Effects of the Heterodimeric Neurotoxic Phospholipase A2 from the Venom of Vipera nikolskii on the Contractility of Rat Papillary Muscles and Thoracic Aortas
Abstract
:1. Introduction
2. Results
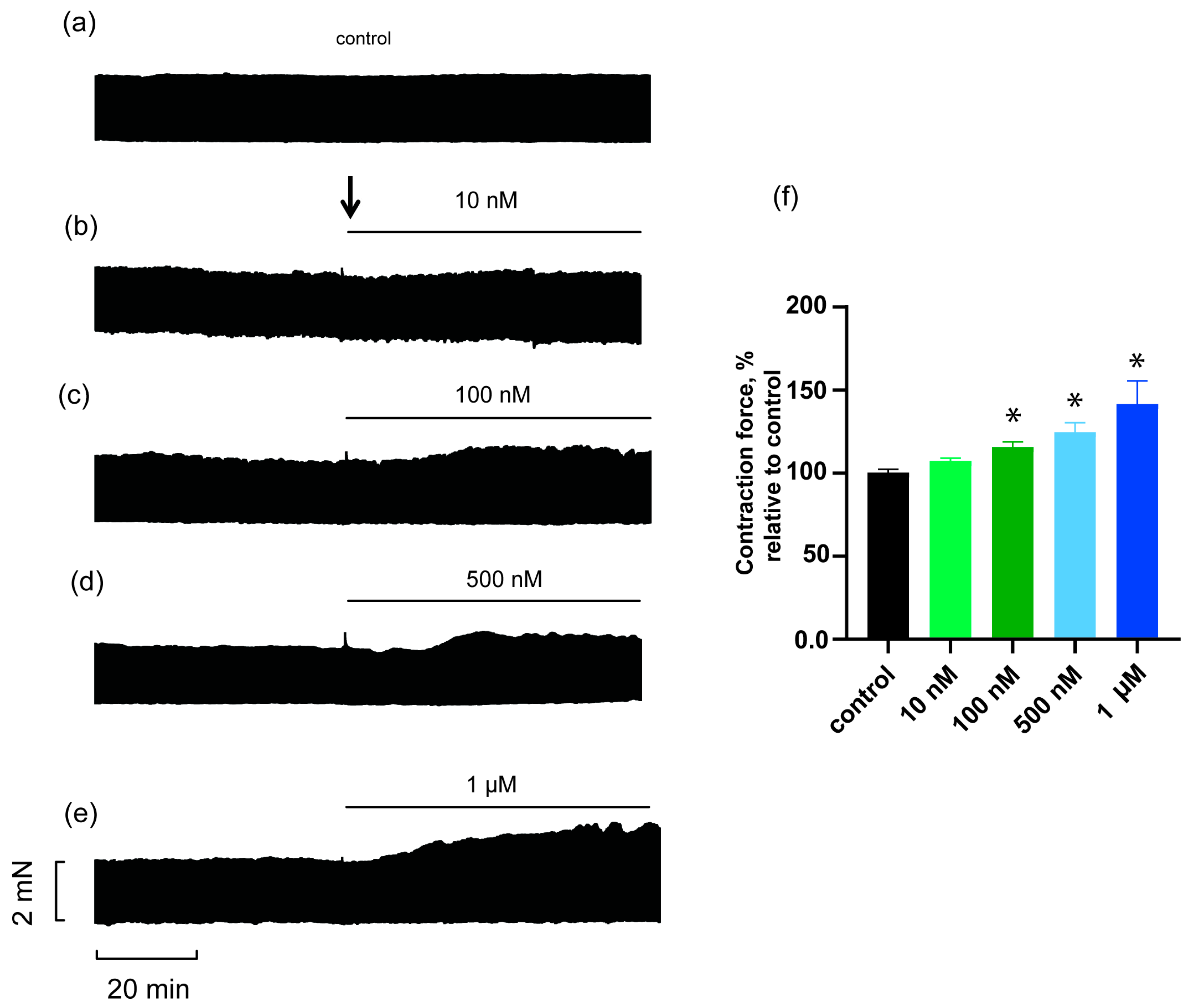
2.1. Effects of HDP-1 on Rat PM Contractility
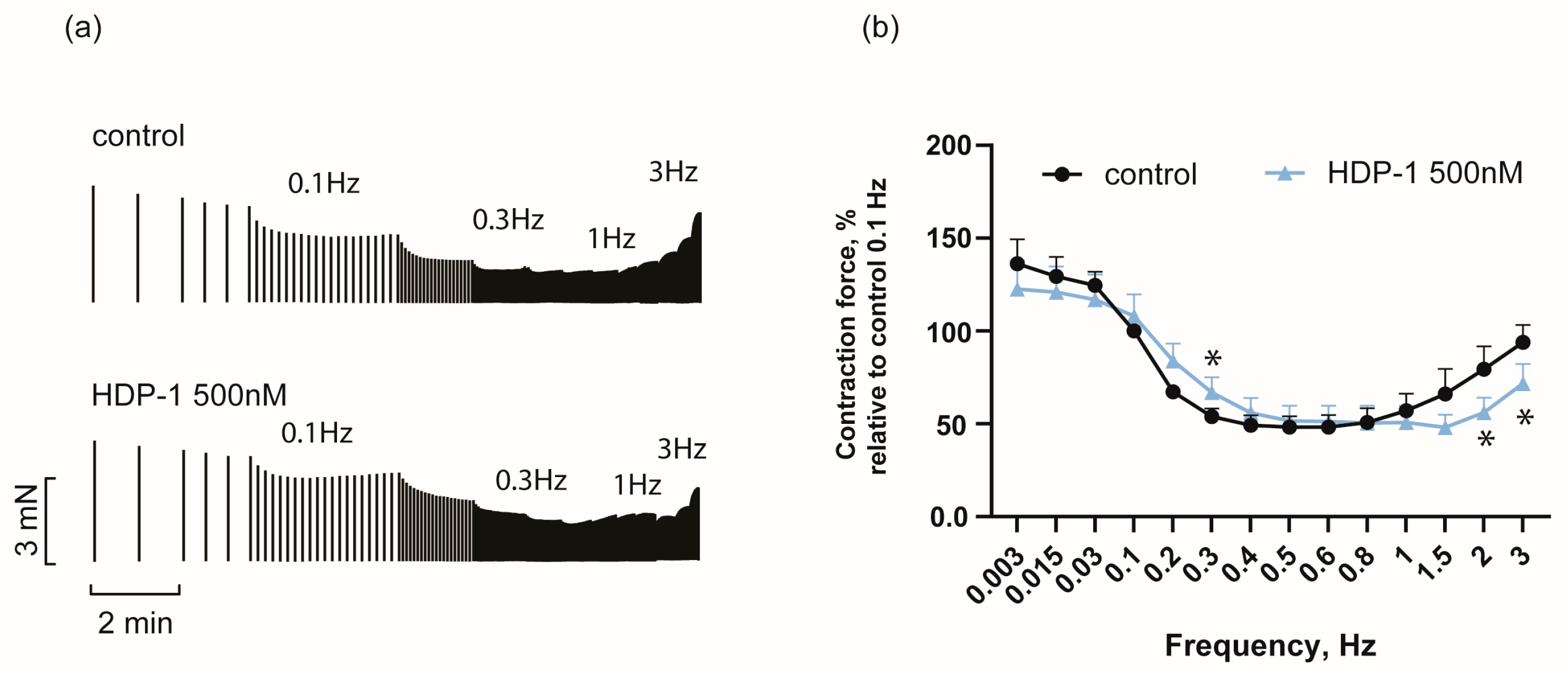
2.2. Frequency Dependence of HDP-1′s Effect on Force of PM Contraction
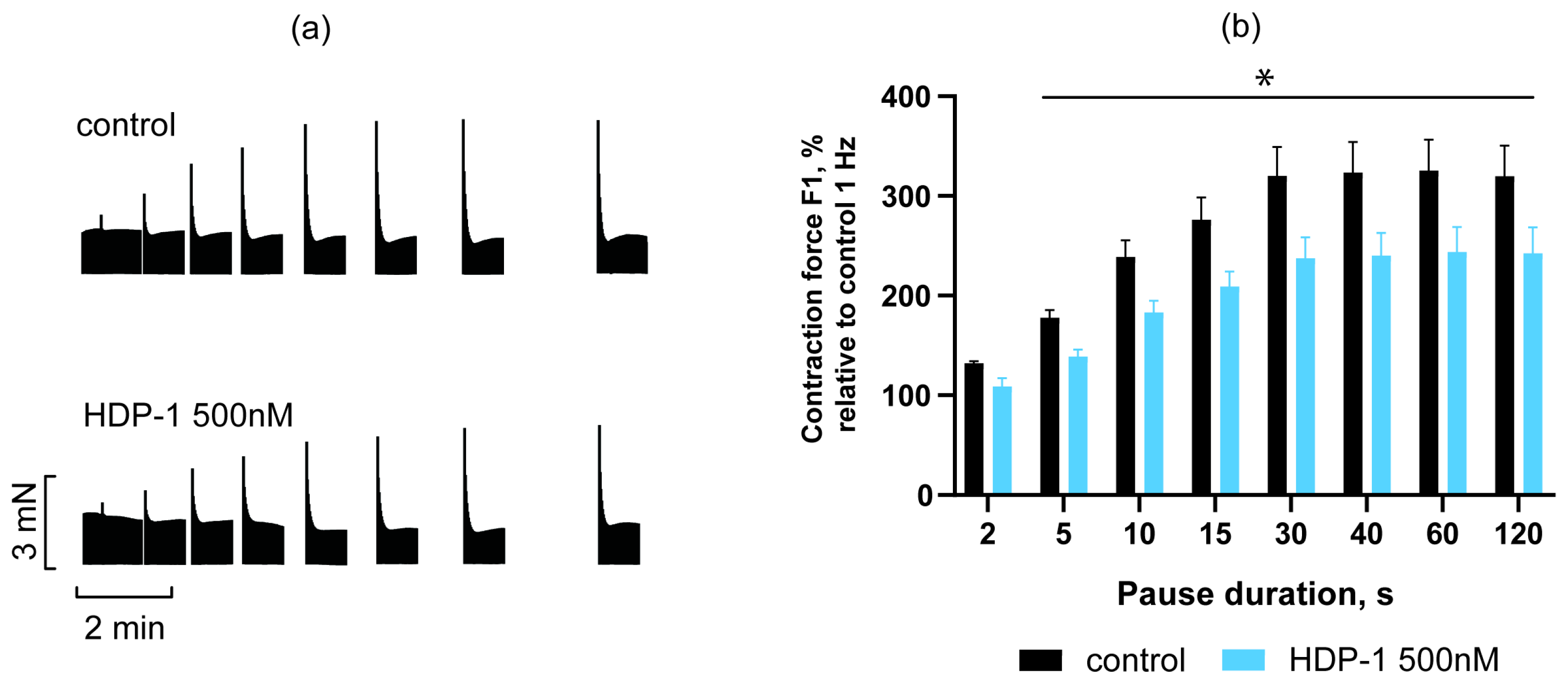
2.3. Effect of HDP-1 on Post-Rest Potentiation of PMs
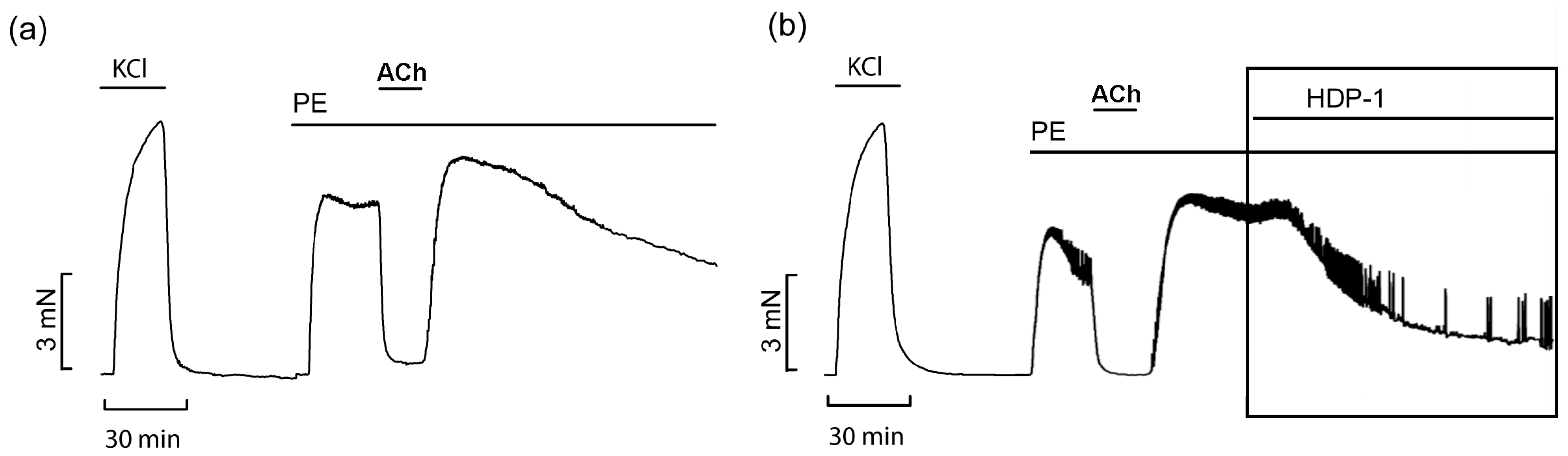
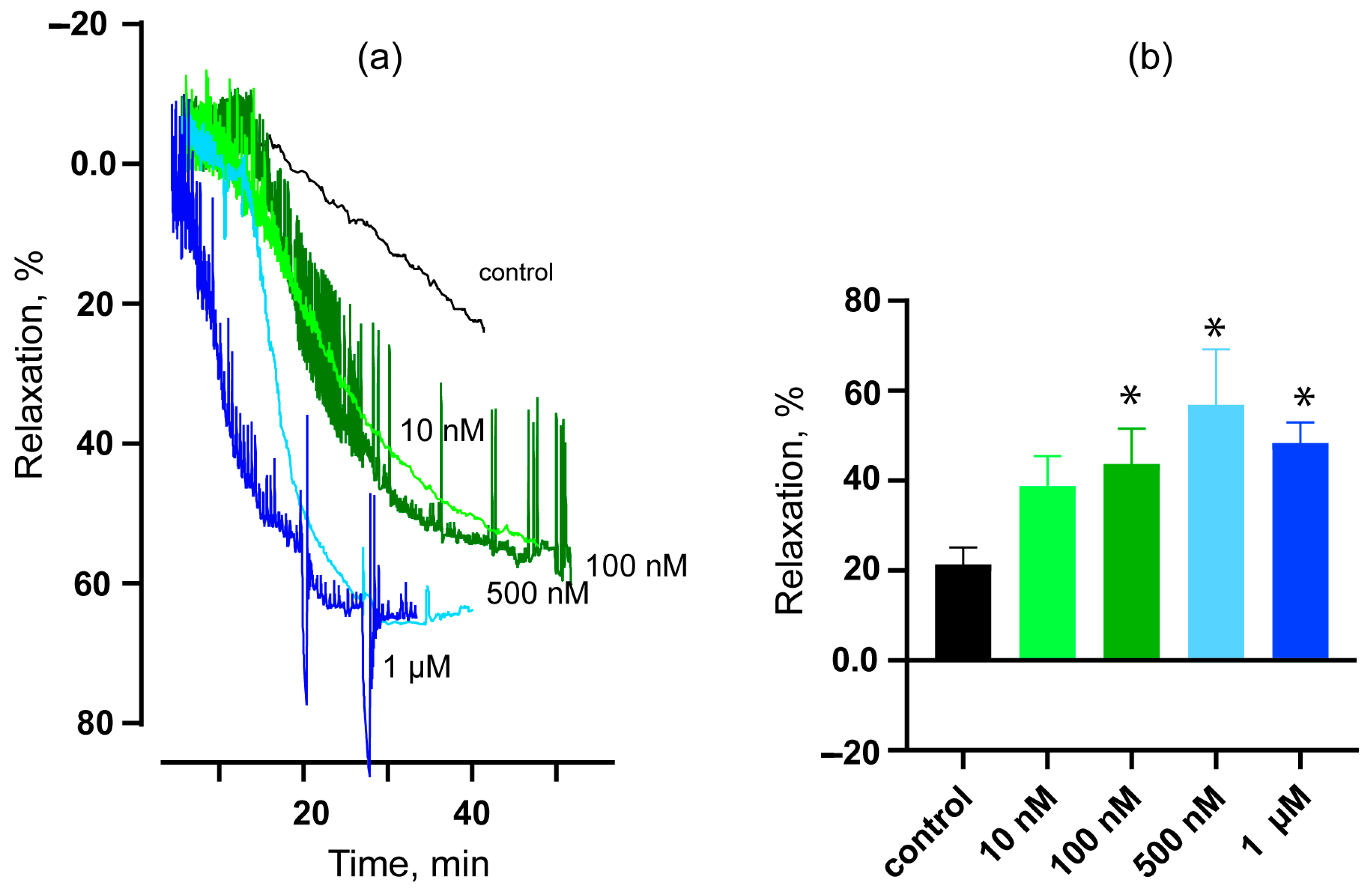
2.4. Vasorelaxant Effects of HDP-1 on Rat ARs
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Animal Handling
5.3. Contractility of PMs
5.4. Contractility of ARs
5.5. Data Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tasoulis, T.; Isbister, G.K. A current perspective on snake venom composition and constituent protein families. Arch. Toxicol. 2023, 97, 133–153. [Google Scholar] [CrossRef]
- Averin, A.S.; Utkin, Y.N. Cardiovascular Effects of Snake Toxins: Cardiotoxicity and Cardioprotection. Acta Nat. 2021, 13, 4–14. [Google Scholar] [CrossRef]
- Frangieh, J.; Rima, M.; Fajloun, Z.; Henrion, D.; Sabatier, J.-M.; Legros, C.; Mattei, C. Snake Venom Components: Tools and Cures to Target Cardiovascular Diseases. Molecules 2021, 26, 2223. [Google Scholar] [CrossRef]
- Averin, A.S.; Nenov, M.N.; Starkov, V.G.; Tsetlin, V.I.; Utkin, Y.N. Effects of Cardiotoxins from Naja oxiana Cobra Venom on Rat Heart Muscle and Aorta: A Comparative Study of Toxin-Induced Contraction Mechanisms. Toxins 2022, 14, 88. [Google Scholar] [CrossRef]
- Madrigal, M.; Sanz, L.; Flores-Díaz, M.; Sasa, M.; Núñez, V.; Alape-Girón, A.; Calvete, J.J. Snake venomics across genus Lachesis. Ontogenetic changes in the venom composition of Lachesis stenophrys and comparative proteomics of the venoms of adult Lachesis melanocephala and Lachesis acrochorda. J. Proteom. 2012, 77, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.; Sajevic, T.; Pungerčar, J.; Križaj, I. Comprehensive Study of the Proteome and Transcriptome of the Venom of the Most Venomous European Viper: Discovery of a New Subclass of Ancestral Snake Venom Metalloproteinase Precursor-Derived Proteins. J. Proteome Res. 2019, 18, 2287–2309. [Google Scholar] [CrossRef] [PubMed]
