregation in Vitro and Inhibits Thrombus Formation in Vivo in Murine Models of Thrombosis. Toxicon 2016, 122, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Komori, Y.; Nikai, T.; Ohara, A.; Yagihashi, S.; Sugihara, H. Effect of Bilineobin, a Thrombin-like Proteinase from the Venom of Common Cantil (Agkistrodon bilineatus). Toxicon 1993, 31, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Nikai, T.; Ohara, A.; Komori, Y.; Fox, J.W.; Sugihara, H. Primary Structure of a Coagulant Enzyme, Bilineobin, from Agkistrodon bilineatus Venom. Arch. Biochem. Biophys. 1995, 318, 89–96. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.; de Sousa, B.B.; Mamede, C.C.N.; de Morais, N.C.G.; de Queiroz, M.R.; da Cunha Pereira, D.F.; Matias, M.S.; Homi Brandeburgo, M.I. Biochemical and Functional Characterization of BmooSP, a New Serine Protease from Bothrops moojeni Snake Venom. Toxicon 2016, 111, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Zaqueo, K.D.; Kayano, A.M.; Simões-Silva, R.; Moreira-Dill, L.S.; Fernandes, C.F.C.; Fuly, A.L.; Maltarollo, V.G.; Honório, K.M.; da Silva, S.L.; Acosta, G.; et al. Isolation and Biochemical Characterization of a New Thrombin-like Serine Protease from Bothrops pirajai Snake Venom. Biomed. Res. Int. 2014, 2014, 595186. [Google Scholar] [CrossRef] [PubMed]
- Zaganelli, G.L.; Zaganelli, M.G.; Magalhães, A.; Diniz, C.R.; de Lima, M.E. Purification and Characterization of a Fibrinogen-Clotting Enzyme from the Venom of Jararacuçu (Bothrops jararacussu). Toxicon 1996, 34, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Pirkle, H.; Theodor, I.; Miyada, D.; Simmons, G. Thrombin-like Enzyme from the Venom of Bitis Gabonica. Purification, Properties, and Coagulant Actions. J. Biol. Chem. 1986, 261, 8830–8835. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Mackessy, S.P. Biochemical and Pharmacological Properties of a New Thrombin-like Serine Protease (Russelobin) from the Venom of Russell’s Viper (Daboia russelii russelii) and Assessment of Its Therapeutic Potential. Biochim. Biophys. Acta 2013, 1830, 3476–3488. [Google Scholar] [CrossRef]
- Farid, T.M.; Tu, A.T.; el-Asmar, M.F. Characterization of Cerastobin, a Thrombin-like Enzyme from the Venom of Cerastes vipera (Sahara Sand Viper). Biochemistry 1989, 28, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Bortoleto, R.K.; Murakami, M.T.; Watanabe, L.; Soares, A.M.; Arni, R.K. Purification, Characterization and Crystallization of Jararacussin-I, a Fibrinogen-Clotting Enzyme Isolated from the Venom of Bothrops jararacussu. Toxicon 2002, 40, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Kadi-Saci, A.; Laraba-Djebari, F. Purification and Characterization of a Thrombin-like Enzyme Isolated from Vipera lebetina Venom: Its Interaction with Platelet Receptor. Blood Coagul. Fibrinolysis 2020, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Amel, K.-S.; Fatima, L.-D. Purification and Characterization of a New Serine Protease (VLCII) Isolated from Vipera lebetina Venom: Its Role in Hemostasis. J. Biochem. Mol. Toxicol. 2015, 29, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Kogan, A.E.; Bashkov, G.V.; Bobruskin, I.D.; Romanova, E.P.; Makarov, V.A.; Strukova, S.M. Protein C Activator from the Venom of Agkistrodon blomhoffi ussuriensis Retards Thrombus Formation in the Arterio-Venous Shunt in Rats. Thromb. Res. 1993, 70, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Bakker, H.M.; Tans, G.; Yukelson, L.Y.; Janssen-Claessen, T.W.; Bertina, R.M.; Hemker, H.C.; Rosing, J. Protein C Activation by an Activator Purified from the Venom of Agkistrodon halys halys. Blood Coagul. Fibrinolysis 1993, 4, 605–614. [Google Scholar] [CrossRef]
- Klein, J.D.; Walker, F.J. Purification of a Protein C Activator from the Venom of the Southern Copperhead Snake (Agkistrodon contortrix contortrix). Biochemistry 1986, 25, 4175–4179. [Google Scholar] [CrossRef]
- Nakagaki, T.; Kazim, A.L.; Kisiel, W. Isolation and Characterization of a Protein C Activator from Tropical Moccasin Venom. Thromb. Res. 1990, 58, 593–602. [Google Scholar] [CrossRef]
- He, J.; Chen, S.; Gu, J. Identification and Characterization of Harobin, a Novel Fibrino(Geno)Lytic Serine Protease from a Sea Snake (Lapemis hardwickii). FEBS Lett. 2007, 581, 2965–2973. [Google Scholar] [CrossRef]
- Megale, Â.A.A.; Magnoli, F.C.; Kuniyoshi, A.K.; Iwai, L.K.; Tambourgi, D.V.; Portaro, F.C.V.; da Silva, W.D. Kn-Ba: A Novel Serine Protease Isolated from Bitis Arietans Snake Venom with Fibrinogenolytic and Kinin-Releasing Activities. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 38. [Google Scholar] [CrossRef]
- Felicori, L.F.; Souza, C.T.; Velarde, D.T.; Magalhaes, A.; Almeida, A.P.; Figueiredo, S.; Richardson, M.; Diniz, C.R.; Sanchez, E.F. Kallikrein-like Proteinase from Bushmaster Snake Venom. Protein Expr. Purif. 2003, 30, 32–42. [Google Scholar] [CrossRef]
- Vaiyapuri, S.; Harrison, R.A.; Bicknell, A.B.; Gibbins, J.M.; Hutchinson, G. Purification and Functional Characterisation of Rhinocerase, a Novel Serine Protease from the Venom of Bitis gabonica rhinoceros. PLoS ONE 2010, 5, e9687. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.C.; Chiou, S.H. Fibrinogenolytic Proteases Isolated from the Snake Venom of Taiwan Habu: Serine Proteases with Kallikrein-like and Angiotensin-Degrading Activities. Biochem. Biophys. Res. Commun. 2001, 281, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Hendon, R.A.; Tu, A.T. Biochemical Characterization of the Lizard Toxin Gilatoxin. Biochemistry 1981, 20, 3517–3522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wisner, A.; Xiong, Y.; Bon, C. A Novel Plasminogen Activator from Snake Venom. Purification, Characterization, and Molecular Cloning. J. Biol. Chem. 1995, 270, 10246–10255. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.F.; Santos, C.I.; Magalhaes, A.; Diniz, C.R.; Figueiredo, S.; Gilroy, J.; Richardson, M. Isolation of a Proteinase with Plasminogen-Activating Activity from Lachesis muta muta (Bushmaster) Snake Venom. Arch. Biochem. Biophys. 2000, 378, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Kim, H.; Chung, K.; Kim, D.S.; Yun, Y. Expression and Characterization of a Novel Plasminogen Activator from Agkistrodon halys Venom. Toxicon 1998, 36, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Schmaier, A.H.; Colman, R.W. Crotalocytin: Characterization of the Timber Rattlesnake Platelet Activating Protein. Blood 1980, 56, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M.; Mentele, R.; Sampaio, C.A.; Fink, E. Purification, Characterization, and Amino Acid Sequence of a Serine Proteinase, PA-BJ, with Platelet-Aggregating Activity from the Venom of Bothrops jararaca. Biochemistry 1995, 34, 7186–7193. [Google Scholar] [CrossRef]
- Serrano, S.M.; Matos, M.F.; Mandelbaum, F.R.; Sampaio, C.A. Basic Proteinases from Bothrops moojeni (Caissaca) Venom—I. Isolation and Activity of Two Serine Proteinases, MSP 1 and MSP 2, on Synthetic Substrates and on Platelet Aggregation. Toxicon 1993, 31, 471–481. [Google Scholar] [CrossRef]
- Hill-Eubanks, D.C.; Parker, C.G.; Lollar, P. Differential Proteolytic Activation of Factor VIII-von Willebrand Factor Complex by Thrombin. Proc. Natl. Acad. Sci. USA 1989, 86, 6508–6512. [Google Scholar] [CrossRef]
