Preparation of Soybean Dreg-Based Biochar@TiO2 Composites and the Photocatalytic Degradation of Aflatoxin B1 Exposed to Simulated Sunlight Irradiation
Abstract
1. Introduction
2. Results and Discussion
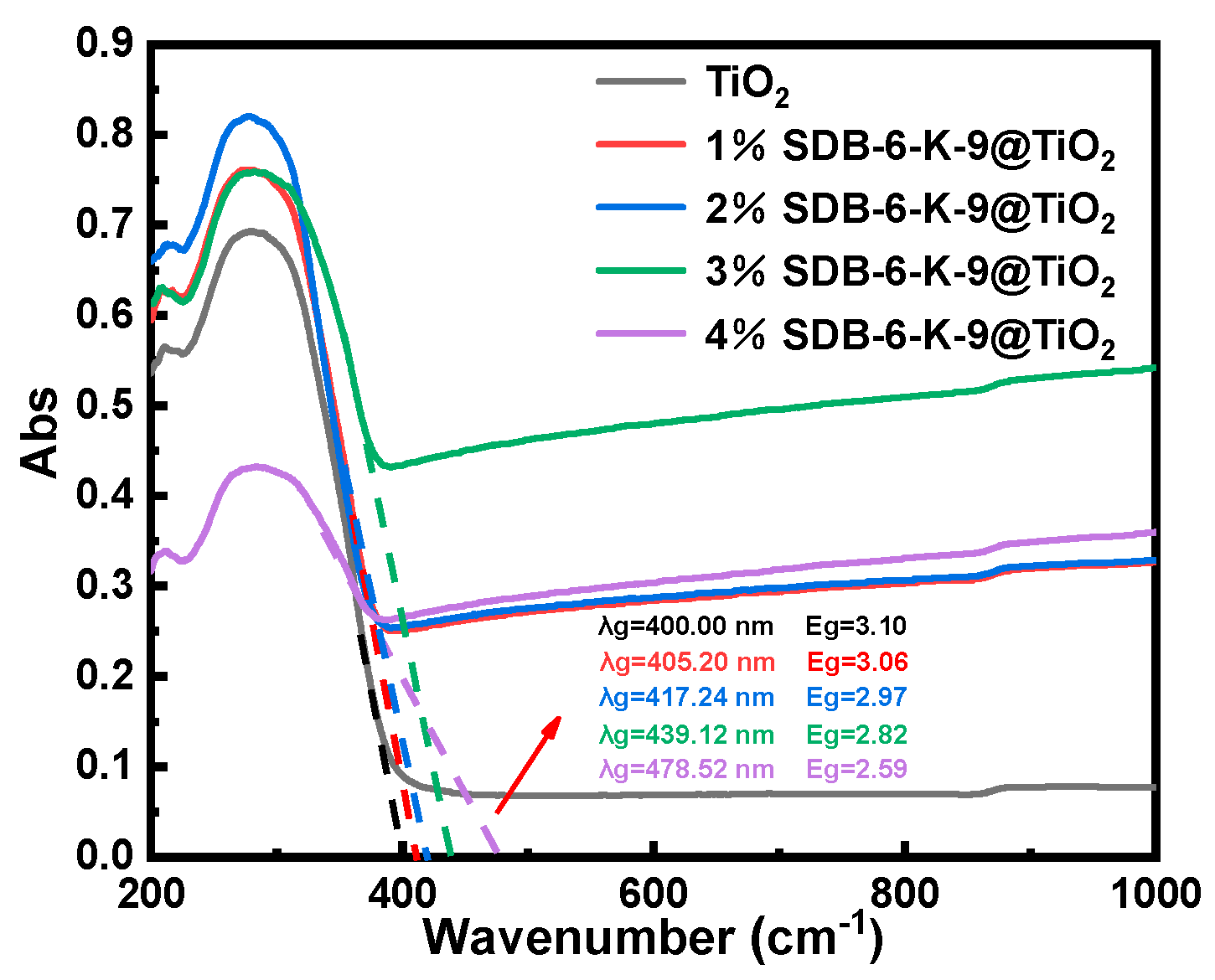
2.1. The Photocatalytic Characteristics
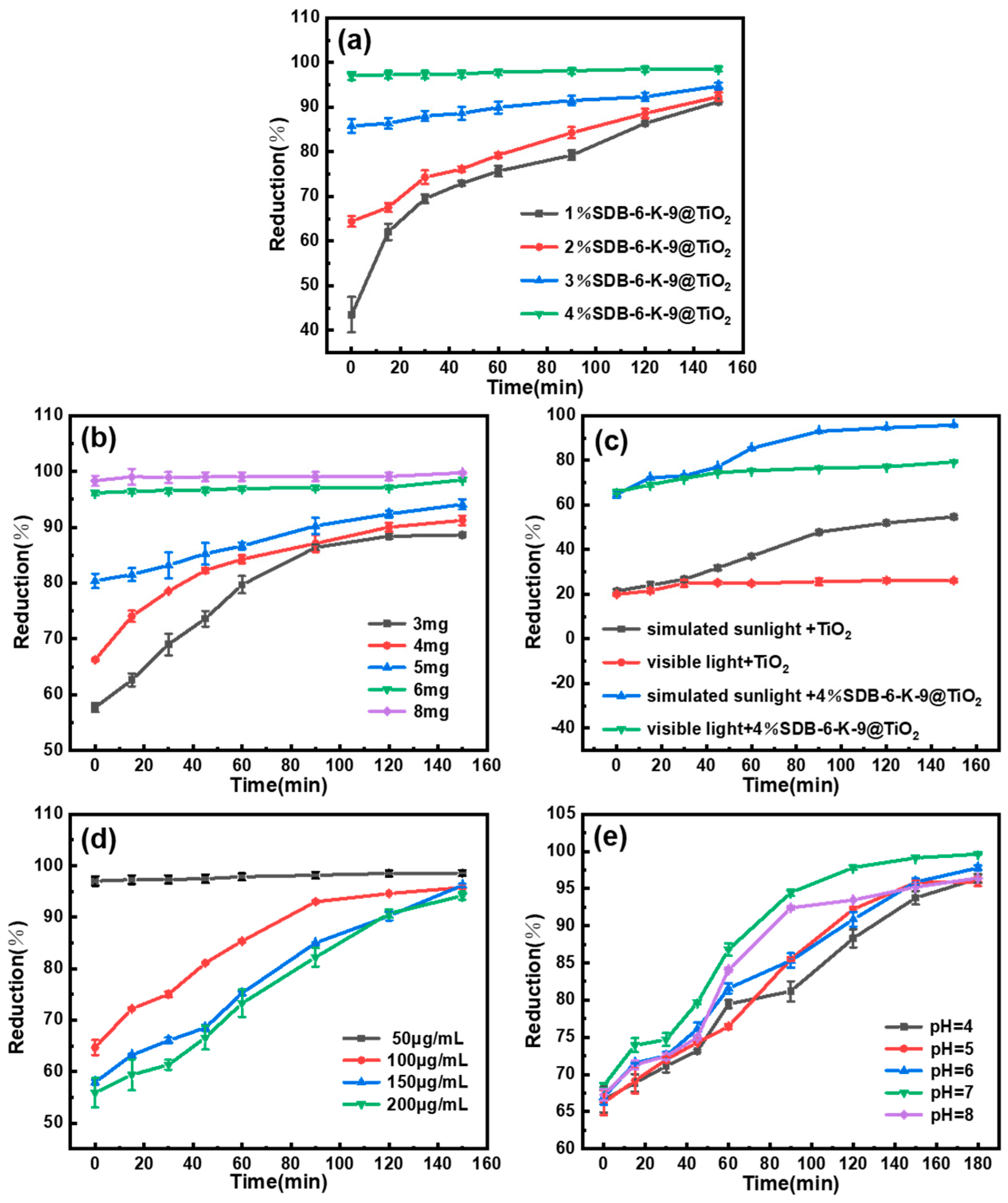
2.2. Degradation Studies
2.3. Photocatalytic Kinetics
2.4. Recycling Performance
2.5. The Photocatalytic Degradation Mechanism
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. The Preparation and Characterization of the SDB-6-K-9@TiO2 Composites
4.3. The Photocatalytic Degradation Tests
4.4. The Examination of the Photocatalytic Mechanism
4.5. SDB-6-K-9@TiO2 Photocatalytic Recycling
4.6. The Determination of the AFB1 and Photocatalytic Degradation Products
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Luo, J.; Fan, J.; Chen, X.; Wan, Y. Aflatoxin B1 removal by multifunctional membrane based on polydopamine intermediate layer. Sep. Purif. Technol. 2018, 199, 311–319. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Shi, H.; Keener, K.M. A review of novel physical and chemical decontamination technologies for aflatoxin in food. Trends Food Sci. Technol. 2018, 71, 73–83. [Google Scholar] [CrossRef]
- Li, P.; Wang, S.; Lv, B.; Zhang, M.; Xing, C.; Sun, X.; Fang, Y. Magnetic rice husk-based biochar for removal of aflatoxin B1 from peanut oil. Food Control 2023, 152, 109883. [Google Scholar] [CrossRef]
- Ying, Z.; Zhang, T.; Li, H.; Liu, X. Adsorptive removal of aflatoxin B1 from contaminated peanut oil via magnetic porous biochar from soybean dreg. Food Chem. 2023, 409, 135321. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, H.; Cao, S.; Chen, J.; Qu, C.; Tang, Y.; Wang, M.; Zhu, L.; Liu, X.; Zhang, J. A magnetic reduced graphene oxide nanocomposite: Synthesis, characterization, and application for high-efficiency detoxification of aflatoxin B1. Toxins 2024, 16, 57. [Google Scholar] [CrossRef]
