From Toxin to Treatment: A Narrative Review on the Use of Botulinum Toxin for Autonomic Dysfunction
Abstract
:1. Introduction
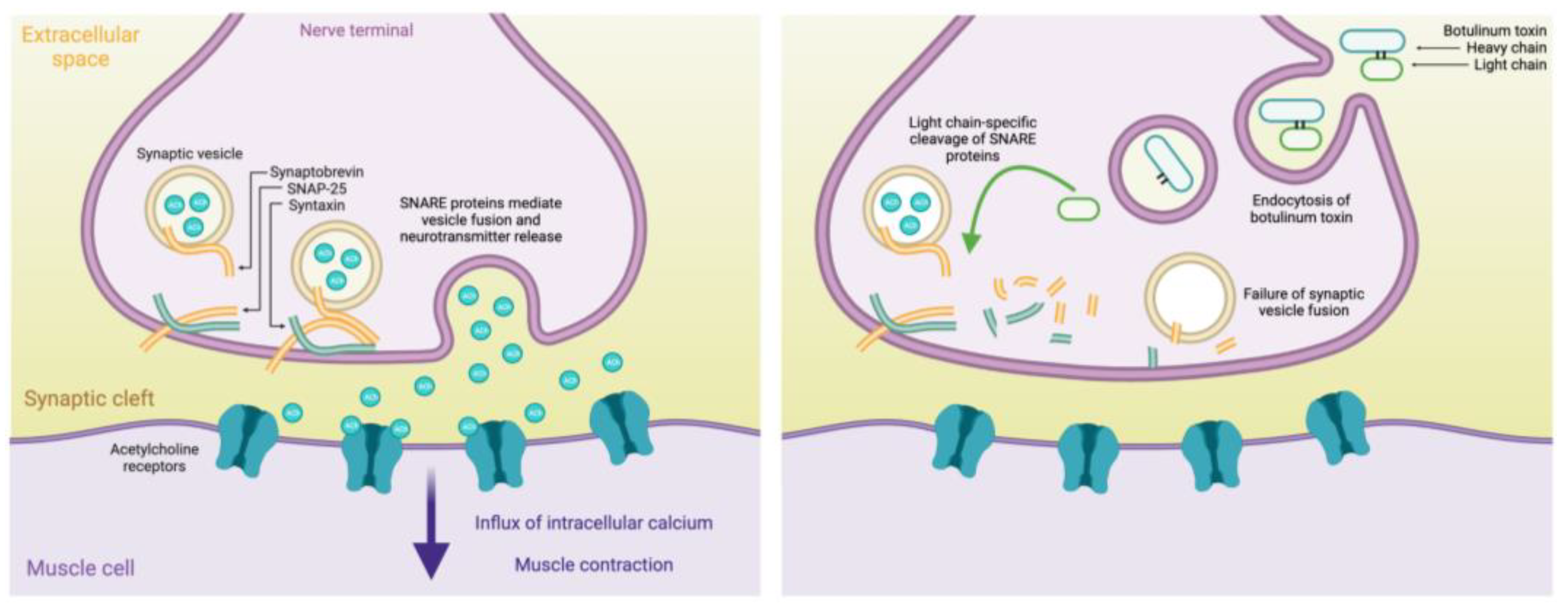
2. Mechanisms and Formulations of Botulinum Toxin
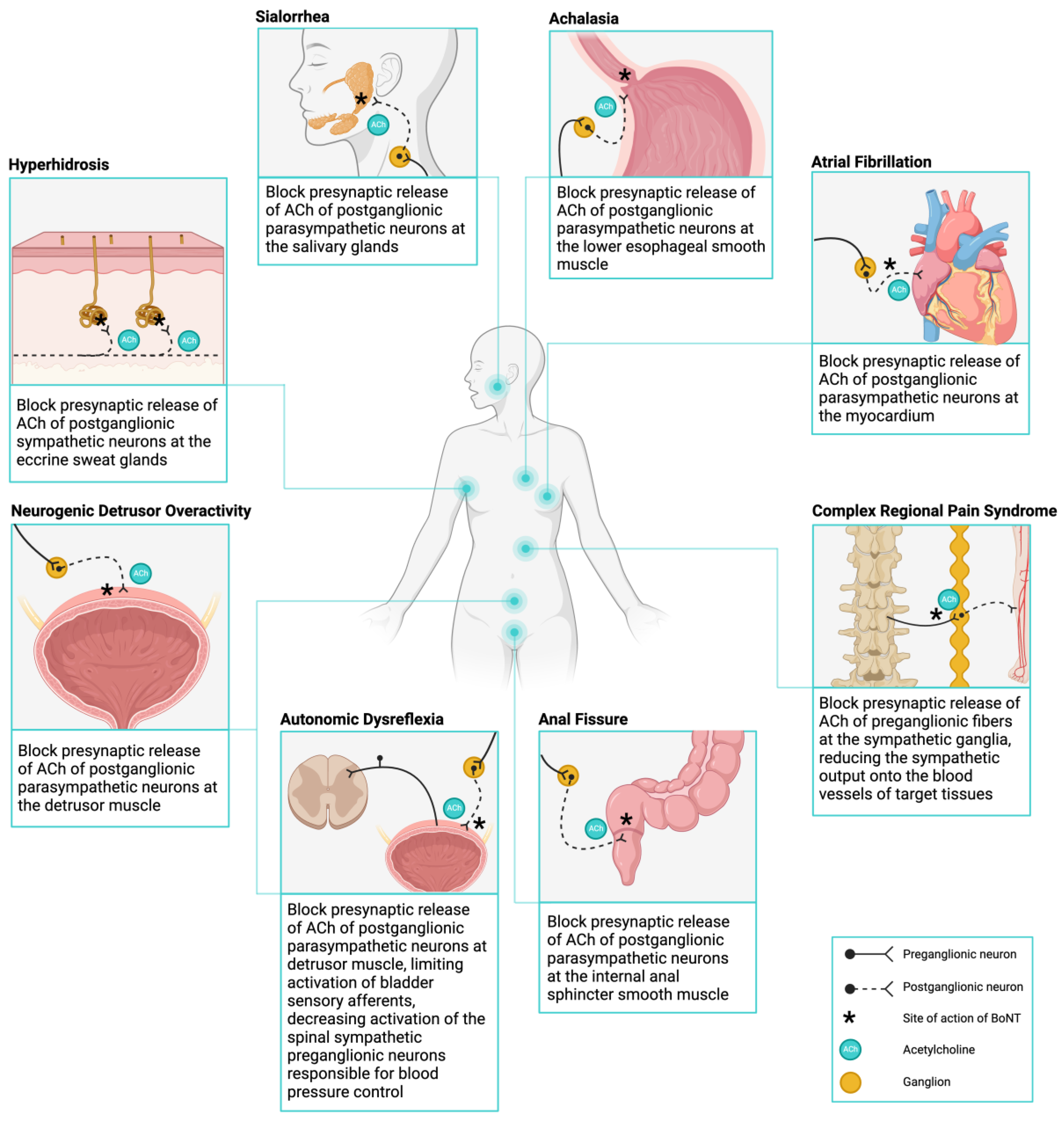
3. Targeting Autonomic Dysfunction with Botulinum Toxin
4. The Use of Botulinum Toxin in Conditions with Autonomic Dysfunction
4.1. Management of Hyperhidrosis
4.1.1. Management of Axillary Hyperhidrosis
4.1.2. Management of Palmar Hyperhidrosis
4.1.3. Management of Craniofacial Hyperhidrosis
4.2. Management of Sialorrhea
4.3. Management of Achalasia
4.4. Management of Anal Fissures
4.5. Management of Neurogenic Detrusor Overactivity
4.6. Management of Autonomic Dysreflexia
4.7. Management of Atrial Fibrillation
4.8. Management of Complex Regional Pain Syndrome
| First Author, Year [References] | Study Design, Number (N) | Formulation: Dose | Outcome | Adverse Events |
|---|---|---|---|---|
| Axillary hyperhidrosis | ||||
| Baumann, 2005 [49] | Pilot Study, N = 23 | MyoblocTM: 2500 U | Improvement of AH from 2.2 to 8.1 months (mean duration 5.0 months) | Bruising, flu-like symptoms, dry eyes, indigestion |
| Dressler, 2002 [45] | Comparative cohort study, N = 19 | MyoblocTM: 2000 U | Improvement of AH of a median duration of 17.1 weeks | Mouth dryness, conjunctival irritation |
| MyoblocTM: 4000 U | Improvement of AH of a median duration of 16.0 weeks | |||
| Heckmann, 2001 [46] | Multicenter clinical trial, N = 145 | Dysport®: 100 U | Reduced rate of sweat production ≥ 50% at 26-week follow-up | None reported |
| Dysport®: 200 U | Reduced rate of sweat production ≥ 50% at 26-week follow-up | |||
| Heckmann, 2005 [47] | Randomized clinical trial, N = 43 | Dysport®: 100 U | Sweat production returned to 98% of baseline 48 weeks after first dose, 63% 48 weeks after second dose | Temporary stinging, irritation, fatigue |
| Dysport®: 200 U | Sweat production returned to 92% of baseline 48 weeks after first dose, 66% 48 weeks after second dose | |||
| Lowe, 2007 [53] | Multicenter double-blind study, N = 322 | Botox®: 50 U | Improvement of AH of a median duration of 205 days | None reported |
| Botox®: 75 U | Improvement of AH of a median duration of 197 days | |||
| Naumann, 2001 [41] | Multicenter, randomized, placebo controlled clinical trial, N = 307 | Botox®: 50 U | Improvement of AH, as evidenced by decreased sweat production and improved satisfaction scores by 16 weeks compared to placebo control group | Perceived increase in non-axillary sweating, flu-like symptoms |
| Naumann, 2003 [42] | Prospective double-blind study, N = 207 | Botox®: 50 U | Improvement of AH of a mean duration of 30.6 weeks between any 2 consecutive treatments and improvements in satisfaction scores. | Perceived increase in non-axillary sweating, flu-like symptoms |
| Palmar hyperhidrosis | ||||
| Campanati, 2014 [50] | Comparative double-blind clinical trial, N = 25 | Botox®: 100–150 U | Improvement in symptoms and 80% reduction in sweat production by 4 weeks, no difference between formulations | None reported |
| Xeomin®: 100–150 U | Improvement in symptoms and 80% reduction in sweat production by 4 weeks, no difference between formulations | |||