- Niewiarowski, S.; Kirby, E.P.; Stocker, K. Throbocytin-a Novel Platelet Activating Enzyme from Bothrops Atrox Venom. Thromb. Res. 1977, 10, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Monkovic, D.D.; Tracy, P.B. Activation of Human Factor V by Factor Xa and Thrombin. Biochemistry 1990, 29, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Ruben, E.A.; Rau, M.J.; Fitzpatrick, J.A.J.; Di Cera, E. Cryo-EM Structures of Human Coagulation Factors V and Va. Blood 2021, 137, 3137–3144. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, T.L.; Bock, P.E.; Jackson, C.M. Kinetic Intermediates in Prothrombin Activation. Bovine Prethrombin 1 Conversion to Thrombin by Factor X. J. Biol. Chem. 1990, 265, 22044–22055. [Google Scholar] [CrossRef] [PubMed]
- Rosing, J.; Zwaal, R.F.; Tans, G. Formation of Meizothrombin as Intermediate in Factor Xa-Catalyzed Prothrombin Activation. J. Biol. Chem. 1986, 261, 4224–4228. [Google Scholar] [CrossRef] [PubMed]
- Tans, G.; Janssen-Claessen, T.; Hemker, H.C.; Zwaal, R.F.; Rosing, J. Meizothrombin Formation during Factor Xa-Catalyzed Prothrombin Activation. Formation in a Purified System and in Plasma. J. Biol. Chem. 1991, 266, 21864–21873. [Google Scholar] [CrossRef] [PubMed]
- Tijburg, P.N.; van Heerde, W.L.; Leenhouts, H.M.; Hessing, M.; Bouma, B.N.; de Groot, P.G. Formation of Meizothrombin as Intermediate in Factor Xa-Catalyzed Prothrombin Activation on Endothelial Cells. The Influence of Thrombin on the Reaction Mechanism. J. Biol. Chem. 1991, 266, 4017–4022. [Google Scholar] [CrossRef]
- Rosing, J.; Tans, G. Structural and Functional Properties of Snake Venom Prothrombin Activators. Toxicon 1992, 30, 1515–1527. [Google Scholar] [CrossRef]
- Manjunatha Kini, R.; Morita, T.; Rosing, J. Registry of Exogenous Hemostatic Factors of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis Classification and Nomenclature of Prothrombin Activators Isolated from Snake Venoms. Thromb. Haemost. 2001, 86, 710–711. [Google Scholar] [CrossRef]
- Yamada, D.; Sekiya, F.; Morita, T. Isolation and Characterization of Carinactivase, a Novel Prothrombin Activator in Echis carinatus Venom with a Unique Catalytic Mechanism. J. Biol. Chem. 1996, 271, 5200–5207. [Google Scholar] [CrossRef]
- Schieck, A.; Habermann, E.; Kornalik, F. The Prothrombin-Activating Principle from Echis carinatus Venom. II. Coagulation Studies in Vitro and in Vivo. Naunyn Schmiedebergs Arch. Pharmacol. 1972, 274, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Petrovan, R.J.; Govers-Riemslag, J.W.; Nowak, G.; Hemker, H.C.; Rosing, J.; Tans, G. Purification and Characterization of Multisquamase, the Prothrombin Activator Present in Echis Multisquamatus Venom. Thromb. Res. 1997, 88, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Walker, F.J.; Owen, W.G.; Esmon, C.T. Characterization of the Prothrombin Activator from the Venom of Oxyuranus scutellatus scutellatus (Taipan Venom). Biochemistry 1980, 19, 1020–1023. [Google Scholar] [CrossRef]
- Rao, V.S.; Swarup, S.; Manjunatha Kini, R. The Catalytic Subunit of Pseutarin C, a Group C Prothrombin Activator from the Venom of Pseudonaja textilis, Is Structurally Similar to Mammalian Blood Coagulation Factor Xa. Thromb. Haemost. 2004, 92, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.S.; Kini, R.M. Snake Venom Prothrombin Activators Homologous to Blood Coagulation Factor Xa. Haemostasis 2001, 31, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M. The Intriguing World of Prothrombin Activators from Snake Venom. Toxicon 2005, 45, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Swarup, S.; Kini, R.M. The Nonenzymatic Subunit of Pseutarin C, a Prothrombin Activator from Eastern Brown Snake (Pseudonaja textilis) Venom, Shows Structural Similarity to Mammalian Coagulation Factor V. Blood 2003, 102, 1347–1354. [Google Scholar] [CrossRef]
- Masci, P.P.; Whitaker, A.N.; de Jersey, J. Purification and Characterization of a Prothrombin Activator from the Venom of the Australian Brown Snake, Pseudonaja textilis textilis. Biochem. Int. 1988, 17, 825–835. [Google Scholar] [PubMed]
- Lang, F. (Ed.) Activated Protein C Resistance. In Encyclopedia of Molecular Mechanisms of Disease; Springer: Berlin/Heidelberg, Germany, 2009; p. 29. ISBN 978-3-540-29676-8. [Google Scholar]
- van der Neut Kolfschoten, M.; Dirven, R.J.; Vos, H.L.; Tans, G.; Rosing, J.; Bertina, R.M. Factor Va Is Inactivated by Activated Protein C in the Absence of Cleavage Sites at Arg-306, Arg-506, and Arg-679. J. Biol. Chem. 2004, 279, 6567–6575. [Google Scholar] [CrossRef]
- Furie, B. Pathogenesis of Thrombosis. Hematol. Am. Soc. Hematol. Educ. Program 2009, 2009, 255–258. [Google Scholar] [CrossRef]
- Wolberg, A.S. Thrombin Generation and Fibrin Clot Structure. Blood Rev. 2007, 21, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Chapin, J.C.; Hajjar, K.A. Fibrinolysis and the Control of Blood Coagulation. Blood Rev. 2015, 29, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bagoly, Z.; Koncz, Z.; Hársfalvi, J.; Muszbek, L. Factor XIII, Clot Structure, Thrombosis. Thromb. Res. 2012, 129, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S. Multifunctional Roles of Thrombin. Ann. Clin. Lab. Sci. 1999, 29, 275–280. [Google Scholar] [PubMed]
- Al-Amer, O.M. The Role of Thrombin in Haemostasis. Blood Coagul. Fibrinolysis 2022, 33, 145–148. [Google Scholar] [CrossRef]
- Narayanan, S.; Hamasaki, N. Current Concepts of Coagulation and Fibrinolysis. Adv. Clin. Chem. 1998, 33, 133–168. [Google Scholar] [CrossRef]
- Rezaie, A.R. Regulation of the Protein C Anticoagulant and Antiinflammatory Pathways. Curr. Med. Chem. 2010, 17, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Pradniwat, P.; Rojnuckarin, P. Snake Venom Thrombin-like Enzymes. Toxin Rev. 2014, 33, 16–22. [Google Scholar] [CrossRef]
- Phillips, D.J.; Swenson, S.D.; Markland, F.S. Thrombin-like Snake Venom Proteinases. In Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA, 2010; pp. 139–154. [Google Scholar]
- Mackessy, S.P. Thrombin-Like Enzymes in Snake Venoms. In Toxins and Hemostasis: From Bench to Bedside; Kini, R.M., Clemetson, K.J., Markland, F.S., McLane, M.A., Morita, T., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 519–557. ISBN 978-90-481-9295-3. [Google Scholar]
- Markland, F.S. Snake Venoms and the Hemostatic System. Toxicon 1998, 36, 1749–1800. [Google Scholar] [CrossRef]
- Soares, S.G.; Oliveira, L.L. Venom-Sweet-Venom: N-Linked Glycosylation in Snake Venom Toxins. Protein Pept. Lett. 2009, 16, 913–919. [Google Scholar] [CrossRef]
- Huang, K.; Zhao, W.; Gao, Y.; Wei, W.; Teng, M.; Niu, L. Structure of Saxthrombin, a Thrombin-like Enzyme from Gloydius saxatilis. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2011, 67, 862–865. [Google Scholar] [CrossRef]
- Hung, C.C.; Huang, K.F.; Chiou, S.H. Characterization of One Novel Venom Protease with Beta-Fibrinogenase Activity from the Taiwan Habu (Trimeresurus mucrosquamatus): Purification and cDNA Sequence Analysis. Biochem. Biophys. Res. Commun. 1994, 205, 1707–1715. [Google Scholar] [CrossRef]