- Mao, J.; He, B.; Zhang, L.; Li, P.; Zhang, Q.; Ding, X.; Zhang, W. A structure identification and toxicity assessment of the degradation products of aflatoxin B1 in peanut oil under UV irradiation. Toxins 2016, 8, 332. [Google Scholar] [CrossRef]
- Cancelliere, R.; Di Tinno, A.; Cataldo, A.; Bellucci, S.; Kumbhat, S.; Micheli, L. Nafion-based label-free immunosensor as a reliable warning system: The case of AFB1 detection in cattle feed. Microchem. J. 2023, 191, 108868. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, L.; Yamdeu, J.H.G.; Gong, Y.Y.; Orfila, C. Adsorption of aflatoxin B1 to corn by-products. Food Chem. 2024, 440, 138212. [Google Scholar] [CrossRef]
- Bian, Z.; Zhu, J.; Li, H. Solvothermal alcoholysis synthesis of hierarchical TiO2 with enhanced activity in environmental and energy photocatalysis. J. Photochem. Photobiol. C 2016, 28, 72–86. [Google Scholar] [CrossRef]
- Xie, X.; Li, S.; Zhang, H.; Wang, Z.; Huang, H. Promoting charge separation of biochar-based Zn-TiO2/pBC in the presence of ZnO for efficient sulfamethoxazole photodegradation under visible light irradiation. Sci. Total Environ. 2019, 659, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Peng, J.; Zheng, Y.; He, X.; Hou, Y.; Wu, L.; Fu, X. Enhanced photocatalytic ozonation degradation of organic pollutants by ZnO modified TiO2 nanocomposites. Appl. Catal. B 2018, 221, 223–234. [Google Scholar] [CrossRef]
- Zhu, L.; Kong, X.; Yang, C.; Ren, B.; Tang, Q. Fabrication and characterization of the magnetic separation photocatalyst C-TiO2@Fe3O4/AC with enhanced photocatalytic performance under visible light irradiation. J. Hazard. Mater. 2020, 381, 120910. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Yao, W.; Zhu, W.; Tang, Y.; Ge, H.; Shi, X.; Duan, T. Fe-N co-doped SiO2@TiO2 yolk-shell hollow nanospheres with enhanced visible light photocatalytic degradation. Appl. Surf. Sci. 2018, 444, 355–363. [Google Scholar] [CrossRef]
