Inhibition of Aflatoxin B1 Production by Procyanidins Present in Annona muricata and Uncaria tomentosa Aqueous Extracts
Abstract
:1. Introduction
2. Results and Discussion
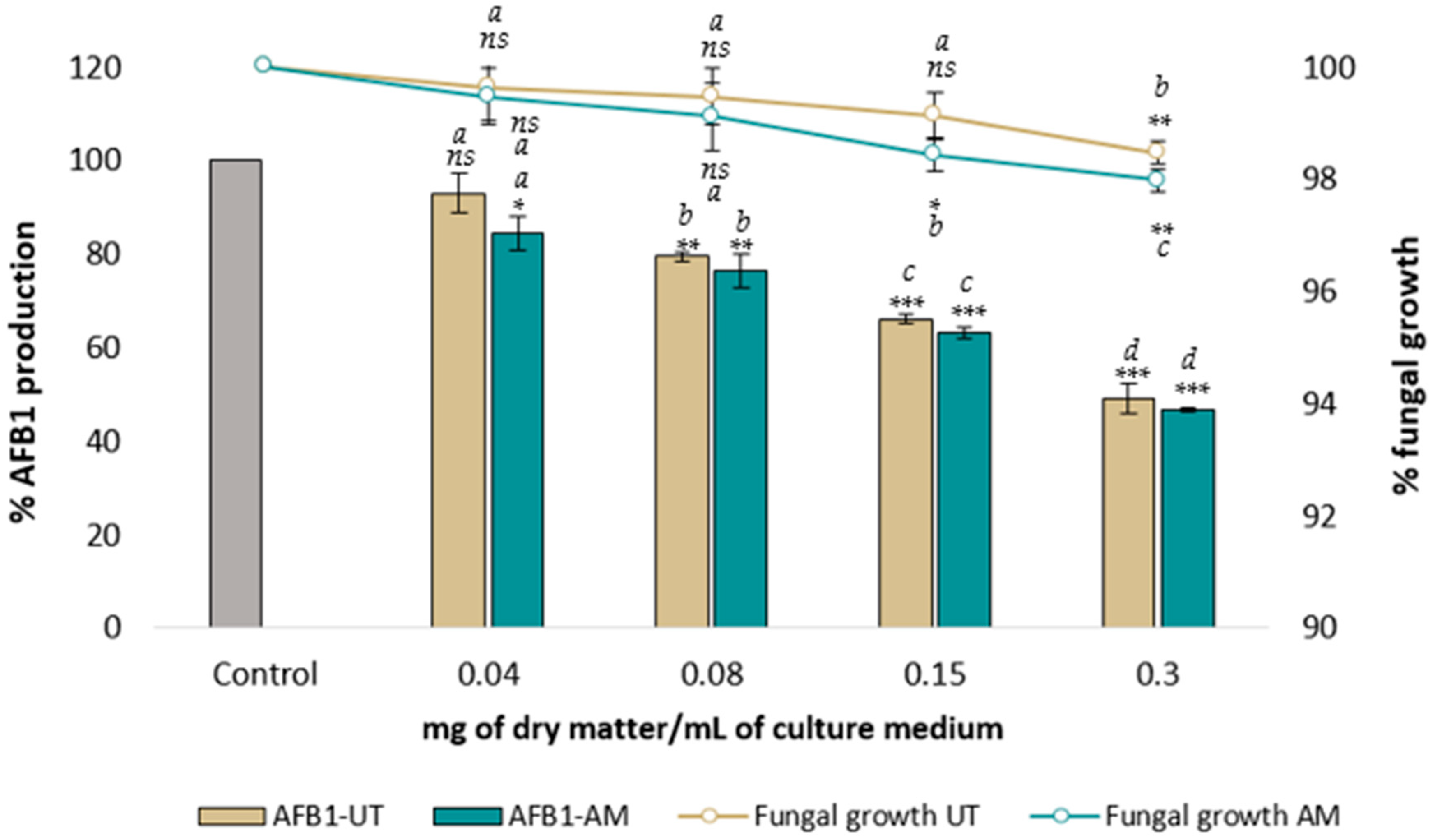
2.1. Effect of AM and UT Aqueous Extracts on A. flavus Growth and AFB1 Synthesis
2.2. Characterization and Fractionation of AM and UT Extracts
2.2.1. Composition of Extracts and Fractions
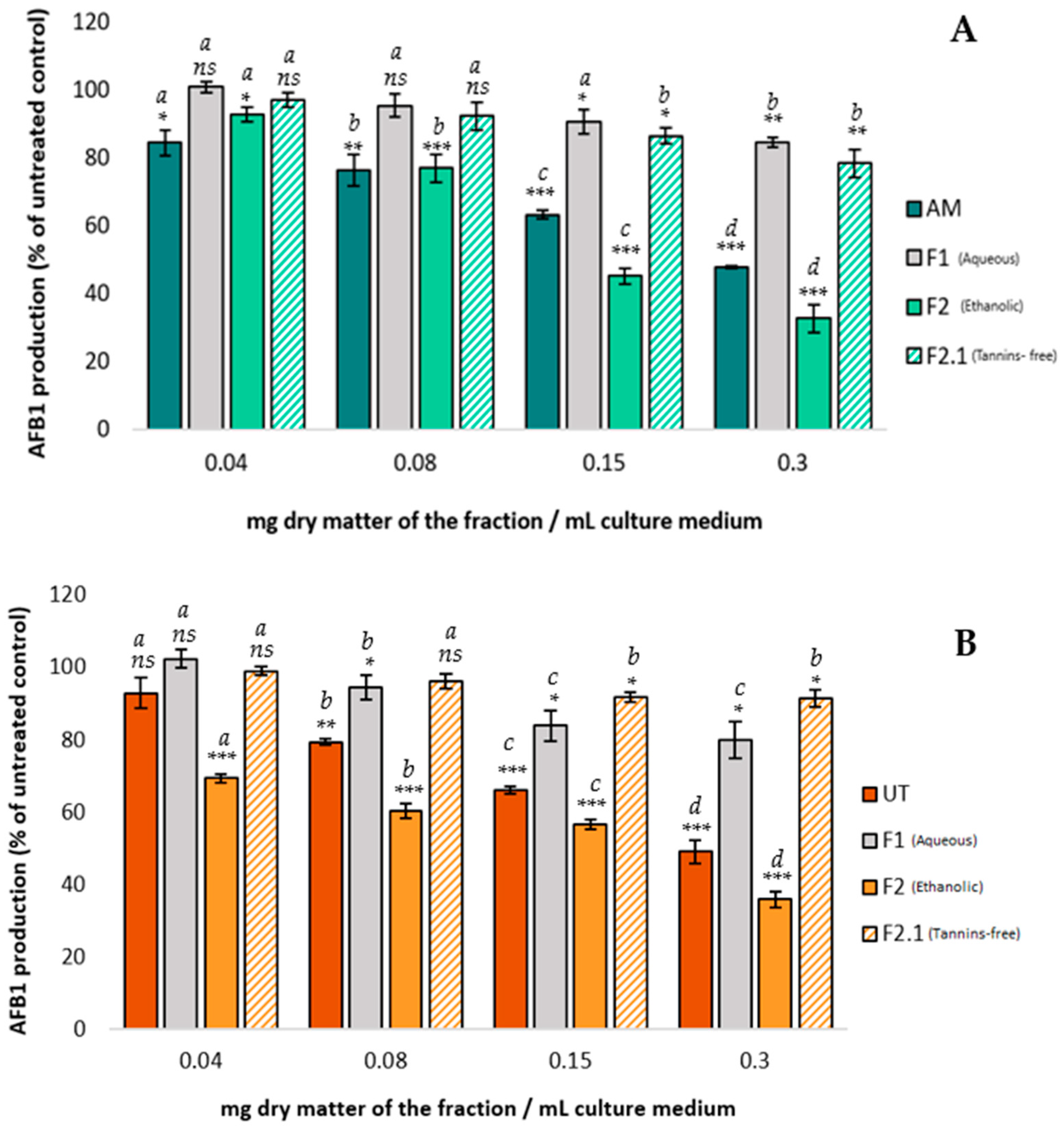
2.2.2. Impact of AM and UT Fractions on AFB1 Production
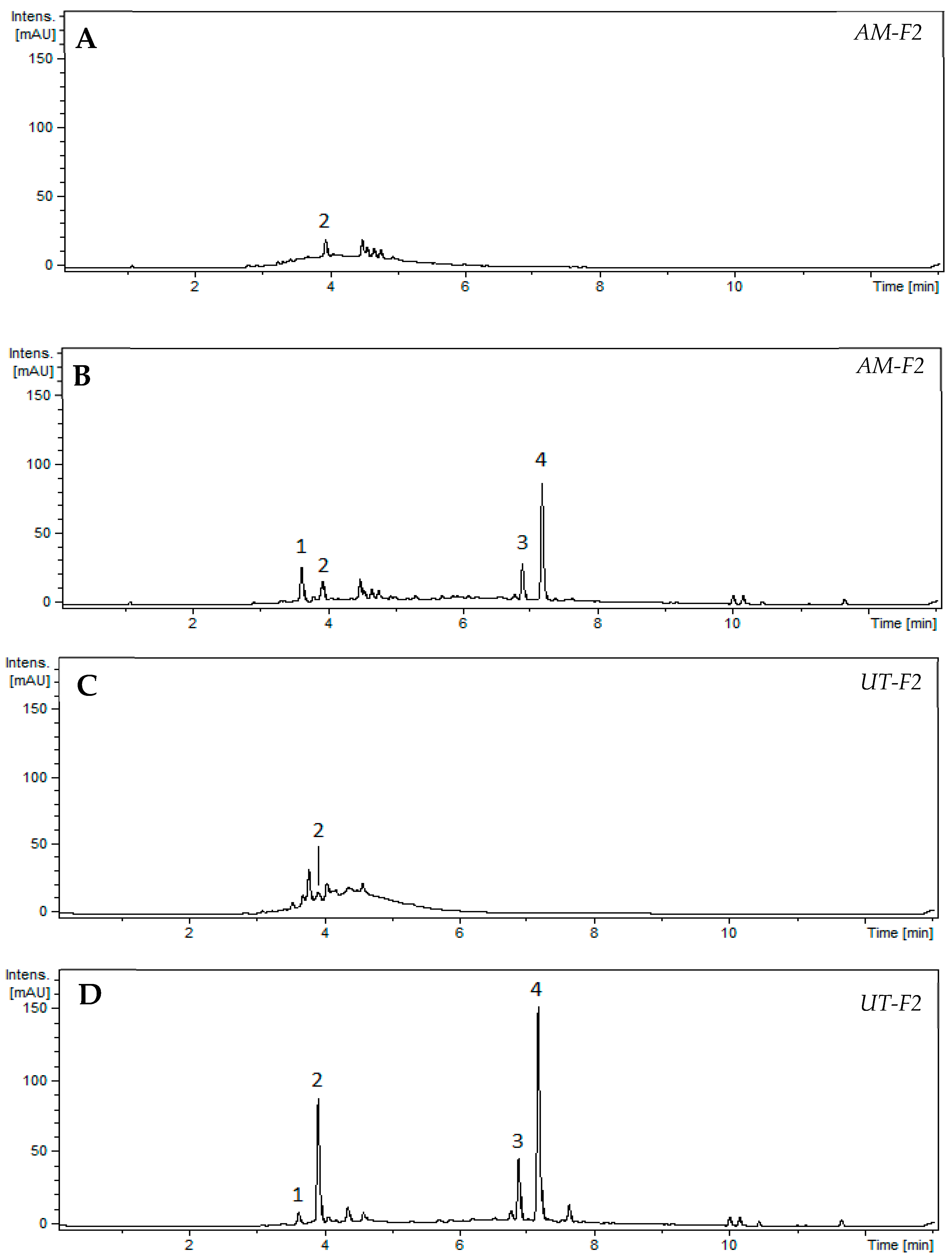
2.2.3. Identification of the Primary Condensed Tannins in the Ethanolic Fractions of AM and UT
2.2.4. Effect of Catechin and Epicatechin on AFB1 Synthesis
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Preparation of Aqueous Extracts
4.4. Fractionation of Extracts
4.5. PVPP Fractionation
4.6. Characterization of Extracts and Fractions
4.6.1. Dry Matter Content
4.6.2. Dosage of Total Phenolic Content
4.6.3. Condensed Tannin Content
4.6.4. Dosage of Free Radical Scavenging Activity
4.7. Characterization of Condensed Tannins in the Ethanolic Fraction of AM and UT Extracts
4.7.1. Depolymerization of Condensed Tannins from Fraction 2
4.7.2. UHPLC-DAD-MS Analyses
4.7.3. Identification of Peaks and Quantification of Products
4.8. Effect of U. tomentosa and A. muricata Extracts and Fractions on Aspergillus flavus Growth and Aflatoxin B1 Synthesis
4.8.1. Fungal Strain and Culture Conditions
4.8.2. Aflatoxin Extraction and HPLC Quantification
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IARC. Fungi Producing Significant Mycotoxins; IARC Scientific Publications: Lyon, France, 2012; pp. 1–30. [Google Scholar]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- EFSA. The 2021 European Union Report on Pesticide Residues in Food. EFSA J. 2018, 21, e07939. [Google Scholar] [CrossRef]
- Dikhoba, P.M.; Mongalo, N.I.; Elgorashi, E.E.; Makhafola, T.J. Antifungal and Anti-Mycotoxigenic Activity of Selected South African Medicinal Plants Species. Heliyon 2019, 5, e02668. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, R.; Caceres, I.; Puel, O.; Bailly, S.; Atoui, A.; Oswald, I.P.; El Khoury, A.; Bailly, J.