- Freitas-de-Sousa, L.A.; Nachtigall, P.G.; Portes-Junior, J.A.; Holding, M.L.; Nystrom, G.S.; Ellsworth, S.A.; Guimarães, N.C.; Tioyama, E.; Ortiz, F.; Silva, B.R.; et al. Size Matters: An Evaluation of the Molecular Basis of Ontogenetic Modifications in the Composition of Bothrops jararacussu Snake Venom. Toxins 2020, 12, 791. [Google Scholar] [CrossRef] [PubMed]
- Floriano, R.S.; Torres-Bonilla, K.A.; Rojas-Moscoso, J.A.; Dias, L.; Rocha, T.; Silva, N.J.; Hyslop, S.; Rowan, E.G. Cardiovascular activity of Micrurus lemniscatus lemniscatus (South American coralsnake) venom. Toxicon 2020, 186, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Accary, C.; Hraoui-Bloquet, S.; Sadek, R.; Alameddine, A.; Fajloun, Z.; Desfontis, J.-C.; Mallem, Y. The relaxant effect of the Montivipera bornmuelleri snake venom on vascular contractility. J. Venom Res. 2016, 7, 10–15. [Google Scholar] [PubMed]
- Angel-Camilo, K.L.; Guerrero-Vargas, J.A.; Carvalho, E.F.d.; Lima-Silva, K.; de Siqueira, R.J.B.; Freitas, L.B.N.; Sousa, J.A.C.d.; Mota, M.R.L.; Santos, A.A.D.; Neves-Ferreira, A.G.d.C.; et al. Disorders on cardiovascular parameters in rats and in human blood cells caused by Lachesis acrochorda snake venom. Toxicon 2020, 184, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.A.P.; Dias, L.; Rennó, A.L.; Sousa, N.C.; Smaal, A.; da Silva, D.A.; Hyslop, S. Rat atrial responses to Bothrops jararacussu (jararacuçu) snake venom. Toxicology 2014, 323, 109–124. [Google Scholar] [CrossRef]