- Komatsuda, S.; Asakura, Y.; Vequizo, J.J.M.; Yamakata, A.; Yin, S. Enhanced photocatalytic NO, decomposition of visible-light responsive F-TiO2/(N, C)-TiO2 by charge transfer between F-TiO2 and (N, C)-TiO2 through their doping levels. Appl. Catal. B 2018, 238, 358–364. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Chen, Y.; Zhang, J.; Guo, P.; Liu, M.; Lu, X.; Zhang, J.; Wang, Z. Rational design of TiO2@ nitrogen-doped carbon coaxial nanotubes as anode for advanced lithium ion batteries. Appl. Surf. Sci. 2018, 458, 1018–1025. [Google Scholar] [CrossRef]
- Nguyen, C.-C.; Nguyen, D.T.; Do, T.-O. A novel route to synthesize C/Pt/TiO2 phase tunable anatase-Rutile TiO2 for efficient sunlight-driven photocatalytic applications. Appl. Catal. B 2018, 226, 46–52. [Google Scholar] [CrossRef]
- Khalid, N.R.; Ahmed, E.; Ahmad, M.; Niaz, N.A.; Ramzan, M.; Shakil, M.; Iqbal, T.; Majid, A. Microwave-assisted synthesis of Ag-TiO2/graphene composite for hydrogen production under visible light irradiation. Ceram. Int. 2016, 42, 18257–18263. [Google Scholar] [CrossRef]
- Martins, A.C.; Cazetta, A.L.; Pezoti, O.; Souza, J.R.B.; Zhang, T.; Pilau, E.J.; Asefa, T.; Almeida, V.C. Sol-gel synthesis of new TiO2/activated carbon photocatalyst and its application for degradation of tetracycline. Ceram. Int. 2017, 43, 4411–4418. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Jonnalagadda, S.B. A review on novel composites of mwcnts mediated semiconducting materials as photocatalysts in water treatment. Sci. Total Environ. 2019, 646, 1398–1412. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, R.; Xie, Y.; Liu, Y. Reduction of aflatoxin B1 by magnetic graphene oxide/TiO2 nanocomposite and its effect on quality of corn oil. Food Chem. 2021, 343, 128521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Li, R.; Guo, J.; Li, Y.; Zhu, J.; Xie, X. TiO2 supported on reed straw biochar as an adsorptive and photocatalytic composite for the efficient degradation of sulfamethoxazole in aqueous matrices. Chemosphere 2017, 185, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Penas-Garzon, M.; Gomez-Aviles, A.; Belver, C.; Rodriguez, J.J.; Bedia, J. Degradation pathways of emerging contaminants using TiO2-activated carbon heterostructures in aqueous solution under simulated solar light. Chem. Eng. J. 2020, 392, 124867. [Google Scholar] [CrossRef]