- Stenflo, J. A New Vitamin K-Dependent Protein. Purification from Bovine Plasma and Preliminary Characterization. J. Biol. Chem. 1976, 251, 355–363. [Google Scholar] [CrossRef]
- Marlar, R.A.; Kleiss, A.J.; Griffin, J.H. Mechanism of Action of Human Activated Protein C, a Thrombin-Dependent Anticoagulant Enzyme. Blood 1982, 59, 1067–1072. [Google Scholar] [CrossRef]
- Esmon, N.L.; DeBault, L.E.; Esmon, C.T. Proteolytic Formation and Properties of Gamma-Carboxyglutamic Acid-Domainless Protein C. J. Biol. Chem. 1983, 258, 5548–5553. [Google Scholar] [CrossRef]
- Asmat, A.; Ramzan, F. Venom Protein C Activators as Diagnostic Agents for Defects of Protein C System. Protein Pept. Lett. 2018, 25, 643–651. [Google Scholar] [CrossRef]
- Gempeler-Messina, P.M.; Volz, K.; Bühler, B.; Müller, C. Protein C Activators from Snake Venoms and Their Diagnostic Use. Haemostasis 2001, 31, 266–272. [Google Scholar] [CrossRef]
- Gale, A.J.; Heeb, M.J.; Griffin, J.H. The Autolysis Loop of Activated Protein C Interacts with Factor Va and Differentiates between the Arg506 and Arg306 Cleavage Sites. Blood 2000, 96, 585–593. [Google Scholar] [CrossRef]
- Gale, A.J.; Cramer, T.J.; Rozenshteyn, D.; Cruz, J.R. Detailed Mechanisms of the Inactivation of Factor VIIIa by Activated Protein C in the Presence of Its Cofactors, Protein S and Factor V. J. Biol. Chem. 2008, 283, 16355–16362. [Google Scholar] [CrossRef]
- Kini, R.M. Anticoagulant Proteins from Snake Venoms: Structure, Function and Mechanism. Biochem. J. 2006, 397, 377–387. [Google Scholar] [CrossRef]
- Kogan, A.E.; Makarov, A.N.; Bobruskin, I.D.; Strukova, S.M. Comparative Study of Protein C Activators from the Agkistrodon Snake Venoms. Thromb. Res. 1991, 62, 775–780. [Google Scholar] [CrossRef]
- Golias, C.; Charalabopoulos, A.; Stagikas, D.; Charalabopoulos, K.; Batistatou, A. The Kinin System—Bradykinin: Biological Effects and Clinical Implications. Multiple Role of the Kinin System—Bradykinin. Hippokratia 2007, 11, 124–128. [Google Scholar]
- Soares de Moura, R.; Resende, A.C.; Emiliano, A.F.; Tano, T.; Mendes-Ribeiro, A.C.; Correia, M.L.G.; de Carvalho, L.C.R.M. The Role of Bradykinin, AT2 and Angiotensin 1-7 Receptors in the EDRF-Dependent Vasodilator Effect of Angiotensin II on the Isolated Mesenteric Vascular Bed of the Rat. Br. J. Pharmacol. 2004, 141, 860–866. [Google Scholar] [CrossRef]
- Péterfi, O.; Boda, F.; Szabó, Z.; Ferencz, E.; Bába, L. Hypotensive Snake Venom Components-A Mini-Review. Molecules 2019, 24, 2778. [Google Scholar] [CrossRef]
- Pinheiro-Júnior, E.L.; Boldrini-França, J.; de Campos Araújo, L.M.P.; Santos-Filho, N.A.; Bendhack, L.M.; Cilli, E.M.; Arantes, E.C. LmrBPP9: A Synthetic Bradykinin-Potentiating Peptide from Lachesis muta rhombeata Venom That Inhibits the Angiotensin-Converting Enzyme Activity in Vitro and Reduces the Blood Pressure of Hypertensive Rats. Peptides 2018, 102, 1–7. [Google Scholar] [CrossRef]
- Collen, D.; Lijnen, H.R. Basic and Clinical Aspects of Fibrinolysis and Thrombolysis. Blood 1991, 78, 3114–3124. [Google Scholar] [CrossRef]
- Carmeliet, P.; Collen, D. Gene Targeting and Gene Transfer Studies of the Plasminogen/Plasmin System: Implications in Thrombosis, Hemostasis, Neointima Formation, and Atherosclerosis. FASEB J. 1995, 9, 934–938. [Google Scholar] [CrossRef]
- Lijnen, H.R.; Collen, D. Matrix Metalloproteinase System Deficiencies and Matrix Degradation. Thromb. Haemost. 1999, 82, 837–845. [Google Scholar] [CrossRef]
- Pepper, M.S. Extracellular Proteolysis and Angiogenesis. Thromb. Haemost. 2001, 86, 346–355. [Google Scholar] [CrossRef]
- Fay, W.P.; Garg, N.; Sunkar, M. Vascular Functions of the Plasminogen Activation System. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Myöhänen, H.; Vaheri, A. Regulation and Interactions in the Activation of Cell-Associated Plasminogen. Cell. Mol. Life Sci. 2004, 61, 2840–2858. [Google Scholar] [CrossRef] [PubMed]
- Cesarman-Maus, G.; Hajjar, K.A. Molecular Mechanisms of Fibrinolysis. Br. J. Haematol. 2005, 129, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Plow, E.; Ploplis, V.; Carmeliet, P.; Collen, D. Plasminogen and Cell Migration in Vivo. Fibrinolysis Proteolysis 1999, 13, 49–53. [Google Scholar] [CrossRef]
- Miles, L.A.; Hawley, S.B.; Baik, N.; Andronicos, N.M.; Castellino, F.J.; Parmer, R.J. Plasminogen Receptors: The Sine qua Non of Cell Surface Plasminogen Activation. Front. Biosci. 2005, 10, 1754–1762. [Google Scholar]
- Fay, W.P. Plasminogen Activator Inhibitor 1, Fibrin, and the Vascular Response to Injury. Trends Cardiovasc. Med. 2004, 14, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Meng, W.; Wang, C.; Wu, Z.; Wu, G.; Xu, Y. Expression, Purification and Characterization of Recombinant Plasminogen Activator from Gloydius Brevicaudus Venom in Escherichia Coli. Protein Expr. Purif. 2013, 91, 85–90. [Google Scholar] [CrossRef]
- Hermogenes, A.L.; Richardson, M.; Magalhaes, A.; Yarleque, A.; Rodriguez, E.; Sanchez, E.F. Interaction of a Plasminogen Activator Proteinase, LV-PA with Human Alpha2-Macroglobulin. Toxicon 2006, 47, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Sajevic, T.; Leonardi, A.; Križaj, I. Haemostatically Active Proteins in Snake Venoms. Toxicon 2011, 57, 627–645. [Google Scholar] [CrossRef]
- Garraud, O.; Hamzeh-Cognasse, H.; Cognasse, F. Platelets and Cytokines: How and Why? Transfus. Clin. Biol. 2012, 19, 104–108. [Google Scholar] [CrossRef]
- Ghoshal, K.; Bhattacharyya, M. Overview of Platelet Physiology: Its Hemostatic and Nonhemostatic Role in Disease Pathogenesis. Sci. World J. 2014, 2014, 781857. [Google Scholar] [CrossRef]
- Freynhofer, M.K.; Gruber, S.C.; Grove, E.L.; Weiss, T.W.; Wojta, J.; Huber, K. Antiplatelet Drugs in Patients with Enhanced Platelet Turnover: Biomarkers versus Platelet Function Testing. Thromb. Haemost. 2015, 114, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.R.; Storey, R.F. The Role of Platelets in Inflammation. Thromb. Haemost. 2015, 114, 449–458. [Google Scholar] [CrossRef]
- Yun, S.-H.; Sim, E.-H.; Goh, R.-Y.; Park, J.-I.; Han, J.-Y. Platelet Activation: The Mechanisms and Potential Biomarkers. Biomed. Res. Int. 2016, 2016, 9060143. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.K. Mechanisms of Platelet Activation: Need for New Strategies to Protect against Platelet-Mediated Atherothrombosis. Thromb. Haemost. 2009, 102, 248–257. [Google Scholar] [CrossRef]
- Estevez, B.; Du, X. New Concepts and Mechanisms of Platelet Activation Signaling. Physiology 2017, 32, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Clemetson, K.J. Platelets and Primary Haemostasis. Thromb. Res. 2012, 129, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Suzuki-Inoue, K.; Inoue, O. Platelet Receptors Activated via Mulitmerization: Glycoprotein VI, GPIb-IX-V, and CLEC-2. J. Thromb. Haemost. 2013, 11 (Suppl. S1), 330–339. [Google Scholar] [CrossRef] [PubMed]
- Herter, J.M.; Rossaint, J.; Zarbock, A. Platelets in Inflammation and Immunity. J. Thromb. Haemost. 2014, 12, 1764–1775. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, F.V.; Antunes, E.; Morganti, R.P.; Monteiro, H.S.A.; Martins, A.M.C.; Toyama, D.O.; Marangoni, S.; Toyama, M.H. Characterization of a New Platelet Aggregating Factor from Crotoxin Crotalus durissus cascavella Venom. Protein J. 2006, 25, 183–192. [Google Scholar] [CrossRef]