| Lowe, 2002 [51] | Placebo-controlled study, N = 19 | Botox®: 100 U | Decrease in sweat production by day 28 | Finger tingling and numbness |
| Moreau, 2003 [31] | Double-blind randomized study, N = 8 | Botox®: 69 U | Decrease in sweating (−69.4%) significant by 3 months, for a mean duration of 17 weeks | Decreased pinch strength |
| Dysport®: 284 U | Decrease in sweating (−56.6%) significant by 1 months, but not 3 months (−48.8%) for a mean duration of 18 weeks | |||
| Rystedt, 2013 [52] | Double-blind randomized study, N = 20 | Botox®: 25 U | Greatest decrease in mean anhidrotic area by 12 weeks at 25 U dose | None reported |
| Xeomin®: 25 U | Greatest decrease in mean anhidrotic area by 12 weeks at 25 U dose | |||
| Dysport®: 100 U | Greatest decrease in mean anhidrotic area by 12 weeks at 100 U dose | |||
| MyoblocTM: 50 U | Greatest decrease in mean anhidrotic area by 12 weeks at 50 U dose | |||
| Saadia, 2001 [54] | Single-blind randomized study, N = 24 | Botox®: 50 U | Decrease in sweating by 6-month follow-up | Decreased pinch strength |
| Botox®: 100 U | Decrease in sweating by 5-month follow-up | |||
| Schnider, 1997 [48] | Double-blind randomized study, N = 11 | Dysport®: 120 U | Decrease in sweating by 26% at 8 weeks and 31% at 13 weeks | Minor weakness in hand grip strength |
| Craniofacial hyperhidrosis | ||||
| Cabreus, 2019 [60] | Case study, N = 8 | MyoblocTM: 2250 U | 90% median improvement of dermatology quality of life score in treatment group compared to −18% decline in placebo group | None reported |
| Eustace, 2018 [61] | Case study, N = 20 | Botulinum-A toxin (not specified): 100 U, effective at 5–6-month follow-up | Decrease in sweating in 64% of participants, compared to 30% with an oral anticholinergic | None reported |
| George, 2014 [56] | Case study, N = 4 | Botox®: 12–80 U, MD 6–8 months | Decrease in sweating in all four participants, duration of effect 6–8 months | None reported |
| Sialorrhea | ||||
| Bhatia, 1999 [70] | Case study, N = 4 | Dysport®: 20 U | Decrease in salivation of a duration of effect of 6 weeks in one patient and 3–4 months in other patients | Mild dysphagia, chewing difficulty |
| Costa, 2008 [76] | Open-label prospective study, N = 16 | MyoblocTM: 1250 U | Reduction in salivation in 94% of patients lasting by 3 months | Increased difficulty chewing, viscous saliva, respiratory infection, facial paresis, burning of eyes |
| Guidubaldi, 2011 [78] | Prospective, randomized, double-blind, crossover, pilot study, N = 14 | Dysport®: 250 U | Mean duration of benefit of 75 days, as determined by saliva weight and subjective reporting scales, non-significant compared to BoNT-B | Change in saliva thickness, no difference between formulations |
| NeuroblocTM: 2500 U | Mean duration of benefit of 90 days, shorter latency of effect compared to BoNT-A | |||
| Isaacson, 2020 [77] | Randomized, parallel, double-blind clinical trial, N = 187 | MyoblocTM: 2500–3500 U | Reduction in salivation, onset at 1 week after injection, maintained for 13 weeks | Dry mouth, dysphagia, dental caries |
| Jost, 2019 [74] | Prospective, randomized, double-blind placebo-controlled trial, N = 180 | Xeomin®: 75–100 U, | Reduction in salivation, still effective at 16 weeks | Dry mouth, dysphagia |