-D. Identification of the Anti-Aflatoxinogenic Activity of Micromeria graeca and Elucidation of Its Molecular Mechanism in Aspergillus flavus. Toxins 2017, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Cadenillas, L.F.; Hernandez, C.; Mathieu, C.; Bailly, J.-D.; Durrieu, V. Screening of the Anti-Aflatoxin B1 Activity of Peruvian Plant Extracts: Relation with Their Composition. Food Bioprocess Technol. 2023, 16, 1324–1334. [Google Scholar] [CrossRef]
- Bluma, R.; Amaiden, M.R.; Etcheverry, M. Screening of Argentine Plant Extracts: Impact on Growth Parameters and Aflatoxin B1 Accumulation by Aspergillus Section Flavi. Int. J. Food Microbiol. 2008, 122, 114–125. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.; Zhu, X.; Xie, Y. Antifungal Efficacy of Paeonol on Aspergillus flavus and Its Mode of Action on Cell Walls and Cell Membranes. LWT 2021, 149, 111985. [Google Scholar] [CrossRef]
- Nobili, C.; De Acutis, A.; Reverberi, M.; Bello, C.; Leone, G.P.; Palumbo, D.; Natella, F.; Procacci, S.; Zjalic, S.; Brunori, A. Buckwheat Hull Extracts Inhibit Aspergillus flavus Growth and AFB1 Biosynthesis. Front. Microbiol. 2019, 10, 1997. [Google Scholar] [CrossRef]
- Moon, Y.-S.; Lee, H.-S.; Lee, S.-E. Inhibitory Effects of Three Monoterpenes from Ginger Essential Oil on Growth and Aflatoxin Production of Aspergillus flavus and Their Gene Regulation in Aflatoxin Biosynthesis. Appl. Biol. Chem. 2018, 61, 243–250. [Google Scholar] [CrossRef]
- Ribeiro, L.P.; Domingues, V.C.; Gonçalves, G.L.P.; Fernandes, J.B.; Glória, E.M.; Vendramim, J.D. Essential Oil from Duguetia lanceolata St.-Hil. (Annonaceae): Suppression of Spoilers of Stored-Grain. Food Biosci. 2020, 36, 100653. [Google Scholar] [CrossRef]
- Loi, M.; Paciolla, C.; Logrieco, A.F.; Mulè, G. Plant Bioactive Compounds in Pre- and Postharvest Management for Aflatoxins Reduction. Front. Microbiol. 2020, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Caceres, I.; Al Khoury, A.; El Khoury, R.; Lorber, S.; Oswald, I.P.; El Khoury, A.; Atoui, A.; Puel, O.; Bailly, J.-D. Aflatoxin Biosynthesis and Genetic Regulation: A Review. Toxins 2020, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Jin, L.; Niu, Y.D.; Huang, Q.; McAllister, T.A.; Yang, H.E.; Denise, H.; Xu, Z.; Acharya, S.; Wang, S.; et al. Condensed Tannins Affect Bacterial and Fungal Microbiomes and Mycotoxin Production during Ensiling and upon Aerobic Exposure. Appl. Environ. Microbiol. 