- Matos, J.; Marino, T.; Molinari, R.; Garcia, H. Hydrogen photoproduction under visible irradiation of Au-TiO2/activated carbon. Appl. Catal. A 2012, 417, 263–272. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, W.; Yan, J.; Han, L.; Chen, Y.; Ouyang, D.; Chen, M. Nanoscale zero-valent iron supported by biochars produced at different temperatures: Synthesis mechanism and effect on Cr (VI) removal. Environ. Pollut. 2017, 223, 153–160. [Google Scholar] [CrossRef]
- Cancelliere, R.; Cianciaruso, M.; Carbone, K.; Micheli, L. Biochar: A sustainable alternative in the development of electrochemical printed platforms. Chemosensors 2022, 10, 344. [Google Scholar] [CrossRef]
- Cancelliere, R.; Cosio, T.; Campione, E.; Corvino, M.; D’Amico, M.P.; Micheli, L.; Signori, E.; Contini, G. Label-free electrochemical immunosensor as a reliable point-of-care device for the detection of interleukin-6 in serum samples from patients with psoriasis. Front. Chem. 2023, 11, 1251360. [Google Scholar] [CrossRef]
- Jamil, T.S.; Ghaly, M.Y.; Fathy, N.A.; Abd el-Halim, T.A.; Osterlund, L. Enhancement of TiO2 behavior on photocatalytic oxidation of mo dye using TiO2/AC under visible irradiation and sunlight radiation. Sep. Purif. Technol. 2012, 98, 270–279. [Google Scholar] [CrossRef]
- Omri, A.; Benzina, M. Influence of the origin of carbon support on the structure and properties of TiO2 nanoparticles prepared by dip coating method. Arabian J. Chem. 2019, 12, 2926–2936. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef] [PubMed]
- Nadimi, M.; Saravani, A.Z.; Aroon, M.A.; Pirbazari, A.E. Photodegradation of methylene blue by a ternary magnetic TiO2/Fe3O4/graphene oxide nanocomposite under visible light. Mater. Chem. Phys. 2019, 225, 464–474. [Google Scholar] [CrossRef]
- Liang, Y.; He, X.; Chen, L.; Zhang, Y. Preparation and characterization of TiO2-Graphene@ Fe3O4 magnetic composite and its application in the removal of trace amounts of microcystin-lr. RSC Adv. 2014, 4, 56883–56891. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, J.; Zhu, B.; Yu, J.; Xu, J. CuInS2 sensitized TiO2 hybrid nanofibers for improved photocatalytic CO2 reduction. Appl. Catal. B 2018, 230, 194–202. [Google Scholar] [CrossRef]