| Jost, 2020 [75] | Prospective, randomized, double-blind placebo-controlled trial, N = 173 | Xeomin®: 75–100 U | Reduction in salivation, effective throughout a 64-week period with reinjections every 16 weeks | Dry mouth, dysphagia, speech disorder, worsening constipation |
| Lagalla, 2006 [71] | Double-blind, randomized, placebo-controlled trial, N = 32 | Botulinum-A toxin (not specified): 100 U | Reduction in salivation, effective at 1-month follow-up | None reported |
| Mazlan, 2015 [72] | Prospective, double-blind, randomized, controlled trial, N = 17 | Dysport®: 50 U, 100 U, 200 U | Reduction in salivation at 24-week follow-up with the 200 U-treated group showing the greatest effect | None reported |
| Restivo, 2018 [73] | Randomized, blinded study, N = 90 | Botox®: 25 U per gland | Reduction in salivation at 2-week follow-up, dose-dependent effect stronger when 4 glands were injected compared to 3 | None reported |
| Xeomin®: 25 U per gland | Reduction in salivation at 2-week follow-up, dose-dependent effect stronger when 3 glands were injected compared to 2 | |||
| Achalasia | ||||
| Annese, 2001 [88] | Randomized, comparative study, N = 78 | Botox®: 100 U | Decrease in LES pressure at 1 month and improvement in symptom score lasting by 6 months, no difference between treatments | None reported |
| Dysport®: 250 U | Decrease in LES pressure at 1 month and improvement in symptom score lasting by 6 months, no difference between treatments | |||
| Jung, 2014 [93] | Non-randomized comparative cohort study, N = 73 | Botox®: 100 U | Median duration of symptom-free period was 13 months in BoNT-treated group, compared to 29 months in the balloon-dilation-treated group | None reported |
| Martínek, 2003 [89] | Non-randomized prospective cohort study, N = 41 | Botox®: 100–250 U Dysport®: 100–250 U | Median duration of symptom-free period was 11.5 after first injection, and 10.5 months after second injection among all BoNT-treated patients. Those receiving both BoNT and balloon dilatation had an increased likelihood of remission at 1 and 2 years compared to BoNT alone | None reported |
| Muehldorfer, 1999 [90] | Prospective randomized study, N = 24 | Xeomin®: 80 U | All patients receiving successful BoNT treatment experienced symptom recurrence by 6 months, whereas 40% of the balloon dilatation group experienced symptom recurrence | One case of myotomy to remove esophageal adhesions |
| Pasricha, 1995 [87] | Double-blind clinical trial, N = 21 | Not specified: 80 U | Mean decrease in LES pressure of 33% in treatment group compared to an increase of 12% in placebo group; 14 patients receiving BoNT were still in remission by 6 months | None reported |
| Pasricha, 1996 [91] | Prospective cohort study, N = 31 | Not specified: 80 U | Among 19 initial responders, median duration of symptom relapse was 468 days | None reported |
| Zhu, 2009 [95] | Randomized study, N = 90 | Hengli®: 100 U | Improved LES pressure and symptom score in BoNT and balloon dilatation combination therapy compared to monotherapy during 2-year follow-up | None reported |
| Anal fissure | ||||