- Stegner, D.; Nieswandt, B. Platelet Receptor Signaling in Thrombus Formation. J. Mol. Med. 2011, 89, 109–121. [Google Scholar] [CrossRef]
- Teng, C.M.; Ko, F.N. Comparison of the Platelet Aggregation Induced by Three Thrombin-like Enzymes of Snake Venoms and Thrombin. Thromb. Haemost. 1988, 59, 304–309. [Google Scholar] [CrossRef]
- Dekhil, H.; Wisner, A.; Marrakchi, N.; El Ayeb, M.; Bon, C.; Karoui, H. Molecular Cloning and Expression of a Functional Snake Venom Serine Proteinase, with Platelet Aggregating Activity, from the Cerastes cerastes Viper. Biochemistry 2003, 42, 10609–10618. [Google Scholar] [CrossRef]
- Degen, S.J.; Davie, E.W. Nucleotide Sequence of the Gene for Human Prothrombin. Biochemistry 1987, 26, 6165–6177. [Google Scholar] [CrossRef]
- Parry, M.A.; Jacob, U.; Huber, R.; Wisner, A.; Bon, C.; Bode, W. The Crystal Structure of the Novel Snake Venom Plasminogen Activator TSV-PA: A Prototype Structure for Snake Venom Serine Proteinases. Structure 1998, 6, 1195–1206. [Google Scholar] [CrossRef]
- Krem, M.M.; Di Cera, E. Molecular Markers of Serine Protease Evolution. EMBO J. 2001, 20, 3036–3045. [Google Scholar] [CrossRef]
- Zhu, Z.; Liang, Z.; Zhang, T.; Zhu, Z.; Xu, W.; Teng, M.; Niu, L. Crystal Structures and Amidolytic Activities of Two Glycosylated Snake Venom Serine Proteinases. J. Biol. Chem. 2005, 280, 10524–10529. [Google Scholar] [CrossRef]
- Zeng, F.; Shen, B.; Zhu, Z.; Zhang, P.; Ji, Y.; Niu, L.; Li, X.; Teng, M. Crystal Structure and Activating Effect on RyRs of AhV_TL-I, a Glycosylated Thrombin-like Enzyme from Agkistrodon halys Snake Venom. Arch. Toxicol. 2013, 87, 535–545. [Google Scholar] [CrossRef]
- Ullah, A.; Souza, T.a.C.B.; Zanphorlin, L.M.; Mariutti, R.B.; Santana, V.S.; Murakami, M.T.; Arni, R.K. Crystal Structure of Jararacussin-I: The Highly Negatively Charged Catalytic Interface Contributes to Macromolecular Selectivity in Snake Venom Thrombin-like Enzymes. Protein Sci. 2013, 22, 128–132. [Google Scholar] [CrossRef]
- Hartley, B.S. Homologies in Serine Proteinases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1970, 257, 77–87. [Google Scholar] [CrossRef]
- Schechter, I.; Berger, A. On the Active Site of Proteases. 3. Mapping the Active Site of Papain; Specific Peptide Inhibitors of Papain. Biochem. Biophys. Res. Commun. 1968, 32, 898–902. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Y.; Zhong, S.; Chen, R.; Zhu, S.; Wang, W.; Lu, Q.; Xiong, Y. A Unique Group of Inactive Serine Protease Homologues from Snake Venom. Toxicon 2008, 52, 277–284. [Google Scholar] [CrossRef]
- Vaiyapuri, S.; Wagstaff, S.C.; Harrison, R.A.; Gibbins, J.M.; Hutchinson, E.G. Evolutionary Analysis of Novel Serine Proteases in the Venom Gland Transcriptome of Bitis gabonica rhinoceros. PLoS ONE 2011, 6, e21532. [Google Scholar] [CrossRef]
- Kurtović, T.; Brgles, M.; Leonardi, A.; Lang Balija, M.; Sajevic, T.; Križaj, I.; Allmaier, G.; Marchetti-Deschmann, M.; Halassy, B. VaSP1, Catalytically Active Serine Proteinase from Vipera Ammodytes Ammodytes Venom with Unconventional Active Site Triad. Toxicon 2014, 77, 93–104. [Google Scholar] [CrossRef]
- Gupta, R.; Brunak, S. Prediction of Glycosylation across the Human Proteome and the Correlation to Protein Function. Pac. Symp. Biocomput. 2002, 7, 310–322. [Google Scholar] [CrossRef]
- Tanaka, N.; Nakada, H.; Itoh, N.; Mizuno, Y.; Takanishi, M.; Kawasaki, T.; Tate, S.; Inagaki, F.; Yamashina, I. Novel Structure of the N-Acetylgalactosamine Containing N-Glycosidic Carbohydrate Chain of Batroxobin, a Thrombin-like Snake Venom Enzyme. J. Biochem. 1992, 112, 68–74. [Google Scholar] [CrossRef]
- Murayama, N.; Saguchi, K.; Mentele, R.; Assakura, M.T.; Ohi, H.; Fujita, Y.; Camargo, A.C.M.; Higuchi, S.; Serrano, S.M.T. The Unusual High Molecular Mass of Bothrops Protease A, a Trypsin-like Serine Peptidase from the Venom of Bothrops jararaca, Is Due to Its High Carbohydrate Content. Biochim. Biophys. Acta 2003, 1652, 1–6. [Google Scholar] [CrossRef]
- Pfeiffer, G.; Linder, D.; Strube, K.H.; Geyer, R. Glycosylation of the Thrombin-like Serine Protease Ancrod from Agkistrodon rhodostoma Venom. Oligosaccharide Substitution Pattern at Each N-Glycosylation Site. Glycoconj. J. 1993, 10, 240–246. [Google Scholar] [CrossRef]
- Pirkle, H. Thrombin-like Enzymes from Snake Venoms: An Updated Inventory. Scientific and Standardization Committee’s Registry of Exogenous Hemostatic Factors. Thromb. Haemost. 1998, 79, 675–683. [Google Scholar] [CrossRef]
- Lochnit, G.; Geyer, R. Carbohydrate Structure Analysis of Batroxobin, a Thrombin-like Serine Protease from Bothrops moojeni Venom. Eur. J. Biochem. 1995, 228, 805–816. [Google Scholar] [CrossRef]
- Pfeiffer, G.; Dabrowski, U.; Dabrowski, J.; Stirm, S.; Strube, K.H.; Geyer, R. Carbohydrate Structure of a Thrombin-like Serine Protease from Agkistrodon rhodostoma. Structure Elucidation of Oligosaccharides by Methylation Analysis, Liquid Secondary-Ion Mass Spectrometry and Proton Magnetic Resonance. Eur. J. Biochem. 1992, 205, 961–978. [Google Scholar] [CrossRef]
- Fry, B.G. From Genome to “Venome”: Molecular Origin and Evolution of the Snake Venom Proteome Inferred from Phylogenetic Analysis of Toxin Sequences and Related Body Proteins. Genome Res. 2005, 15, 403–420. [Google Scholar] [CrossRef]
- Fry, B.G.; Scheib, H.; van der Weerd, L.; Young, B.; McNaughtan, J.; Ramjan, S.F.R.; Vidal, N.; Poelmann, R.E.; Norman, J.A. Evolution of an Arsenal: Structural and Functional Diversification of the Venom System in the Advanced Snakes (Caenophidia). Mol. Cell. Proteom. 2008, 7, 215–246. [Google Scholar] [CrossRef]
- Koumandou, V.L.; Scorilas, A. Evolution of the Plasma and Tissue Kallikreins, and Their Alternative Splicing Isoforms. PLoS ONE 2013, 8, e68074. [Google Scholar] [CrossRef]
- Jenzano, J.W.; Brown, C.K.; Mauriello, S.M. Temporal Variations of Glandular Kallikrein, Protein and Amylase in Mixed Human Saliva. Arch. Oral Biol. 1987, 32, 757–759. [Google Scholar] [CrossRef]
- Garrett, J.R.; Smith, R.E.; Kyriacou, K.; Kidd, A.; Liao, J. Factors Affecting the Secretion of Submandibular Salivary Kallikrein in Cats. Q. J. Exp. Physiol. 1987, 72, 357–368. [Google Scholar] [CrossRef]
- Ligabue-Braun, R.; Verli, H.; Carlini, C.R. Venomous Mammals: A Review. Toxicon 2012, 59, 680–695. [Google Scholar] [CrossRef]
- Barua, A.; Mikheyev, A.S. An Ancient, Conserved Gene Regulatory Network Led to the Rise of Oral Venom Systems. Proc. Natl. Acad. Sci. USA 2021, 118, e2021311118. [Google Scholar] [CrossRef]
- Barua, A.; Koludarov, I.; Mikheyev, A.S. Co-Option of the Same Ancestral Gene Family Gave Rise to Mammalian and Reptilian Toxins. BMC Biol. 2021, 19, 268. [Google Scholar] [CrossRef]
- Fry, B.G.; Winter, K.; Norman, J.A.; Roelants, K.; Nabuurs, R.J.A.; van Osch, M.J.P.; Teeuwisse, W.M.; van der Weerd, L.; McNaughtan, J.E.; Kwok, H.F.; et al. Functional and Structural Diversification of the Anguimorpha Lizard Venom System. Mol. Cell Proteom. 2010, 9, 2369–2390. [Google Scholar] [CrossRef]
- Casewell, N.R.; Huttley, G.A.; Wüster, W. Dynamic Evolution of Venom Proteins in Squamate Reptiles. Nat. Commun. 2012, 3, 1066. [Google Scholar] [CrossRef]