2018, 84, e02274-17. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Cadenillas, L.; Maghubi, A.E.; Caceres, I.; Durrieu, V.; Mathieu, C.; Bailly, J.-D. Mimosa tenuiflora Aqueous Extract: Role of Condensed Tannins in Anti-Aflatoxin B1 Activity in Aspergillus flavus. Toxins 2021, 13, 391. [Google Scholar] [CrossRef]
- Wahab, S.M.A.; Jantan, I.; Haque, M.A.; Arshad, L. Exploring the Leaves of Annona muricata L. as a Source of Potential Anti-Inflammatory and Anticancer Agents. Front. Pharmacol. 2018, 9, 661. [Google Scholar] [CrossRef]
- Adewole, S.; Ojewole, J. Protective Effects of Annona muricata Linn. (Annonaceae) Leaf Aqueous Extract on Serum Lipid Profiles and Oxidative Stress in Hepatocytes of Streptozotocin-Treated Diabetic Rats. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 30–41. [Google Scholar] [CrossRef]
- Hajdu, Z.; Hohmann, J. An Ethnopharmacological Survey of the Traditional Medicine Utilized in the Community of Porvenir, Bajo Paraguá Indian Reservation, Bolivia. J. Ethnopharmacol. 2012, 139, 838–857. [Google Scholar] [CrossRef]
- Balderrama-Carmona, A.P.; Silva-Beltrán, N.P.; Gálvez-Ruiz, J.-C.; Ruíz-Cruz, S.; Chaidez-Quiroz, C.; Morán-Palacio, E.F. Antiviral, Antioxidant, and Antihemolytic Effect of Annona muricata L. Leaves Extracts. Plants 2020, 9, 1650. [Google Scholar] [CrossRef]
- Santos, I.L.; Rodrigues, A.M.d.C.; Amante, E.R.; Silva, L.H.M. Soursop (Annona muricata) Properties and Perspectives for Integral Valorization. Foods 2023, 12, 1448. [Google Scholar] [CrossRef]
- Azevedo, B.C.; Morel, L.J.F.; Carmona, F.; Cunha, T.M.; Contini, S.H.T.; Delprete, P.G.; Ramalho, F.S.; Crevelin, E.; Bertoni, B.W.; França, S.C.; et al. Aqueous Extracts from Uncaria tomentosa (Willd. Ex Schult.) DC. Reduce Bronchial Hyperresponsiveness and Inflammation in a Murine Model of Asthma. J. Ethnopharmacol. 2018, 218, 76–89. [Google Scholar] [CrossRef]