| Berkel, 2014 [106] | Randomized clinical trial, N = 60 | Dysport®: 60 U | Complete fissure healing of a median duration of 9 weeks in 67% of patients receiving BoNT, compared to 33% of patients treated with isosorbide dinitrate ointment | Headache, loss of mucus, flatus, and mucus incontinence |
| Brisinda, 1999 [105] | Randomized-blinded clinical trial, N = 50 | Botox®: 20 U | Complete fissure healing in 96% of patients receiving BoNT at 2 months compared to 60% of patients receiving nitroglycerin | None reported |
| Brisinda, 2002 [102] | Randomized double-blind clinical trial, N = 150 | Botulinum-A toxin (not specified): 20–80 U | Complete fissure healing in 73% of patients receiving 20 U then 30 U of BoNT, and 87% in patients receiving 30 U then 50 U at 1 month, increasing to 89% and 96%, respectively, by 2 months | Mild incontinence of flatus |
| Brisinda, 2004 [107] | Randomized controlled clinical trial, N = 50 | Botox®: 50 U | Complete fissure healing in 92% of patients, decrease in mean resting anal pressure (41.8%) and maximum voluntary squeeze pressure (20.2%) compared to baseline at 2 months | Mild incontinence of flatus |
| Dysport®: 150 U | Complete fissure healing in 94% of patients, decrease in mean resting anal pressure (60.0%) and maximum voluntary squeeze pressure (71.0%) compared to baseline at 2 months | |||
| Gui, 1994 [101] | Case Study, N = 10 | Botox®: 15 U | Complete fissure healing in 70% of patients at 2 months | Mild incontinence of flatus |
| Maria, 1998 [104] | Comparative treatment study, N = 57 | Botox®: 35–45 U | Improved fissure healing at two months in patients treated with 45 U of BoNT (68%) compared to patients treated with 35 U (43%) | None reported |
| Neurogenic detrusor overactivity | ||||
| Asafu-Adjei, 2019 [121] | Pilot study, N = 17 | Xeomin®: 200–300 U | Decrease in daily pad use, urinary frequency, incontinence episodes, increase in hours between catheterization and catheterization volume, and improvements in symptom score during follow-ups between 2 to 4 weeks | None reported |
| Chen, 2014 [119] | Randomized, prospective study, N = 72 | Botox®: 200–300 U | Improvement in incontinence severity and quality of life at 6- and 12-month follow-ups with no difference between 200 U and 300 U dosage groups | Difficult urination, hematuria, urinary tract infection |
| Grise, 2010 [120] | Prospective, randomized, double-blind, comparative study, N = 77 | Dysport®: 500–750 U | 56.4% of patients receiving 500 U were continent at day 30, compared to 73.7% of patients receiving 750 U | Hematuria, pyelonephritis, urgency, general fatigue with vertigo, difficulty with catheterization |
| Herschorn, 2011 [118] | Prospective, double-blind study, N = 57 | Botox®: 300 U | Decrease in number of incontinence episodes at 6-, 24-, 36-week follow-up | Urinary tract infection |
| Kennelly, 2022 [21] | Two randomized double-blind phase 3 clinical trial, N = 485 | Dysport®: 600–800 U | Reduction in weekly neurogenic detrusor overactivity incontinence episodes and an increased total voiding volume at 2-, 6-, and 12-week follow ups | Urinary tract infection hematuria, acute pyelonephritis, autonomic dysreflexia |