- Luksic, B.; Brizic, I.; Lang Balija, M.; Modun, D.; Culic, V.; Halassy, B.; Salamunic, I.; Boban, M. Dose dependent effects of standardized nose-horned viper (Vipera ammodytes ammodytes) venom on parameters of cardiac function in isolated rat heart. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 147, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Szold, O.; Ben-Abraham, R.; Frolkis, I.; Sorkine, M.; Sorkine, P. Tumor necrosis factor as a mediator of cardiac toxicity following snake envenomation. Crit. Care Med. 2003, 31, 1449–1453. [Google Scholar] [CrossRef] [PubMed]
- Zinenko, O.; Tovstukha, I.; Korniyenko, Y. PLA2 Inhibitor Varespladib as an Alternative to the Antivenom Treatment for Bites from Nikolsky’s Viper Vipera berus nikolskii. Toxins 2020, 12, 356. [Google Scholar] [CrossRef] [PubMed]
- Zinenko, O.; Durkin, D.M.; Carter, R.W.; Ritter, B.; Lewin, M.R. Cold Finger: Raynaud Phenomenon Following Snakebite Envenoming by Nikolsky’s Viper (Vipera berus nikolskii). Toxins 2023, 15, 598. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Lomonte, B. Phospholipases A2: Unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon 2013, 62, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.E.; Yang, C.C.; Rosenberg, P. Basic phospholipase A2 from Naja nigricollis snake venom: Phospholipid hydrolysis and effects on electrical and contractile activity of the rat heart. Toxicol. Appl. Pharmacol. 1982, 66, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.Z.; Wang, Q.C.; Liu, G.F. Effects of an acidic phospholipase A2 purified from Ophiophagus hannah (king cobra) venom on rat heart. Toxicon 1993, 31, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, D.S.; Condrea, E.; Maraganore, J.M.; Heinrikson, R.L.; Benjamin, S.; Rosenberg, P. Comparison of enzymatic and pharmacological activities of lysine-49 and aspartate-49 phospholipases A2 from Agkistrodon piscivorus piscivorus snake venom. Biochem. Pharmacol. 1987, 36, 1723–1730. [Google Scholar] [CrossRef]
- Karabuva, S.; Lukšić, B.; Brizić, I.; Latinović, Z.; Leonardi, A.; Križaj, I. Ammodytin L is the main cardiotoxic component of the Vipera ammodytes ammodytes venom. Toxicon 2017, 139, 94–100. [Google Scholar] [CrossRef]