- Junqueira-de-Azevedo, I.L.M.; Bastos, C.M.V.; Ho, P.L.; Luna, M.S.; Yamanouye, N.; Casewell, N.R. Venom-Related Transcripts from Bothrops jararaca Tissues Provide Novel Molecular Insights into the Production and Evolution of Snake Venom. Mol. Biol. Evol. 2015, 32, 754–766. [Google Scholar] [CrossRef]
- Hargreaves, A.D.; Swain, M.T.; Logan, D.W.; Mulley, J.F. Testing the Toxicofera: Comparative Transcriptomics Casts Doubt on the Single, Early Evolution of the Reptile Venom System. Toxicon 2014, 92, 140–156. [Google Scholar] [CrossRef]
- Reyes-Velasco, J.; Card, D.C.; Andrew, A.L.; Shaney, K.J.; Adams, R.H.; Schield, D.R.; Casewell, N.R.; Mackessy, S.P.; Castoe, T.A. Expression of Venom Gene Homologs in Diverse Python Tissues Suggests a New Model for the Evolution of Snake Venom. Mol. Biol. Evol. 2015, 32, 173–183. [Google Scholar] [CrossRef]
- Koludarov, I.; Jackson, T.N.; Brouw, B.O.d.; Dobson, J.; Dashevsky, D.; Arbuckle, K.; Clemente, C.J.; Stockdale, E.J.; Cochran, C.; Debono, J.; et al. Enter the Dragon: The Dynamic and Multifunctional Evolution of Anguimorpha Lizard Venoms. Toxins 2017, 9, 242. [Google Scholar] [CrossRef]
- Barua, A.; Mikheyev, A.S. Toxin Expression in Snake Venom Evolves Rapidly with Constant Shifts in Evolutionary Rates. Proc. Biol. Sci. 2020, 287, 20200613. [Google Scholar] [CrossRef]
- Xie, B.; Dashevsky, D.; Rokyta, D.; Ghezellou, P.; Fathinia, B.; Shi, Q.; Richardson, M.K.; Fry, B.G. Dynamic Genetic Differentiation Drives the Widespread Structural and Functional Convergent Evolution of Snake Venom Proteinaceous Toxins. BMC Biol. 2022, 20, 4. [Google Scholar] [CrossRef]
- Vaiyapuri, S.; Thiyagarajan, N.; Hutchinson, E.G.; Gibbins, J.M. Sequence and Phylogenetic Analysis of Viper Venom Serine Proteases. Bioinformation 2012, 8, 763–772. [Google Scholar] [CrossRef]
- Deshimaru, M.; Ogawa, T.; Nakashima, K.; Nobuhisa, I.; Chijiwa, T.; Shimohigashi, Y.; Fukumaki, Y.; Niwa, M.; Yamashina, I.; Hattori, S.; et al. Accelerated Evolution of Crotalinae Snake Venom Gland Serine Proteases. FEBS Lett. 1996, 397, 83–88. [Google Scholar] [CrossRef]
- Doley, R.; Mackessy, S.P.; Kini, R.M. Role of Accelerated Segment Switch in Exons to Alter Targeting (ASSET) in the Molecular Evolution of Snake Venom Proteins. BMC Evol. Biol. 2009, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Tans, G.; Govers-Riemslag, J.W.; van Rijn, J.L.; Rosing, J. Purification and Properties of a Prothrombin Activator from the Venom of Notechis Scutatus Scutatus. J. Biol. Chem. 1985, 260, 9366–9372. [Google Scholar] [CrossRef]
- Reza, A.; Swarup, S.; Manjunatha Kini, R. Two Parallel Prothrombin Activator Systems in Australian Rough-Scaled Snake, Tropidechis Carinatus. Structural Comparison of Venom Prothrombin Activator with Blood Coagulation Factor X. Thromb. Haemost. 2005, 93, 40–47. [Google Scholar] [CrossRef]
- Reza, M.A.; Swarup, S.; Kini, R.M. Structure of Two Genes Encoding Parallel Prothrombin Activators in Tropidechis Carinatus Snake: Gene Duplication and Recruitment of Factor X Gene to the Venom Gland. J. Thromb. Haemost. 2007, 5, 117–126. [Google Scholar] [CrossRef]
- Kwong, S.; Woods, A.E.; Mirtschin, P.J.; Ge, R.; Kini, R.M. The Recruitment of Blood Coagulation Factor X into Snake Venom Gland as a Toxin: The Role of Promoter Cis-Elements in Its Expression. Thromb. Haemost. 2009, 102, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.A.; Minh Le, T.N.; Swarup, S.; Manjunatha Kini, R. Molecular Evolution Caught in Action: Gene Duplication and Evolution of Molecular Isoforms of Prothrombin Activators in Pseudonaja textilis (Brown Snake). J. Thromb. Haemost. 2006, 4, 1346–1353. [Google Scholar] [CrossRef]
- Lan, D.; Song, S.; Liu, Y.; Jiao, B.; Meng, R. Use of Batroxobin in Central and Peripheral Ischemic Vascular Diseases: A Systematic Review. Front. Neurol. 2021, 12, 716778. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wu, H.; Ji, X.; Meng, R. The BE COOL Treatments (Batroxobin, oxygEn, Conditioning, and cOOLing): Emerging Adjunct Therapies for Ischemic Cerebrovascular Disease. J. Clin. Med. 2022, 11, 6193. [Google Scholar] [CrossRef]
- Mazzucco, L.; Balbo, V.; Cattana, E.; Borzini, P. Platelet-Rich Plasma and Platelet Gel Preparation Using Plateltex. Vox Sang. 2008, 94, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Plateltex® Device for the Activation (Gelification) of Blood Components Destined to the Topical Non-Transfusional Use. Available online: www.plateltex.com/data/pdf/IFU4.3plateltexACTJUNE2016ENG.pdf (accessed on 7 July 2024).
- Kjaergard, H.K.; Trumbull, H.R. Vivostat System Autologous Fibrin Sealant: Preliminary Study in Elective Coronary Bypass Grafting. Ann. Thorac. Surg. 1998, 66, 482–486. [Google Scholar] [CrossRef]
- Ferreira, R.S.; de Barros, L.C.; Abbade, L.P.F.; Barraviera, S.R.C.S.; Silvares, M.R.C.; de Pontes, L.G.; Dos Santos, L.D.; Barraviera, B. Heterologous Fibrin Sealant Derived from Snake Venom: From Bench to Bedside—An Overview. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 21. [Google Scholar] [CrossRef]
- Levy, D.E.; del Zoppo, G.J.; Demaerschalk, B.M.; Demchuk, A.M.; Diener, H.-C.; Howard, G.; Kaste, M.; Pancioli, A.M.; Ringelstein, E.B.; Spatareanu, C.; et al. Ancrod in Acute Ischemic Stroke: Results of 500 Subjects Beginning Treatment within 6 Hours of Stroke Onset in the Ancrod Stroke Program. Stroke 2009, 40, 3796–3803. [Google Scholar] [CrossRef]
- Wei, J.; Zhu, M.; Zhang, Z.; Jia, Z.; He, X.; Wan, Y.; Wang, S.; Xiu, D.; Tang, Y.; Li, J.; et al. A Multicenter, Phase III Trial of Hemocoagulase Agkistrodon: Hemostasis, Coagulation, and Safety in Patients Undergoing Abdominal Surgery. Chin. Med. J. 2010, 123, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, R.C.; Douxfils, J. Ecarin Based Coagulation Testing. Am. J. Hematol. 2020, 95, 863–869. [Google Scholar] [CrossRef]
- Quehenberger, P.; Handler, S.; Mannhalter, C.; Kyrle, P.A.; Speiser, W. The Factor V (Leiden) Test: Evaluation of an Assay Based on Dilute Russell Viper Venom Time for the Detection of the Factor V Leiden Mutation. Thromb. Res. 1999, 96, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Pengo, V.; Bison, E.; Banzato, A.; Zoppellaro, G.; Jose, S.P.; Denas, G. Lupus Anticoagulant Testing: Diluted Russell Viper Venom Time (dRVVT). Methods Mol. Biol. 2017, 1646, 169–176. [Google Scholar] [CrossRef]
- Funk, C.; Gmür, J.; Herold, R.; Straub, P.W. Reptilase-R—A New Reagent in Blood Coagulation. Br. J. Haematol. 1971, 21, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.C.; Ferreira, R.S.; Barraviera, S.R.C.S.; Stolf, H.O.; Thomazini-Santos, I.A.; Mendes-Giannini, M.J.S.; Toscano, E.; Barraviera, B. A New Fibrin Sealant from Crotalus durissus terrificus Venom: Applications in Medicine. J. Toxicol. Environ. Health B Crit. Rev. 2009, 12, 553–571. [Google Scholar] [CrossRef]
- Hayashi, M.A.F.; Campeiro, J.D.; Yonamine, C.M. Revisiting the Potential of South American Rattlesnake Crotalus durissus terrificus Toxins as Therapeutic, Theranostic and/or Biotechnological Agents. Toxicon 2022, 206, 1–13. [Google Scholar] [CrossRef]