| Schurch, 2000 [117] | Prospective non-randomized study, N = 21 | Botulinum-A toxin (not specified): 200–300 U | Restoration of continence in 89% of completed participants, along with increases in mean maximum cystometric capacity, reflex volume, and post-void residual volume, as well as a decrease in mean detrusor voiding pressure at 6-week follow up | None reported |
| Autonomic dysreflexia | ||||
| Dorey, 2021 [133] | Secondary post hoc analysis on prospective clinical trial, N = 55 | Botox®: 200 U | Amelioration in AD-associated HRV responses during bladder filling after 1-month post-injection | Fatigue, pain, urinary tract infection |
| Fougere, 2016 [116] | Prospective, pre/post comparison study, N = 17 | Botox®: 200 U, effective at 1-month follow-up | Reduction in change in SBP during bladder filling and the number of bladder-related AD events over 24 h ABPM 1-month post-injection | Headache, urinary tract infection |
| Huang, 2022 [134] | Cross-sectional, non-randomized clinical trial, N = 25 | Botox®: 200 U, effective at 3-month follow-up | Decreased maximum detrusor pressure and change in SBP during bladder filling as well as the number of bladder-related AD events over 24 h ABPM 3 months post-injection | None reported |
| Jung, 2019 [135] | Case study, N = 1 | Botox®: 200 U | Stabilization of BP and daily maximum SBP 1-month post-injection and improvements in AD symptoms and bladder spams 6 months post-injection | None reported |
| Walter, 2020 [113] | Prospective clinical trial | Botox®: 200 U, effective at 1-month follow-up | Reduction in AD severity in 82% of participants during bladder filling and 74% during 24 h ABPM, increase in cystometric capacity and maximum detrusor pressure at cystometric capacity 1-month post injection | Increased fatigue, headache, pain |
| Atrial Fibrillation | ||||
| Romanov, 2018 [139] | Randomized, double-blind placebo-controlled trial, N = 34 | Xeomin®: 200 U | Reduced cumulative incidence of atrial tachyarrhythmia over 36 months | Hospitalization due to recurrent atrial fibrillation |
| Waldron, 2019 [143] | Randomized, double-blind placebo-controlled trial, N = 130 | Botox®: 250 U | Lower but non-significant incidence of postoperative atrial fibrillation | No difference compared to placebo |
| Complex Regional Pain Syndrome | ||||
| Carroll, 2009 [148] | Randomized, double-blind, controlled, crossover trial, N = 9 | Botulinum-A toxin (unspecified): 250 U | Median duration before analgesic failure was 71 days post-LSB in BoNT treatment group compared to 10 days in untreated group | Temporary nausea and emesis in one participant |
| Lee, 2018 [149] | Retrospective observational trial, N = 18 | Botox®: 100 U | Median duration before analgesic failure was 15 days | None reported |
| MyoblocTM: 5000 U | Median duration before analgesic failure was 69 days | |||
| Yoo, 2022 [145] | Randomized, double-blind control trial, N = 48 | Botulinum-A toxin (Daewoong, South Korea): 75 U | Improved analgesia over 3-month study period compared to levobupivacaine | None reported |
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorizas, A.; Krueger, N.; Sadick, N.S. Aesthetic Uses of the Botulinum Toxin. Dermatol. Clin. 2014, 32, 23–36. [Google Scholar] [CrossRef]