- De Paula, R.C.; Castro, H.C.; Rodrigues, C.R.; Melo, P.A.; Fuly, A.L. Structural and pharmacological features of phospholipases A2 from snake venoms. Protein Pept. Lett. 2009, 16, 899–907. [Google Scholar] [CrossRef]
- Dennis, E.A.; Cao, J.; Hsu, Y.-H.; Magrioti, V.; Kokotos, G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef] [PubMed]
- Salvador, G.H.M.; Fernandes, C.A.H.; Borges, R.J.; Soares, A.M.; Fontes, M.R.M. Structural studies with crotoxin B from Crotalus durissus collilineatus venom suggest a heterodimeric assembly formed by two new isoforms. Biochimie 2023, 218, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Petrova, S.D.; Atanasov, V.N.; Balashev, K. Vipoxin and its components: Structure-function relationship. Adv. Protein Chem. Struct. Biol. 2012, 87, 117–153. [Google Scholar] [CrossRef] [PubMed]
- Ramazanova, A.S.; Zavada, L.L.; Starkov, V.G.; Kovyazina, I.V.; Subbotina, T.F.; Kostyukhina, E.E.; Dementieva, I.N.; Ovchinnikova, T.V.; Utkin, Y.N. Heterodimeric neurotoxic phospholipases A2—The first proteins from venom of recently established species Vipera nikolskii: Implication of venom composition in viper systematics. Toxicon 2008, 51, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, S.I.; Ziganshin, R.H.; Starkov, V.G.; Tsetlin, V.I.; Utkin, Y.N. Quantitative Proteomic Analysis of Venoms from Russian Vipers of Pelias Group: Phospholipases A2 are the Main Venom Components. Toxins 2016, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Renetsederm, R.; Brunie, S.; Dijkstra, B.W.; Drenth, J.; Sigler, P.B. A comparison of the crystal structures of phospholipase A2 from bovine pancreas and Crotalus atrox venom. J. Biol. Chem. 1985, 260, 11627–11634. [Google Scholar] [CrossRef]
- Santos, P.E.; Souza, S.D.; Freire-Maia, L.; Almeida, A.P. Effects of crotoxin on the isolated guinea pig heart. Toxicon 1990, 28, 215–224. [Google Scholar] [CrossRef]
- Zhang, P.; Lader, A.S.; Etcheverry, M.A.; Cantiello, H.F. Crotoxin potentiates L-type calcium currents and modulates the action potential of neonatal rat cardiomyocytes. Toxicon 2010, 55, 1236–1243. [Google Scholar] [CrossRef]