- Li, H.; Huang, Y.; Wu, X.; Wu, T.; Cao, Y.; Wang, Q.; Qiu, Y.; Fu, W.; Zhang, Q.; Pang, J. Effects of Hemocoagulase Agkistrodon on the Coagulation Factors and Its Procoagulant Activities. Drug Des. Devel Ther. 2018, 12, 1385–1398. [Google Scholar] [CrossRef]
- Yao, Y.-T.; Yuan, X.; Fang, N.-X. Hemocoagulase Reduces Postoperative Bleeding and Blood Transfusion in Cardiac Surgical Patients: A PRISMA-Compliant Systematic Review and Meta-Analysis. Medicine 2019, 98, e18534. [Google Scholar] [CrossRef]
- Thiagarajan, P.; Pengo, V.; Shapiro, S.S. The Use of the Dilute Russell Viper Venom Time for the Diagnosis of Lupus Anticoagulants. Blood 1986, 68, 869–874. [Google Scholar] [CrossRef]
- Bordon, K.d.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef] [PubMed]
- Datta, G.; Tu, A.T. Structure and Other Chemical Characterizations of Gila Toxin, a Lethal Toxin from Lizard Venom. J. Pept. Res. 1997, 50, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Carone, S.E.I.; Menaldo, D.L.; Sartim, M.A.; Bernardes, C.P.; Caetano, R.C.; da Silva, R.R.; Cabral, H.; Barraviera, B.; Ferreira Junior, R.S.; Sampaio, S.V. BjSP, a Novel Serine Protease from Bothrops jararaca Snake Venom That Degrades Fibrinogen without Forming Fibrin Clots. Toxicol. Appl. Pharmacol. 2018, 357, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wisner, A.; Maroun, R.C.; Choumet, V.; Xiong, Y.; Bon, C. Trimeresurus Stejnegeri Snake Venom Plasminogen Activator. Site-Directed Mutagenesis and Molecular Modeling. J. Biol. Chem. 1997, 272, 20531–20537. [Google Scholar] [CrossRef] [PubMed]
- Muanpasitporn, C.; Rojnuckarin, P. Expression and Characterization of a Recombinant Fibrinogenolytic Serine Protease from Green Pit Viper (Trimeresurus albolabris) Venom. Toxicon 2007, 49, 1083–1089. [Google Scholar] [CrossRef]
- You, W.-K.; Choi, W.-S.; Koh, Y.-S.; Shin, H.-C.; Jang, Y.; Chung, K.-H. Functional Characterization of Recombinant Batroxobin, a Snake Venom Thrombin-like Enzyme, Expressed from Pichia pastoris. FEBS Lett. 2004, 571, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Isabel, T.F.; Costa, G.N.M.; Pacheco, I.B.; Barbosa, L.G.; Santos-Junior, C.D.; Fonseca, F.P.P.; Boldrini França, J.; Henrique-Silva, F.; Yoneyama, K.A.G.; Rodrigues, R.S.; et al. Expression and Partial Biochemical Characterization of a Recombinant Serine Protease from Bothrops pauloensis Snake Venom. Toxicon 2016, 115, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Boldrini-França, J.; Santos Rodrigues, R.; Santos-Silva, L.K.; de Souza, D.L.N.; Gomes, M.S.R.; Cologna, C.T.; de Pauw, E.; Quinton, L.; Henrique-Silva, F.; de Melo Rodrigues, V.; et al. Expression of a New Serine Protease from Crotalus durissus collilineatus Venom in Pichia pastoris and Functional Comparison with the Native Enzyme. Appl. Microbiol. Biotechnol. 2015, 99, 9971–9986. [Google Scholar] [CrossRef] [PubMed]
- Boldrini-França, J.; Pinheiro-Junior, E.L.; Arantes, E.C. Functional and Biological Insights of rCollinein-1, a Recombinant Serine Protease from Crotalus durissus collilineatus. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e147118. [Google Scholar] [CrossRef]
- Yang, D.; Peng, M.; Yang, H.; Yang, Q.; Xu, J. Expression, Purification and Characterization of Gloydius shedaoensis Venom Gloshedobin as Hsp70 Fusion Protein in Pichia pastoris. Protein Expr. Purif. 2009, 66, 138–142. [Google Scholar] [CrossRef]
- Alomran, N.; Blundell, P.; Alsolaiss, J.; Crittenden, E.; Ainsworth, S.; Dawson, C.A.; Edge, R.J.; Hall, S.R.; Harrison, R.A.; Wilkinson, M.C.; et al. Exploring the Utility of Recombinant Snake Venom Serine Protease Toxins as Immunogens for Generating Experimental Snakebite Antivenoms. Toxins 2022, 14, 443. [Google Scholar] [CrossRef]
- Liu, S.; Sun, M.-Z.; Sun, C.; Zhao, B.; Greenaway, F.T.; Zheng, Q. A Novel Serine Protease from the Snake Venom of Agkistrodon Blomhoffii ussurensis. Toxicon 2008, 52, 760–768. [Google Scholar] [CrossRef]
- Nikai, T.; Imai, K.; Sugihara, H.; Tu, A.T. Isolation and Characterization of Horridum Toxin with Arginine Ester Hydrolase Activity from Heloderma horridum (Beaded Lizard) Venom. Arch. Biochem. Biophys. 1988, 264, 270–280. [Google Scholar] [CrossRef]
- Stocker, K.; Fischer, H.; Meier, J.; Brogli, M.; Svendsen, L. Characterization of the Protein C Activator Protac from the Venom of the Southern Copperhead (Agkistrodon contortrix) Snake. Toxicon 1987, 25, 239–252. [Google Scholar] [CrossRef]
- Nakayama, D.; Ben Ammar, Y.; Takeda, S. Crystallization and Preliminary X-Ray Crystallographic Analysis of Blood Coagulation Factor V-Activating Proteinase (RVV-V) from Russell’s Viper Venom. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2009, 65, 1306–1308. [Google Scholar] [CrossRef]
- Siigur, E.; Tõnismägi, K.; Trummal, K.; Samel, M.; Vija, H.; Aaspõllu, A.; Rönnholm, G.; Subbi, J.; Kalkkinen, N.; Siigur, J. A New Tyrosine-Specific Chymotrypsin-like and Angiotensin-Degrading Serine Proteinase from Vipera lebetina Snake Venom. Biochimie 2011, 93, 321–330. [Google Scholar] [CrossRef]
- Rivera-de-Torre, E.; Rimbault, C.; Jenkins, T.P.; Sørensen, C.V.; Damsbo, A.; Saez, N.J.; Duhoo, Y.; Hackney, C.M.; Ellgaard, L.; Laustsen, A.H. Strategies for Heterologous Expression, Synthesis, and Purification of Animal Venom Toxins. Front. Bioeng. Biotechnol. 2021, 9, 811905. [Google Scholar] [CrossRef]
- Hung, C.C.; Chiou, S.H. Expression of a Kallikrein-like Protease from the Snake Venom: Engineering of Autocatalytic Site in the Fusion Protein to Facilitate Protein Refolding. Biochem. Biophys. Res. Commun. 2000, 275, 924–930. [Google Scholar] [CrossRef]
- Yonamine, C.M.; Prieto-da-Silva, A.R.B.; Magalhães, G.S.; Rádis-Baptista, G.; Morganti, L.; Ambiel, F.C.; Chura-Chambi, R.M.; Yamane, T.; Camillo, M.a.P. Cloning of Serine Protease cDNAs from Crotalus durissus terrificus Venom Gland and Expression of a Functional Gyroxin Homologue in COS-7 Cells. Toxicon 2009, 54, 110–120. [Google Scholar] [CrossRef]
- Sahyoun, C.; Rima, M.; Mattei, C.; Sabatier, J.M.; Fajloun, Z.; Legros, C. Separation and Analytical Techniques Used in Snake Venomics: A Review Article. Processes 2022, 10, 1380. [Google Scholar] [CrossRef]
- Vander Dos Santos, R.; Villalta-Romero, F.; Stanisic, D.; Borro, L.; Neshich, G.; Tasic, L. Citrus Bioflavonoid, Hesperetin, as Inhibitor of Two Thrombin-like Snake Venom Serine Proteases Isolated from Crotalus simus. Toxicon 2018, 143, 36–43. [Google Scholar] [CrossRef] [PubMed]

| Activity | Specific Enzyme Name | Species | Mass (kDa) | Cleavage Site | Uniprot | Reference |
|---|---|---|---|---|---|---|
| Factor X activator | n.a. | Walterinnesia aegyptia | 60 | (Arg194-Ile195) | n.a. | 2015, [17] |
| n.a. | Bungarus fasciatus | 70 | (Arg194-Ile195) | n.a. | 1995, [31] | |
| n.a. | Cerastes vipera | 12.5 | n.a. | n.a. | 1993, [29] | |
| n.a. | Ophiophagus hannah | 64.5 | n.a. | n.a. | 1995, [32] | |
| VaaSP-VX | Vipera ammodytes ammodytes | 34 | Arg194-Ile195 | n.a. | 2020, [30] | |
| Factor V activator | Contortrixobin | Agkistrodon contortrix | 26 | n.a. | P82981 | 2000, [33] |
| n.a. | Naja oxiana | 48 | n.a. | n.a. | 1992, [34] | |
| RV-FVPα | Daboia russelii | 38 | n.a. | n.a. | 2014, [18] | |
| RVV-V | Daboia russelii | 28 | Arg1545-Ser1546 | P86530 | 1988, [35,36,37] | |