- Ccahuana-Vasquez, R.A.; Santos, S.S.F.d.; Koga-Ito, C.Y.; Jorge, A.O.C. Antimicrobial Activity of Uncaria tomentosa against Oral Human Pathogens. Braz. Oral Res. 2007, 21, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Snow, A.D.; Castillo, G.M.; Nguyen, B.P.; Choi, P.Y.; Cummings, J.A.; Cam, J.; Hu, Q.; Lake, T.; Pan, W.; Kastin, A.J.; et al. The Amazon Rain Forest Plant Uncaria tomentosa (Cat’s Claw) and Its Specific Proanthocyanidin Constituents Are Potent Inhibitors and Reducers of Both Brain Plaques and Tangles. Sci. Rep. 2019, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Peñaloza, E.M.C.; Kaiser, S.; Resende, P.E.d.; Pittol, V.; Carvalho, Â.R.; Ortega, G.G. Chemical Composition Variability in the Uncaria tomentosa (Cat’s Claw) Wild Population. Quím. Nova 2015, 38, 378–386. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Magdy Beshbishy, A.; Wasef, L.; Elewa, Y.H.A.; Abd El-Hack, M.E.; Taha, A.E.; Al-Sagheer, A.A.; Devkota, H.P.; Tufarelli, V. Uncaria tomentosa (Willd. Ex Schult.) DC.: A Review on Chemical Constituents and Biological Activities. Appl. Sci. 2020, 10, 2668. [Google Scholar] [CrossRef]
- Shukla, R.; Singh, P.; Prakash, B.; Anuradha; Dubey, N.K. Antifungal, Aflatoxin Inhibitory and Free Radical-Scavenging Activities of Some Medicinal Plants Extracts. J. Food Qual. 2012, 35, 182–189. [Google Scholar] [CrossRef]
- Srivastava, B.; Singh, P.; Srivastava, A.K.; Shukla, R.; Dubey, N.K. Efficacy of Artabotrys odoratissimus Oil as a Plant Based Antimicrobial against Storage Fungi and Aflatoxin B1 Secretion. Int. J. Food Sci. 2009, 44, 1909–1915. [Google Scholar] [CrossRef]
- Muñoz-Acevedo, A.; Méndez, L.Y.V.; Stashenko, E.E.; Kouznetsov, V. Improved Trolox® Equivalent Antioxidant Capacity Assay for Efficient and Fast Search of New Antioxidant Agents. Anal. Chem. Lett. 2011, 1, 86–102. [Google Scholar] [CrossRef]
- Choi, C.W.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Park, S.H.; Kim, S.K. Antioxidant Activity and Free Radical Scavenging Capacity between Korean Medicinal Plants and Flavonoids by Assay-Guided Comparison. Plant Sci. 2002, 163, 1161–1168. [Google Scholar] [CrossRef]
- Nam, J.-S.; Park, S.-Y.; Jang, H.-L.; Rhee, Y.H. Phenolic Compounds in Different Parts of Young Annona muricata Cultivated in Korea and Their Antioxidant Activity. Appl. Biol. Chem. 2017, 60, 535–543. [Google Scholar] [CrossRef]