- Erbguth, F.J.; Naumann, M. Historical Aspects of Botulinum Toxin: Justinus Kerner (1786–1862) and the “Sausage Poison”. Neurology 1999, 53, 1850. [Google Scholar] [CrossRef]
- Erbguth, F.J. Historical Notes on Botulism, Clostridium Botulinum, Botulinum Toxin, and the Idea of the Therapeutic Use of the Toxin. Mov. Disord. 2004, 19, S2–S6. [Google Scholar] [CrossRef]
- Scott, A.B.; Fahn, S.; Brin, M.F. Treatment of Strabismus and Blepharospasm with Botox (onabotulinumtoxinA): Development, Insights, and Impact. Medicine 2023, 102, e32374. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, L.L.; Ostrem, J.L.; Bledsoe, I.O. FDA Approvals and Consensus Guidelines for Botulinum Toxins in the Treatment of Dystonia. Toxins 2020, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- Naumann, M.; Jost, W.H.; Toyka, K.V. Botulinum Toxin in the Treatment of Neurological Disorders of the Autonomic Nervous System. Arch. Neurol. 1999, 56, 914. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Hill, K.K.; Raphael, B.H. Historical and Current Perspectives on Clostridium Botulinum Diversity. Res. Microbiol. 2015, 166, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.-H.; Jin, R. Architecture of the Botulinum Neurotoxin Complex: A Molecular Machine for Protection and Delivery. Curr. Opin. Struct. Biol. 2015, 31, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Burgen, A.S.V.; Dickens, F.; Zatman, L.J. The Action of Botulinum Toxin on the Neuro-muscular Junction. J. Physiol. 1949, 109, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Nigam, P.; Nigam, A. Botulinum Toxin. Indian J. Dermatol. 2010, 55, 8. [Google Scholar] [CrossRef] [PubMed]
- Binz, T. Clostridial Neurotoxin Light Chains: Devices for SNARE Cleavage Mediated Blockade of Neurotransmission. In Botulinum Neurotoxins; Rummel, A., Binz, T., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 364, pp. 139–157. ISBN 978-3-642-33569-3. [Google Scholar]
- Blasi, J.; Chapman, E.R.; Link, E.; Binz, T.; Yamasaki, S.; Camilli, P.D.; Südhof, T.C.; Niemann, H.; Jahn, R. Botulinum Neurotoxin A Selectively Cleaves the Synaptic Protein SNAP-25. Nature 1993, 365, 160–163. [Google Scholar] [CrossRef]
- Pantano, S.; Montecucco, C. The Blockade of the Neurotransmitter Release Apparatus by Botulinum Neurotoxins. Cell. Mol. Life Sci. 2014, 71, 793–811. [Google Scholar] [CrossRef] [PubMed]
- Samizadeh, S.; De Boulle, K. Botulinum Neurotoxin Formulations: Overcoming the Confusion. Clin. Cosmet. Investig. Dermatol. 2018, 11, 273–287. [Google Scholar] [CrossRef]
- BOTOX (onabotulinumtoxinA) Label. Allergan, Inc. U.S. Food and Drug Administration Website. Available online: https://www.accessdata.fda.gov/Drugsatfda_docs/Label/2011/103000s5236lbl.Pdf (accessed on 11 December 2023).
- DYSPORT (abobotulinumtoxinA) Label. Ipsen Biopharmaceuticals. U.S. Food and Drug Administration Website. Available online: https://www.accessdata.fda.gov/Drugsatfda_docs/Label/2016/125274s107lbl.Pdf (accessed on 11 December 2023).
- XEOMIN (incobotulinumtoxinA) Label. Merz Pharmaceuticals GmbH. U.S. Food and Drug Administration Website. Available online: https://www.accessdata.fda.gov/Drugsatfda_docs/Label/2018/125360s073lbl.Pdf (accessed on 11 December 2023).