| UVV-V | Vipera ursini | 34 | n.a. | n.a. | 2002, [38] | |
| VaaSP-VX | Vipera ammodytes ammodytes | 34 | Arg348, Arg1753 (bovine FV) | n.a. | 2020, [30] | |
| VLFVA (LVV-V) | Macrovipera lebetina | 28.4 | n.a. | n.a. | 1998, [39] | |
| Prothrombin activators | Hopsarin D | Hoplocephalus stephensi | 46.1 | Arg274-Thr275, Arg323-Ile324 | P83370 | 2003, [40] |
| Notanarin D | Notechis ater | 46 | n.a. | P0CY52 | 2003, [40] | |
| Notecarin D | Notechis scutatus | 54 | n.a. | P82807 | 2003, [40] | |
| Oscutarin C | Oxyuranus scutellatus | 300 | Arg274-Thr275, Arg323-Ile324 | Q58L96 | 1986, [41] | |
| PLIPA | Pseudonaja textilis | nr | (Arg363-Ile364) | n.a. | 1994, [42] | |
| Pseutarin C | Pseudonaja textilis | nr | Arg273-Thr274, Arg322-Ile323 (bovine prothrombin) | Q56VR3 | 2002, [43] | |
| Textarin D | Pseudonaja textilis | 50–53 | (Arg363-Ile364) | n.a. | 1994, [42] | |
| Trocarin D | Tropidechis carinatus | 46.5 | Arg274-Thr275, Arg323-Ile324 | P81428 | 1999, [44] | |
| Thrombin-like α-chain fibrinogenolytic | Acutobin (Acutin/Acuthrombin) | Deinagkistrodon acutus | 40 | n.a. | Q9I8X2 | 1999, [45,46,47] |
| Agacutase | Deinagkistrodon acutus | 31 | n.a. | n.a. | 2013, [48] | |
| Ancrod (Viprinex™, Arvin™, Arwin™) | Calloselasma rhodostoma | nr | Arg23-His24, Arg16-Gly17 | P26324 | 1976, [49,50,51] | |
| Batroxobin (Defibrase®) | Bothrops atrox | 43 | Arg16-Gly17 | P04971 | 1976, [52,53] | |
| Bhalternin | Bothrops alternatus | 31.5 | n.a. | P0CG03 | 2010, [54] | |
| Rhombeobin | Lachesis muta rhombeata | 47 | n.a. | n.a. | 2013, [55] | |
| Barnettobin (Bb-TLE) | Bothrops barnetti | 52 | n.a. | K4LLQ2 | 2013, [56] | |
| Bothrombin (Reptilase®) | Bothrops jararaca | 35 | Arg16-Gly17 | P81661 | 1994, [57] | |
| BpSP-I | Bothrops pauloensis | 34 | n.a. | P0DJF1 | 2009, [58] | |
| Calobin | Gloydius ussuriensis | 34 | n.a. | Q91053 | 1996, [59] | |
| CDC SI | Crotalus durissus cumanensis | 28.5 | n.a. | P0DKX2 | 2013, [60] | |
| CDC SII | Crotalus durissus cumanensis | 28.8 | n.a. | P0DKX3 | 2013, [60] | |
| Cerastotin | Cerastes cerastes | 40 | n.a. | P81038 | 1997, [61] | |
| Crotalase | Crotalus adamanteus | 33 | n.a. | F8S114 | 1971, [21,62] | |
| Cerastocytin | Cerastes cerastes | 38 | n.a. | Q7SYF1 | 1995, [15] | |
| Moojase | Bothrops moojeni | 30.3 | n.a. | n.a. | 2018, [63] | |
| Elegaxobin II | Protobothrops elegans | 35 | n.a. | P84787 | 2003, [64] | |
| Flavoxobin | Protobothrops flavoviridis | 26.7 | Arg16-Gly17 | P05620 | 1988, [65] | |
| Gyroxin | Crotalus durissus terrificus | 28 | n.a. | B0FXM1 | 1988, [66] | |
| Jerdonobin | Protobothrops jerdonii | 38 | (Arg16-Gly17) | P0DM43 | 2000, [67] | |
| Jerdonobin-II | Protobothrops jerdonii | 32 | (Arg16-Gly17) | n.a. | 2005, [68] | |
| KN-BJ | Bothrops jararaca | 38 | n.a. | O13069 | 1998, [69] | |
| Leucurobin | Bothrops leucurus | 35 | (Arg16-Gly17) | P0DJ86 | 2007, [70] | |
| LM-TL | Lachesis muta | 41–47 | n.a. | P33589 | 1989, [71] | |
| Thrombocytin | Bothrops atrox | 36 | n.a. | n.a. | 1979, [72,73] | |
| RP-34 | Cerastes cerastes | 97 | n.a. | n.a. | 1992, [74] | |
| Thrombin-like β-chain fibrinogenolytic | Contortrixobin | Agkistrodon contortrix | 26 | n.a. | P86530 | 2000, [33] |
| Halystase | Gloydius blomhoffi | 38 | Arg42 (fibrinogen Bβ chain) | P81176 | 1998, [75] | |
| Mucrosobin | Protobothrops mucrosquamatus | 28 | n.a. | n.a. | 2001, [76] | |
| BpirSP27 | Bothrops pirajai | 27.1 | n.a. | P0DL26 | 2012, [77] | |
| BJ-48 | Bothrops jararacussu | 48 | n.a. | P0DJF0 | 2007, [78,79] | |
| Brevinase | Gloydius brevicaudus | 33.5 | n.a. | Q9PT51 | 1999, [80] | |
| Pallabin | Gloydius halys | 26 | n.a. | Q9YGJ2 | 1999, [81] | |
| Thrombin-like α/β-chain fibrinogenolytic | Afaâcytin (RP34) | Cerastes cerastes | 40 | n.a. | Q9PRM8 | 1995, [82] |
| Agkihpin | Gloydius halys | 25.46 | n.a. | N0AAE6 | 2016, [83] | |
| Bilineobin | Gloydius bilineatus | 57 | Arg19-Gly20 (fibrinogen Aα chain), Arg21-Gly22 (fibrinogen Bβ chain) | Q9PSN3 | 1993, [84,85] | |
| BmooSP | Bothrops moojeni | 36 | n.a. | n.a. | 2016, [86] | |
| BpirSP-39 | Bothrops pirajai | 39.4 | n.a. | n.a. | 2014, [87] | |
| BpirSP41 | Bothrops pirajai | 40.6 | n.a. | P0DL27 | 2012, [77] | |
| FC-Bj | Bothrops jararacussu | nr | n.a. | n.a. | 1996, [88] | |
| Gabonase | Bitis gabonica | 30.6 | n.a. | P0C577 | 1986, [89] | |
| Russelobin | Daboia russelii | 51.3 | n.a. | n.a. | 2013, [90] | |
| Cerastobin | Cerastes vipera | 38 | n.a. | P18692 | 1989, [91] | |
| Jararacussin-I | Bothrops jararacussu | 28 | n.a. | n.a. | 2002, [92] | |
| VLCV | Macrovipera lebetina | 45 | n.a. | n.a. | 2020, [93] | |
| VLCII | Macrovipera lebetina | 60 | n.a. | n.a. | 2015, [94] | |
| Protein C activator | n.a. | Gloydius ussuriensis | nr | n.a. | n.a. | 1993, [95] |
| n.a. | Gloydius halys halys | 36 | n.a. | n.a. | 1993, [96] | |
| n.a. | Agkistrodon contortrix | 20 | n.a. | n.a. | 1986, [97] | |
| n.a. | Gloydius bilineatus | 38 | n.a. | P33588 | 1990, [98] | |
| Kallikrein-like | AHP-Ka | Gloydius halys | 34 | n.a. | P0DJG5 | 2012, [14] |
| Harobin | Hydrophis hardwickii | 25 | n.a. | Q5MCS0 | 2007, [99] | |
| Kn-Ba | Bitis arietans | 33 | n.a. | n.a. | 2018, [100] | |
| LV-Ka | Lachesis muta | 33 | n.a. | n.a. | 2003, [101] | |
| Rhinocerase | Bitis rhinoceros | 36 | n.a. | P86497 | 2010, [102] | |
| Tm-VIG | Protobothrops mucrosquamatus | nr | n.a. | n.a. | 2001, [103] | |
| Tm-IIG | n.a. | n.a. | ||||
| Gilatoxin | Heloderma horridum and Heloderma suspectum | 35–37.5 | n.a. | P43685 | 1981, [104] | |
| Helodermatine | Heloderma horridum | 63 | n.a. | n.a. | 1986, [9] | |
| Plasminogen activator | TSV-PA | Trimeresurus stejnegeri | 33 | Arg561-Val562 | Q91516 | 1995, [105] |
| LV-PA | Lachesis muta muta | 33 | (Arg561-Val562) | Q27J47 | 2000, [106] | |
| Haly-PA | Gloydius halys | 32 | (Arg561-Val562) | Q9YGJ8 | 1998, [107] | |
| Platelet activator | Crotalocytin | Crotalus horridus | 64 | n.a. | n.a. | 1980, [108] |
| Cerastocytin | Cerastes cerastes | 38 | n.a. | Q7SYF1 | 1995, [15] | |
| PA-BJ | Bothrops jararaca | 30 | n.a. | P81824 | 1995, [109] | |
| MSP 1 | Bothrops moojeni | 32.5–34 | n.a. | n.a. | 1993, [110] | |
| BJV-VIIIcp | Bothrops jararacussu | 28 | n.a. | n.a. | 1989, [111] | |
| Thrombocytin | Bothrops atrox | 36 | n.a. | n.a. | 1979, [72,73,112] | |
| Cerastotin | Cerastes vipera | 38 | n.a. | P81038 | 1989, [91] |
| PDB Code | Resolution | Glycosylation | Ligand | Organism | Reference |
|---|---|---|---|---|---|
| 1BQY | 2.50 | - | Glu-Gly-Arg-chloromethylketone | Trimeresurus stejnegeri | [188] |
| 1OP0 | 2.00 | Asn35 | - | Deinagkistrodon acutus | [190] |
| 1OP2 | 2.10 | Asn35 | |||
| 5XRF | 2.20 | Asn81, Asn124 | - | To be published | |
| 2AIQ | 1.54 | Asn37, Asn96A, Asn148 | Benzamidine | Agkistrodon contortrix | [20] |
| 2AIP | 1.65 | - | |||
| 3S69 | 1.43 | - | - | Goydius intermedius | [145] |
| 3S9A | 1.90 | Asn245 | - | Daboia siamensis | [35] |
| 3S9B | 1.90 | Asn245 | - | ||
| 3S9C | 1.80 | Asn245 | Factor V 14 peptide | ||
| 3SBK | 2.55 | Asn245 | D-Phe-Pro-Arg-chloromethylketone | ||
| 4E7N | 1.75 | Asn95A | - | Gloydius halys | [191] |
| 4GSO | 2.60 | - | - | Bothrops jararacussu | [192] |