- Orak, H.H.; Bahrisefit, I.S.; Sabudak, T. Antioxidant Activity of Extracts of Soursop (Annona muricata L.) Leaves, Fruit Pulps, Peels and Seeds. Pol. J. Food Nutr. Sci. 2019, 69, 359–366. [Google Scholar] [CrossRef]
- Pineda-Ramírez, N.; Calzada, F.; Alquisiras-Burgos, I.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Ortiz-Plata, A.; Pinzón Estrada, E.; Torres, I.; Aguilera, P. Antioxidant Properties and Protective Effects of Some Species of the Annonaceae, Lamiaceae, and Geraniaceae Families against Neuronal Damage Induced by Excitotoxicity and Cerebral Ischemia. Antioxidants 2020, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.; Dinis, T.; Batista, M.T. Antioxidant Properties of Proanthocyanidins of Uncaria tomentosa Bark Decoction: A Mechanism for Anti-Inflammatory Activity. Phytochemistry 2005, 66, 89–98. [Google Scholar] [CrossRef]
- Tomsone, L.; Kruma, Z.; Galoburda, R. Comparison of Different Solvents and Extraction Methods for Isolation of Phenolic Compounds from Horseradish Roots (Armoracia rusticana). J. Agric. Biol. Eng. 2012, 6, 236–241. [Google Scholar]
- Sharif, M.F.; Bennett, M.T. The Effect of Different Methods and Solvents on the Extraction of Polyphenols in Ginger (Zingiber officinale). J. Teknol. 2016, 78, 11-2. [Google Scholar] [CrossRef]
- Du, G.; Li, M.; Ma, F.; Liang, D. Antioxidant Capacity and the Relationship with Polyphenol and Vitamin C in Actinidia Fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Kiselova, Y.; Ivanova, D.; Chervenkov, T.; Gerova, D.; Galunska, B.; Yankova, T. Correlation between the In Vitro Antioxidant Activity and Polyphenol Content of Aqueous Extracts from Bulgarian Herbs. Phytother. Res. 2006, 20, 961–965. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.M.; Kim, J.H.; Oh, Y.S.; Joo, D.H.; Lee, E.Y.; Shin, H.S.; Kim, A.R.; Lee, S.L.; Park, S.N. Antioxidative Effects and Component Analysis of Graviola (Annona muricata) Leaf Extract/Fractions. J. Soc. Cosmet. Sci. Korea 2017, 43, 309–320. [Google Scholar] [CrossRef]
- Navarro-Hoyos, M.; Lebrón-Aguilar, R.; Quintanilla-López, J.E.; Cueva, C.; Hevia, D.; Quesada, S.; Azofeifa, G.; Moreno-Arribas, M.V.; Monagas, M.; Bartolomé, B. Proanthocyanidin Characterization and Bioactivity of Extracts from Different Parts of Uncaria tomentosa L. (Cat’s Claw). Antioxidants 2017, 6, 12. [Google Scholar] [CrossRef]