- Lu, L.; Shan, R.; Shi, Y.; Wang, S.; Yuan, H. A novel TiO2/biochar composite catalysts for photocatalytic degradation of methyl orange. Chemosphere 2019, 222, 391–398. [Google Scholar] [CrossRef]
- Ying, Z.; Zhao, D.; Li, H.; Liu, X.; Zhang, J. Efficient adsorption of deoxynivalenol by porous carbon prepared from soybean dreg. Toxins 2021, 13, 500. [Google Scholar] [CrossRef]
- Silvestri, S.; Goncalves, M.G.; da Silva Veiga, P.A.; da Silva Matos, T.T.; Peralta-Zamora, P.; Mangrich, A.S. TiO2 supported on Salvinia molesta biochar for heterogeneous photocatalytic degradation of acid orange 7 dye. J. Environ. Chem. Eng. 2019, 7, 102879. [Google Scholar] [CrossRef]
- Jia, T.; Fu, F.; Yu, D.; Cao, J.; Sun, G. Facile synthesis and characterization of N-doped TiO2/C nanocomposites with enhanced visible-light photocatalytic performance. Appl. Surf. Sci. 2018, 430, 438–447. [Google Scholar] [CrossRef]
- Tayel, A.; Ramadan, A.R.; El Seoud, O.A. Titanium dioxide/graphene and titanium dioxide/graphene oxide nanocomposites: Synthesis, characterization and photocatalytic applications for water decontamination. Catalysts 2018, 8, 491. [Google Scholar] [CrossRef]
- Alalm, M.G.; Tawfik, A.; Ookawara, S. Enhancement of photocatalytic activity of TiO2 by immobilization on activated carbon for degradation of pharmaceuticals. J. Environ. Chem. Eng. 2016, 4, 1929–1937. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, R.; Xie, Y.; Liu, Y. Photocatalytic degradation of aflatoxin B1 by activated carbon supported TiO2 catalyst. Food Control 2019, 100, 183–188. [Google Scholar] [CrossRef]
- Liu, R.; Jin, Q.; Huang, J.; Liu, Y.; Wang, X.; Mao, W.; Wang, S. Photodegradation of aflatoxin B1 in peanut oil. Eur. Food Res. Technol. 2011, 232, 843–849. [Google Scholar] [CrossRef]