- Sartim, M.A.; Nogueira, R.C.; Cavalcante, T.T.A.; Sousa, L.O.; Monteiro, W.M.; Cintra, A.C.O.; Neto-Neves, E.M.; Sampaio, S.V. Hemodynamic impairment induced by Crotoxin using in vivo and ex vivo approach in a rat model. Int. J. Biol. Macromol. 2023, 232, 123408. [Google Scholar] [CrossRef]
- Pitari, G.M.; Giuliano, F.; Bianchi, A.; Corrado, A.P. Responses of rat aortic rings to crotoxin. Fundam. Clin. Pharmacol. 1996, 10, 180. [Google Scholar]
- Pereira, T.P.; Bezerra de Menezes, R.; Torres, A.F.C.; Brito, T.S.; Batista-Lima, F.J.; Vinhote, J.F.C.; Sousa, D.F.; Ximenes, R.M.; Toyama, M.H.; Diz Filho, E.B.S.; et al. Renal and vascular effects of Crotalus durissus cumanensis venom and its crotoxin fraction. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011, 17, 333–347. [Google Scholar] [CrossRef]
- Palmer, B.M.; Bell, S.P. Preparing Excitable Cardiac Papillary Muscle and Cardiac Slices for Functional Analyses. Front. Physiol. 2022, 13, 817205. [Google Scholar] [CrossRef]
- Rameshrad, M.; Babaei, H.; Azarmi, Y.; Fouladi, D.F. Rat aorta as a pharmacological tool for in vitro and in vivo studies. Life Sci. 2016, 145, 190–204. [Google Scholar] [CrossRef]
- Endoh, M. Force-frequency relationship in intact mammalian ventricular myocardium: Physiological and pathophysiological relevance. Eur. J. Pharmacol. 2004, 500, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Schouten, V.J.; ter Keurs, H.E. The force-frequency relationship in rat myocardium. The influence of muscle dimensions. Pflugers Arch. 1986, 407, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Hiranandani, N.; Varian, K.D.; Monasky, M.M.; Janssen, P.M. Frequency-dependent contractile response of isolated cardiac trabeculae under hypo-, normo-, and hyperthermic conditions. J. Appl. Physiol. 