- Caceres, I.; El Khoury, R.; Bailly, S.; Oswald, I.P.; Puel, O.; Bailly, J.-D. Piperine Inhibits Aflatoxin B1 Production in Aspergillus flavus by Modulating Fungal Oxidative Stress Response. Fungal Genet. Biol. 2017, 107, 77–85. [Google Scholar] [CrossRef]
- Prakash, B.; Shukla, R.; Singh, P.; Kumar, A.; Mishra, P.K.; Dubey, N.K. Efficacy of Chemically Characterized Piper betle L. Essential Oil against Fungal and Aflatoxin Contamination of Some Edible Commodities and Its Antioxidant Activity. Int. J. Food Microbiol. 2010, 142, 114–119. [Google Scholar] [CrossRef]
- Ravinayagam, V.; Jaganathan, R.; Panchanadham, S.; Palanivelu, S. Potential Antioxidant Role of Tridham in Managing Oxidative Stress against Aflatoxin-B1-Induced Experimental Hepatocellular Carcinoma. Int. J. Hepatol. 2012, 2012, 428373. [Google Scholar] [CrossRef] [PubMed]
- Narasaiah, K.V.; Sashidhar, R.B.; Subramanyam, C. Biochemical Analysis of Oxidative Stress in the Production of Aflatoxin and Its Precursor Intermediates. Mycopathologia 2006, 162, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Iram, W.; Anjum, T.; Iqbal, M.; Ghaffar, A.; Abbas, M. Structural Elucidation and Toxicity Assessment of Degraded Products of Aflatoxin B1 and B2 by Aqueous Extracts of Trachyspermum ammi. Front. Microbiol. 2016, 7, 346. [Google Scholar] [CrossRef] [PubMed]
- Negera, M.; Washe, A. Use of Natural Dietary Spices for Reclamation of Food Quality Impairment by Aflatoxin. J. Food Qual. 2019, 2019, 4371206. [Google Scholar] [CrossRef]
- Killedar, S.; More, H. Estimation of Tannins in Different Parts of Memecylon umbellatum Burm. J. Pharm. Res. 2010, 3, 554–556. [Google Scholar]
- Navarro-Hoyos, M.; Alvarado-Corella, D.; Moreira-Gonzalez, I.; Arnaez-Serrano, E.; Monagas-Juan, M. Polyphenolic Composition and Antioxidant Activity of Aqueous and Ethanolic Extracts from Uncaria tomentosa Bark and Leaves. Antioxidants 2018, 7, 65. [Google Scholar] [CrossRef]
- Nolasco-Gonzalez, Y.; Chacon-Lopez, M.; Ortiz-Basurto, R.; Aguilera-Aguirre, S.; Gonzalez-Aguilar, G.; Rodriguez-Aguayo, C.; Navarro-Cortez, M.; Garcia-Galindo, H.; Garcia-Magaña, M.d.L.; Meza-Espinoza, L.; et al. Annona muricata Leaves as a Source of Bioactive Compounds: Extraction and Quantification Using Ultrasound. Horticulturae 2022, 8, 560. [Google Scholar] [CrossRef]