- MYOBLOC (rimabotulinumtoxinB) Label. Solstice Neurosciences). U.S. Food and Drug Administration Website. Available online: https://www.accessdata.fda.gov/Drugsatfda_docs/Label/2019/103846s5190lbl.Pdf (accessed on 11 December 2023).
- JEUVEAU (prabotulinumtoxinA-Xvfs) Label. Evolus Inc. U.S. Food and Drug Administration Website. Available online: https://www.accessdata.fda.gov/Drugsatfda_docs/Label/2019/761085s000lbl.Pdf (accessed on 11 December 2023).
- DAXXIFY (daxibotulinumtoxinA-Lanm) Label. Revance Therapeutics. U.S. Food and Drug Administration Website. Available online: https://www.accessdata.fda.gov/Drugsatfda_docs/Label/2022/761127s000lbl.Pdf (accessed on 11 December 2023).
- Kennelly, M.; Cruz, F.; Herschorn, S.; Abrams, P.; Onem, K.; Solomonov, V.K.; Del Rosario Figueroa Coz, E.; Manu-Marin, A.; Giannantoni, A.; Thompson, C.; et al. Efficacy and Safety of AbobotulinumtoxinA in Patients with Neurogenic Detrusor Overactivity Incontinence Performing Regular Clean Intermittent Catheterization: Pooled Results from Two Phase 3 Randomized Studies (CONTENT1 and CONTENT2). Eur. Urol. 2022, 82, 223–232. [Google Scholar] [CrossRef]
- Sternini, C. Organization of the Peripheral Nervous System: Autonomic and Sensory Ganglia. J. Investig. Dermatol. Symp. Proc. 1997, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Karemaker, J.M. An Introduction into Autonomic Nervous Function. Physiol. Meas. 2017, 38, R89–R118. [Google Scholar] [CrossRef]
- Krassioukov, A.V.; Weaver, L.C. Anatomy of the Autonomic Nervous System. Phys. Med. Rehabil. 1996, 10, 1–14. [Google Scholar]
- Krassioukov, A.V.; Bygrave, M.A.; Puckett, W.R.; Bunge, R.P.; Rogers, K.A. Human Sympathetic Preganglionic Neurons and Motoneurons Retrogradely Labelled with DiI. J. Auton. Nerv. Syst. 1998, 70, 123–128. [Google Scholar] [CrossRef]
- Lundberg, J.M.; Hökfelt, T. Chapter 16 Multiple Co-Existence of Peptides and Classical Transmitters in Peripheral Autonomic and Sensory Neurons—Functional and Pharmacological Implications. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 1986; Volume 68, pp. 241–262. ISBN 978-0-444-80762-5. [Google Scholar]
- Kimura, K.; Low, D.A.; Keller, D.M.; Davis, S.L.; Crandall, C.G. Cutaneous Blood Flow and Sweat Rate Responses to Exogenous Administration of Acetylcholine and Methacholine. J. Appl. Physiol. 2007, 102, 1856–1861. [Google Scholar] [CrossRef]
- Landis, S.C.; Fredieu, J.R. Coexistence of Calcitonin Gene-Related Peptide and Vasoactive Intestinal Peptide in Cholinergic Sympathetic Innervation of Rat Sweat Glands. Brain Res. 1986, 377, 177–181. [Google Scholar] [CrossRef]
- Lindh, B.; Lundberg, J.M.; Hökfelt, T.; Elfvin, L.G.; Fahrenkrug, J.; Fischer, J. Coexistence of CGRP- and VIP-like Immunoreactivities in a Population of Neurons in the Cat Stellate Ganglia. Acta Physiol. Scand. 1987, 131, 475–476. [Google Scholar] [CrossRef]
- Leblanc, G.G.; Trimmer, B.A.; Landis, S.C. Neuropeptide Y-like Immunoreactivity in Rat Cranial Parasympathetic Neurons: Coexistence with Vasoactive Intestinal Peptide and Choline Acetyltransferase. Proc. Natl. Acad. Sci. USA 1987, 84, 3511–3515. [Google Scholar] [CrossRef]
- Wecht, J.M.; La Fountaine, M.F.; Handrakis, J.P.; West, C.R.; Phillips, A.; Ditor, D.S.; Sharif, H.; Bauman, W.A.; Krassioukov, A.V. Autonomic Nervous System Dysfunction Following Spinal Cord Injury: Cardiovascular, Cerebrovascular, and Thermoregulatory Effects. Curr. Phys. Med. Rehabil. Rep. 2015, 3, 197–205. [Google Scholar] [CrossRef]
- Krassioukov, A.V.; Furlan, J.C.; Fehlings, M.G. Autonomic Dysreflexia in Acute Spinal Cord Injury: An Under-Recognized Clinical Entity. J. Neurotrauma 2003, 20, 707–716. [Google Scholar] [CrossRef]