| RMSD (Å) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3S9A | 4E7N | 1OP0 | 1OP2 | 4GSO | 3S69 | 1BQY | 2AIQ | 5XRF | |
| 3S9A | - | 0.559 | 0.582 | 0.699 | 0.878 | 0.723 | 0.729 | 0.671 | 0.882 |
| 4E7N | 67.09% | - | 0.377 | 0.380 | 0.738 | 0.443 | 0.550 | 0.517 | 0.621 |
| 1OP0 | 57.69% | 69.65% | - | 0.197 | 0.696 | 0.289 | 0.534 | 0.514 | 0.553 |
| 1OP2 | 58.11% | 70.08% | 99.57% | - | 0.690 | 0.313 | 0.458 | 0.566 | 0.562 |
| 4GSO | 58.62% | 68.53% | 85.77% | 86.2% | - | 0.625 | 0.538 | 0.817 | 0.806 |
| 3S69 | 61.96% | 70.51% | 84.18% | 84.61% | 76.72% | - | 0.538 | 0.566 | 0.553 |
| 1BQY | 61.11% | 67.94% | 71.79% | 72.22% | 68.96% | 73.07% | - | 0.622 | 0.626 |
| 2AIQ | 61.47% | 72.72% | 68.83% | 69.26% | 70.56% | 67.96% | 67.09% | - | 0.710 |
| 5XRF | 52.13% | 58.4% | 56.83% | 56.83% | 54.74% | 56.83% | 53.41% | 58.87% | - |
| 3S9A | 4E7N | 1OP0 | 1OP2 | 4GSO | 3S69 | 1BQY | 2AIQ | 5XRF | |
| Percentage of identity | |||||||||
| Venom-Based Drug | Main Source | Mechanism of Action | Example of Application | References |
|---|---|---|---|---|
| Reptilase® (Batroxobin) | Bothrops atrox | Fibrinogen α-chain cleavage into fibrin | Diagnostic of coagulation disorders to assess the functionality of fibrinogen | [229] |
| Defibrase® (Batroxobin) | Bothrops moojeni | Treatment of ischemia caused by vascular occlusive diseases | [230] | |
| Plateltex-ACT® (Batroxobin and calcium gluconate) | Bothrops atrox | Fibrinogen α-chain cleavage into fibrin in presence of calcium, leading to a fibrin reticulum and promoting a blood cell gelation | Topical treatment of damaged tissues | [231,232] |
| Vivostat® fibrin sealant (Batroxobin and citrate) | Bothrops moojeni | A medical device is used to prepare autologous fibrin, exploring the fibrinogen α-chain cleavage promoted by batroxobin in presence of citrate | Used as an autologous fibrin sealant in surgery | [233] |
| Gyroxin heterologous fibrin sealant under clinical evaluation | Crotalus durissus terrificus | Fibrinogen α-chain cleavage into fibrin | Used as a heterologous fibrin sealant in surgery, and in chronic venous ulcers | [234] |
| Viprinex® (Ancrod) | Calloselasma rhodostoma | Fibrinogen α and β chains cleavage into fibrin degradation products | Treatment of acute ischemic stroke | [235] |
| Suling® (Hemocoagulase Agkistrodon) | Deinagkistrodon acutus | Activation of prothrombin into thrombin, resulting in the conversion of fibrinogen into fibrin | Treatment of acute ischemic stroke | [236] |
| Protac® | Agkistrodon contortrix | Fast-acting protein C activator | Diagnostic of protein C pathway disorders, and assessment of the thrombosis risk | [151] |
| Ecarin | Echis carinatus | Activation of prothrombin into meizothrombin, followed by conversion of fibrinogen into fibrin | Diagnostic tool to detect thrombin and thrombin inhibitors | [237] |
| Russel’s viper venom-Factor V (RVV-V) | Daboia russelii | Activation of factor V into factor Va, interfering with thrombin production | Diagnostic tool to evaluate the functionality of coagulation factors, used in diagnosis of activated protein C resistance | [238] |
| Russel’s viper venom-Factor X (RVV-X) | Daboia russelii | Activation of factor X into factor Xa, interfering with thrombin production | Diagnostic tool to evaluate the functionality of coagulation factors, used in Lupus anticoagulant testing | [239] |
| Origin | Expression System | Protein | Organism | Purification Method | Reference |
|---|---|---|---|---|---|
| Heterologous Expression | E. coli | rCC-PPP | Cerastes cerastes | IEC | [186] |
| rGBV-PA | Gloydius brevicaudus | IMAC | [170] | ||
| IEC | |||||
| TSV-PA | Trimeresurus stejnegeri | SEC | [249] | ||
| K. phaffii | Albofibrase | Trimeresurus albolabris | IMAC | [250] | |
| Batroxobin | Bothrops moojeni | HIC | [251] | ||
| AC | |||||
| BpSP-II | Bothrops pauloensis | IMAC | [252] | ||
| Collinein-1 | Crotalus durissus | IEC | [253] | ||
| RP-FPLC | |||||
| IMAC | [254] | ||||
| IEC | |||||
| Gloshedobin | Gloydius shedaoensis | IEC | [255] | ||
| SEC | |||||
| HEK | Ancrod, Batroxobin, RVV-V | Calloselasma rhodostoma, Bothrops atrox, Daboia russelii | IMAC | [256] | |
| Venom Purification | ABUSV-Spase | Gloydius ussuriensis | IEC | [257] | |
| SEC | |||||
| Agkihpin | Gloydius halys | SEC | [83] | ||
| Bhalternin | Bothrops alternatus | IEC | [54] | ||
| SEC | |||||
| AC | |||||
| RP-HPLC | |||||
| BjSP | Bothrops jararaca | SEC | [248] | ||
| IEC | |||||
| RP-HPLC | |||||
| BpSP-I | Bothrops pauloensis | IEC | [58] | ||
| HIC | |||||
| RP-HPLC | |||||
| BpSP-II | Bothrops pauloensis | IMAC | [252] | ||
| BpirSP27, BpirSP41 | Bothrops pirajai | SEC | [77] | ||
| AC | |||||
| RP-HPLC | |||||
| BpirSP-39 | Bothrops pirajai | SEC | [87] | ||
| AC | |||||
| RP-HPLC | |||||
| Collinein-1 | Crotalus durissus | SEC | [253] | ||
| IEC | |||||
| RP-FPLC | |||||
| Cotiarinase | Bothrops cotiara | SEC | [19] | ||
| IEC | |||||
| Crotoxin | Crotalus durissus | SEC | [183] | ||
| RP-HPLC | |||||
| Factor V activator | Naja oxiana | IEC | [34] | ||
| Factor X activator | Cerastes vipera | SEC | [29] | ||
| IEC | |||||
| Ophiophagus hannah | SEC | [30] | |||
| IEC | |||||
| Walterinnesia aegyptia | RP-HPLC | [17] | |||
| Horridum toxin | Heloderma horridum | SEC | [247,258] | ||
| IEC | |||||
| Kallikrein-like | Gloydius halys | IEC | [14] | ||
| AC | |||||
| RP-HPLC | |||||
| Kn-Ba | Bitis arietans | SEC | [100] | ||
| Moojase | Bothrops moojeni | IEC | [63] | ||
| RP-HPLC | |||||
| Rhinocerase | Bitis rhinocerus | LPIP | [99] | ||
| SEC | |||||
| Rhombeobin | Lachesis muta rhombeata | RP-HPLC | [55] | ||
| Protac® | Agkistrodon contortrix | IEC | [259] | ||
| SEC | |||||
| RV-FVP | Daboia russelii | SEC | [18] | ||
| IEC | |||||
| RVV-V | Daboia siamensis | SEC | [260] | ||
| IEC | |||||
| RP-HPLC | |||||
| Tm-VIG | Protobothrops mucrosquamatus | IEC | [146] | ||
| SEC | |||||
| RP-HPLC | |||||
| VaaSP-VX | Vipera ammodytes ammodytes | SEC | [30] | ||
| IEC | |||||
| VLCTLP | Macrovipera lebetina | SEC | [261] | ||
| IEC | |||||
| AC | |||||
| VLCV | Macrovipera lebetina | SEC | [93] | ||
| IEC | |||||
| RP-HPLC | |||||
| VLFVA | Macrovipera lebetina | SEC | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal, J.F.D.; Schwartz, M.F.; Garay, A.V.; Valadares, N.F.; Bueno, R.V.; Monteiro, A.C.L.; Freitas, S.M.d.; Barbosa, J.A.R.G. Exploring the Diversity and Function of Serine Proteases in Toxicofera Reptile Venoms: A Comprehensive Overview. Toxins 2024, 16, 428. https://doi.org/10.3390/toxins16100428
Vidal JFD, Schwartz MF, Garay AV, Valadares NF, Bueno RV, Monteiro ACL, Freitas SMd, Barbosa JARG. Exploring the Diversity and Function of Serine Proteases in Toxicofera Reptile Venoms: A Comprehensive Overview. Toxins. 2024; 16(10):428. https://doi.org/10.3390/toxins16100428
Chicago/Turabian StyleVidal, Julia F. D., Matheus F. Schwartz, Aisel V. Garay, Napoleão F. Valadares, Renata V. Bueno, Ana Carolina L. Monteiro, Sônia Maria de Freitas, and João Alexandre R. G. Barbosa. 2024. "Exploring the Diversity and Function of Serine Proteases in Toxicofera Reptile Venoms: A Comprehensive Overview" Toxins 16, no. 10: 428. https://doi.org/10.3390/toxins16100428