- Valdez-Guerrero, Y.D.; Esparza-González, S.; Morlett-Chávez, J.; Nery-Flores, S.; Flores-Gallegos, A.; Ascacio-Valdés, J.; Rodríguez-Herrera, R. Isolation of Polyphenols from Soursop (Annona muricata L.) Leaves Using Green Chemistry Techniques and Their Anticancer Effect. Braz. Arch. Biol. Technol. 2021, 64, e21200163. [Google Scholar] [CrossRef]
- Rubert-Nason, K.F.; Yang, P.; Morrow, C.J.; Lindroth, R.L. Environment and Genotype Influence Quantitative and Qualitative Variation in Condensed Tannins in Aspen. J. Chem. Ecol. 2023, 49, 325–339. [Google Scholar] [CrossRef]
- Top, S.M.; Preston, C.M.; Dukes, J.S.; Tharayil, N. Climate Influences the Content and Chemical Composition of Foliar Tannins in Green and Senesced Tissues of Quercus rubra. Front. Plant Sci. 2017, 8, 423. [Google Scholar] [CrossRef]
- Norton, R.A. Inhibition of Aflatoxin B1 Biosynthesis in Aspergillus flavus by Anthocyanidins and Related Flavonoids. J. Agric. Food Chem. 1999, 47, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Hu, L.B.; Zhao, Y.; Wang, M.Y.; Zhang, H.; Mo, H.Z. Inhibition of Fungal Aflatoxin B1 Biosynthesis by Diverse Botanically-Derived Polyphenols. Trop. J. Pharm. Res. 2015, 14, 605–609. [Google Scholar] [CrossRef]
- Dammak, I.; Lasram, S.; Hamdi, Z.; Ben Moussa, O.; Mkadmini Hammi, K.; Trigui, I.; Houissa, H.; Mliki, A.; Hassouna, M. In Vitro Antifungal and Anti-Ochratoxigenic Activities of Aloe Vera Gel against Aspergillus carbonarius Isolated from Grapes. Ind. Crops 2018, 123, 416–423. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Blackwell Scientific: Oxford, UK; Boston, MA, USA, 1994; ISBN 978-0-632-02969-3. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Billerach, G.; Rouméas, L.; Dubreucq, E.; Fulcrand, H. Furanolysis with Menthofuran: A New Depolymerization Method for Analyzing Condensed Tannins. J. Agric. Food Chem. 2020, 68, 2917–2926. [Google Scholar] [CrossRef]
| Fraction | Dry Matter (g) | Polyphenols Content (mg GAE/g DM) * | Antioxidant Activity IC50DPPH (mg/L) ** | Condensed Tannins (mg/g DM) |
|---|---|---|---|---|
| AM | 3.91 ± 0.01 a | 188 ± 9 a | 35 a | 137 ± 2 a |