2006, 100, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Barrington, P.L.; Soons, K.R.; Rosenberg, P. Cardiotoxicity of Naja nigricollis phospholipase A2 is not due to alterations in prostaglandin synthesis. Toxicon 1986, 24, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Barrington, P.L.; Yang, C.C.; Rosenberg, P. Cardiotoxic effects of Naja nigricollis venom phospholipase A2 are not due to phospholipid hydrolytic products. Life Sci. 1984, 35, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Gao, W.; Taylor, P.B. Force-frequency response in isoproterenol-induced hypertrophied rat heart. Eur. J. Pharmacol. 1996, 318, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Lee, C.Y. Relaxant effect of phospholipase A2 from Vipera russelli snake venom on rat aorta. Eur. J. Pharmacol. 1985, 118, 139–146. [Google Scholar] [CrossRef]
- Kaplia, A.A.; Kravtsova, V.V.; Kravtsov, A.V. Influence of Phospholipase A2 from Naja naja oxiana Venom on the Activity of Rat Brain Isoenzymes of Na+,K+-ATPase. Biochemistry 1996, 61, 715–720. [Google Scholar]
- Bluhm, W.F.; Kranias, E.G.; Dillmann, W.H.; Meyer, M. Phospholamban: A major determinant of the cardiac force-frequency relationship. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H249–H255. [Google Scholar] [CrossRef] [PubMed]
- Hiranandani, N.; Raman, S.; Kalyanasundaram, A.; Periasamy, M.; Janssen, P.M.L. Frequency-dependent contractile strength in mice over- and underexpressing the sarco(endo)plasmic reticulum calcium-ATPase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R30–R36. [Google Scholar] [CrossRef]
- Stuyvers, B.D.; McCulloch, A.D.; Guo, J.; Duff, H.J.; ter Keurs, H.E.D.J. Effect of stimulation rate, sarcomere length and Ca2+ on force generation by mouse cardiac muscle. J. Physiol. 2002, 544, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, I.L.; Martins, A.M.C.; Nascimento, N.R.F.; Havt, A.; Evangelista, J.S.A.M.; de Norões, T.B.S.; Toyama, M.H.; Diz-Filho, E.B.; Toyama, D.D.O.; Fonteles, M.C.; et al. Renal and cardiovascular effects of Bothrops marajoensis venom and phospholipase A2. Toxicon 2010, 55, 1061–1070. [Google Scholar] [CrossRef]