- Jamil, T.S.; Abbas, H.A.; Nasr, R.A.; El-Kady, A.A.; Ibrahim, M.I.M. Detoxification of aflatoxin B1 using nano-sized Sc-doped SrTi0.7Fe0.3O3 under visible light. J. Photochem. Photobiol. A 2017, 341, 127–135. [Google Scholar] [CrossRef]
- Magzoub, R.A.M.; Yassin, A.A.A.; Abdel-Rahim, A.M.; Gubartallah, E.A.; Miskam, M.; Saad, B.; Sabar, S. Photocatalytic detoxification of aflatoxins in sudanese peanut oil using immobilized titanium dioxide. Food Control 2019, 95, 206–214. [Google Scholar] [CrossRef]

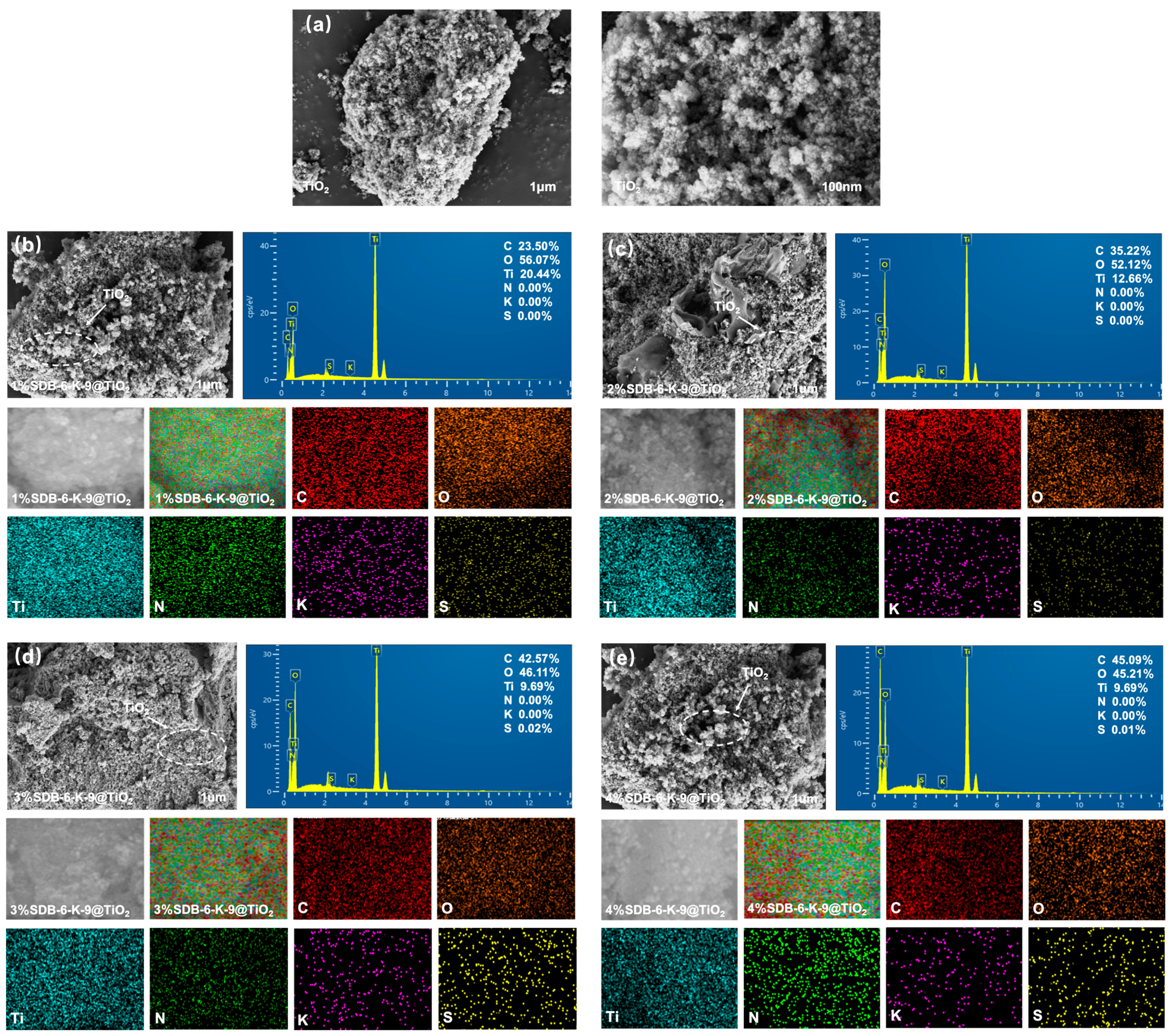


| Sample | Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Particle Size (nm) |
|---|---|---|---|
| TiO2 | 174.91 ± 6.32 | 0.5812 ± 0.0112 | 1.329 ± 0.039 |
| 1%SDB-6-K-9@TiO2 | 294.57 ± 8.61 | 0.5313 ± 0.0032 | 8.207 ± 0.075 |
| 2%SDB-6-K-9@TiO2 | 399.16 ± 4.75 | 0.6044 ± 0.0124 | 7.561 ± 0.037 |
| 3%SDB-6-K-9@TiO2 | 468.28 ± 9.46 | 0.6271 ± 0.0289 | 4.539 ± 0.029 |
| 4%SDB-6-K-9@TiO2 | 642.64 ± 10.53 | 0.7799 ± 0.0247 | 3.903 ± 0.036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Ying, Z.; Li, H.; Liu, X.; Ma, D.; Yu, H. Preparation of Soybean Dreg-Based Biochar@TiO2 Composites and the Photocatalytic Degradation of Aflatoxin B1 Exposed to Simulated Sunlight Irradiation. Toxins 2024, 16, 429. https://doi.org/10.3390/toxins16100429
Zhang J, Ying Z, Li H, Liu X, Ma D, Yu H. Preparation of Soybean Dreg-Based Biochar@TiO2 Composites and the Photocatalytic Degradation of Aflatoxin B1 Exposed to Simulated Sunlight Irradiation. Toxins. 2024; 16(10):429. https://doi.org/10.3390/toxins16100429
Chicago/Turabian StyleZhang, Jian, Zhiwei Ying, He Li, Xinqi Liu, Dongge Ma, and Hailong Yu. 2024. "Preparation of Soybean Dreg-Based Biochar@TiO2 Composites and the Photocatalytic Degradation of Aflatoxin B1 Exposed to Simulated Sunlight Irradiation" Toxins 16, no. 10: 429. https://doi.org/10.3390/toxins16100429
APA StyleZhang, J., Ying, Z., Li, H., Liu, X., Ma, D., & Yu, H. (2024). Preparation of Soybean Dreg-Based Biochar@TiO2 Composites and the Photocatalytic Degradation of Aflatoxin B1 Exposed to Simulated Sunlight Irradiation. Toxins, 16(10), 429. https://doi.org/10.3390/toxins16100429