| F1 | 1.70 ± 0.02 b | 56 ± 5 b | 310 b | Nd |
| F2 | 1.83 ± 0.04 b | 320 ± 1 b | 20 b | 220 ± 4 b |
| F2.1 | 0.21 ± 0.01 b | 10 ± 1 b | 460 b | 2 ± 1 b |
| UT | 5.31 ± 0.03 a | 226 ± 3 a | 15 a | 219 ± 5 a |
| F1 | 1.92 ± 0.01 b | 18 ± 2 b | 363 b | Nd |
| F2 | 2.24 ± 0.01 b | 530 ± 7 b | 8 b | 502 ± 6 b |
| F2.1 | 0.18 ± 0.03 b | 6 ± 1 b | >500 b | 3 ± 1 b |
| Composition in Condensed Tannins of the Tested Fractions (%w/w) | |||||
|---|---|---|---|---|---|
| Peak Number | 1 | 2 | 3 | 4 | |
| Detected compound | Catechin (terminal units) | Epicatechin (terminal units) | Catechin-Mf * (extension units) | Epicatechin-Mf * (extension units) | Total procyanidin content |
| Tested fractions | |||||
| AM-F2 | 2.8 ± 0.1 | 1.7 ± 0.1 | 2.5 ± 0.1 | 8.9 ± 0.1 | 15.9 |
| UT-F2 | 1.1 ± 0.1 | 9.3 ± 0.1 | 4.3 ± 0.1 | 16.4 ± 0.2 | 31.1 |
| Standard | Concentration of the Standard (µg/mL of Culture Medium) | Inhibition of AFB1 * (%) |
|---|---|---|
| Catechin | 5.00 | 28 ± 1 |
| 10.00 | 37 ± 2 | |
| 15.00 | 45 ± 1 | |
| Epicatechin | 6.25 | 19 ± 1 |
| 12.50 | 27 ± 2 | |
| 25.00 | 36 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadenillas, L.F.; Billerach, G.; Hernandez, C.; Durrieu, V.; Bailly, J.-D. Inhibition of Aflatoxin B1 Production by Procyanidins Present in Annona muricata and Uncaria tomentosa Aqueous Extracts. Toxins 2024, 16, 454. https://doi.org/10.3390/toxins16110454
Cadenillas LF, Billerach G, Hernandez C, Durrieu V, Bailly J-D. Inhibition of Aflatoxin B1 Production by Procyanidins Present in Annona muricata and Uncaria tomentosa Aqueous Extracts. Toxins. 2024; 16(11):454. https://doi.org/10.3390/toxins16110454
Chicago/Turabian StyleCadenillas, Laura F., Guillaume Billerach, Christopher Hernandez, Vanessa Durrieu, and Jean-Denis Bailly. 2024. "Inhibition of Aflatoxin B1 Production by Procyanidins Present in Annona muricata and Uncaria tomentosa Aqueous Extracts" Toxins 16, no. 11: 454. https://doi.org/10.3390/toxins16110454
APA StyleCadenillas, L. F., Billerach, G., Hernandez, C., Durrieu, V., & Bailly, J.-D. (2024). Inhibition of Aflatoxin B1 Production by Procyanidins Present in Annona muricata and Uncaria tomentosa Aqueous Extracts. Toxins, 16(11), 454. https://doi.org/10.3390/toxins16110454