- Averin, A.S.; Konakov, M.V.; Pimenov, O.Y.; Galimova, M.H.; Berezhnov, A.V.; Nenov, M.N.; Dynnik, V.V. Regulation of Papillary Muscle Contractility by NAD and Ammonia Interplay: Contribution of Ion Channels and Exchangers. Membranes 2022, 12, 1239. [Google Scholar] [CrossRef]
- Pieske, B.; Sütterlin, M.; Schmidt-Schweda, S.; Minami, K.; Meyer, M.; Olschewski, M.; Holubarsch, C.; Just, H.; Hasenfuss, G. Diminished post-rest potentiation of contractile force in human dilated cardiomyopathy. Functional evidence for alterations in intracellular Ca2+ handling. J. Clin. Investig. 1996, 98, 764–776. [Google Scholar] [CrossRef]
- Ayres, R.; Feijó, P.R.O.; Cintra, A.C.O.; Tomaz, M.A.; Melo, P.A.; Cunha, V.M.N.; Quintas, L.E.M. Different effects of myotoxins bothropstoxin-I and II from Bothrops snake venom on cation transport ATPases from murine fast twitch skeletal muscle. Toxicon 2015, 103, 80–84. [Google Scholar] [CrossRef]
- Chaisakul, J.; Isbister, G.K.; Tare, M.; Parkington, H.C.; Hodgson, W.C. Hypotensive and vascular relaxant effects of phospholipase A2 toxins from Papuan taipan (Oxyuranus scutellatus) venom. Eur. J. Pharmacol. 2014, 723, 227–233. [Google Scholar] [CrossRef]
- de Wit, C.; Bolz, S.S.; Pohl, U. Interaction of endothelial autacoids in microvascular control. Z. Kardiol. 2000, 89, 113–116. [Google Scholar] [CrossRef]
- Zou, Z.; Zeng, F.; Zhang, L.; Niu, L.; Teng, M.; Li, X. Purification, crystallization and preliminary X-ray diffraction analysis of an acidic phospholipase A2 with vasoconstrictor activity from Agkistrodon halys pallas venom. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012, 68, 1329–1332. [Google Scholar] [CrossRef]
- Zeng, F.; Zou, Z.; Niu, L.; Li, X.; Teng, M. AhV_aPA-induced vasoconstriction involves the IP3Rs-mediated Ca2+ releasing. Toxicon 2013, 70, 107–113. [Google Scholar] [CrossRef]
- Vuong, N.T.; Jackson, T.N.W.; Wright, C.E. Role of Phospholipases A2 in Vascular Relaxation and Sympatholytic Effects of Five Australian Brown Snake, Pseudonaja spp., Venoms in Rat Isolated Tissues. Front. Pharmacol. 2021, 12, 754304. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, V.N.; Stoykova, S.; Goranova, Y.; Mitewa, M.; Petrova, S. Acute toxicity of vipoxin and its components: Is the acidic component an “inhibitor” of PLA2 toxicity? Interdiscip. Toxicol. 2012, 5, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Starkov, V.G.; Osipov, A.V.; Utkin, Y.N. Toxicity of venoms from vipers of Pelias group to crickets Gryllus assimilis and its relation to snake entomophagy. Toxicon 2007, 49, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2010.276.01.0033.01.ENG&toc=OJ%3AL%3A2010%3A276%3ATOC (accessed on 8 February 2024).
- Nakipova, O.V.; Averin, A.S.; Evdokimovskii, E.V.; Pimenov, O.Y.; Kosarski, L.; Ignat’ev, D.; Anufriev, A.; Kokoz, Y.M.; Reyes, S.; Terzic, A.; et al. Store-operated Ca2+ entry supports contractile function in hearts of hibernators. PLoS ONE 2017, 12, e0177469. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Averin, A.; Starkov, V.; Tsetlin, V.; Utkin, Y. Effects of the Heterodimeric Neurotoxic Phospholipase A2 from the Venom of Vipera nikolskii on the Contractility of Rat Papillary Muscles and Thoracic Aortas. Toxins 2024, 16, 100. https://doi.org/10.3390/toxins16020100
Averin A, Starkov V, Tsetlin V, Utkin Y. Effects of the Heterodimeric Neurotoxic Phospholipase A2 from the Venom of Vipera nikolskii on the Contractility of Rat Papillary Muscles and Thoracic Aortas. Toxins. 2024; 16(2):100. https://doi.org/10.3390/toxins16020100
Chicago/Turabian StyleAverin, Alexey, Vladislav Starkov, Victor Tsetlin, and Yuri Utkin. 2024. "Effects of the Heterodimeric Neurotoxic Phospholipase A2 from the Venom of Vipera nikolskii on the Contractility of Rat Papillary Muscles and Thoracic Aortas" Toxins 16, no. 2: 100. https://doi.org/10.3390/toxins16020100
APA StyleAverin, A., Starkov, V., Tsetlin, V., & Utkin, Y. (2024). Effects of the Heterodimeric Neurotoxic Phospholipase A2 from the Venom of Vipera nikolskii on the Contractility of Rat Papillary Muscles and Thoracic Aortas. Toxins, 16(2), 100. https://doi.org/10.3390/toxins16020100