- Vinik, A.I.; Maser, R.E.; Mitchell, B.D.; Freeman, R. Diabetic Autonomic Neuropathy. Diabetes Care 2003, 26, 1553–1579. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.; Kaufmann, H. Treatment of Autonomic Dysfunction in Parkinson Disease and Other Synucleinopathies. Mov. Disord. 2018, 33, 372–390. [Google Scholar] [CrossRef] [PubMed]
- Sukhera, J. Narrative Reviews in Medical Education: Key Steps for Researchers. J. Grad. Med. Educ. 2022, 14, 418–419. [Google Scholar] [CrossRef]
- Ferrari, R. Writing Narrative Style Literature Reviews. Med. Writ. 2015, 24, 230–235. [Google Scholar] [CrossRef]
- Green, B.N.; Johnson, C.D.; Adams, A. Writing Narrative Literature Reviews for Peer-Reviewed Journals: Secrets of the Trade. J. Chiropr. Med. 2006, 5, 101–117. [Google Scholar] [CrossRef]
- McConaghy, J.R.; Fosselman, D. Hyperhidrosis: Management Options. Am. Fam. Physician 2018, 97, 729–734. [Google Scholar]
- Paula Loureiro, M.; de Campos, J.R.M.; de Wolosker, N.; Kauffman, P. (Eds.) Hyperhidrosis: A Complete Guide to Diagnosis and Management; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-89526-0. [Google Scholar]
- Lenefsky, M.; Rice, Z.P. Hyperhidrosis and Its Impact on Those Living with It. Am. J. Manag. Care 2018, 24, S491–S495. [Google Scholar]
- Naumann, M.; Lowe, N.J. Botulinum Toxin Type A in Treatment of Bilateral Primary Axillary Hyperhidrosis: Randomised, Parallel Group, Double Blind, Placebo Controlled. BMJ 2001, 323, 596. [Google Scholar] [CrossRef]
- Naumann, M.; Lowe, N.J.; Kumar, C.R.; Hamm, H. Botulinum Toxin Type A Is a Safe and Effective Treatment for Axillary Hyperhidrosis Over 16 Months: A Prospective Study. Arch. Dermatol. 2003, 139, 731–736. [Google Scholar] [CrossRef]
- Lowe, N.J.; Glaser, D.A.; Eadie, N.; Daggett, S.; Kowalski, J.W.; Lai, P.-Y. Botulinum Toxin Type A in the Treatment of Primary Axillary Hyperhidrosis: A 52-Week Multicenter Double-Blind, Randomized, Placebo-Controlled Study of Efficacy and Safety. J. Am. Acad. Dermatol. 2007, 56, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Dressler, D. Botulinum Toxin Therapy: Its Use for Neurological Disorders of the Autonomic Nervous System. J. Neurol. 2013, 260, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Dressler, D.; Adib Saberi, F.; Benecke, R. Botulinum Toxin Type B for Treatment of Axillar Hyperhidrosis. J. Neurol. 2002, 249, 1729–1732. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, M.; Ceballos-Baumann, A.O.; Plewig, G. Botulinum Toxin A for Axillary Hyperhidrosis (Excessive Sweating). N. Engl. J. Med. 2001, 344, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, M. Low-Dose Efficacy of Botulinum Toxin A for Axillary Hyperhidrosis: A Randomized, Side-By-Side, Open-Label Study. Arch. Dermatol. 2005, 141, 1255. [Google Scholar] [CrossRef] [PubMed]
- Schnider, P.; Binder, M.; Auff, E.; Kittler, H.; Berger, T.; Wolff, K. Double-Blind Trial of Botulinum A Toxin for the Treatment of Focal Hyperhidrosis of the Palms. Br. J. Dermatol. 1997, 136, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.; Slezinger, A.; Halem, M.; Vujevich, J.; Martin, L.K.; Black, L.; Bryde, J. Pilot Study of the Safety and Efficacy of MyoblocTM (Botulinum Toxin Type B) for Treatment of Axillary Hyperhidrosis. Int. J. Dermatol. 2005, 44, 418–424. [Google Scholar] [CrossRef]
- Campanati, A.; Giuliodori, K.; Martina, E.; Giuliano, A.; Ganzetti, G.; Offidani, A. Onabotulinumtoxin Type A (Botox®) versus Incobotulinumtoxin Type A (Xeomin®) in the Treatment of Focal Idiopathic Palmar Hyperhidrosis: Results of a Comparative Double-Blind Clinical Trial. J. Neural Transm. 2014, 121, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.J.; Yamauchi, P.S.; Lask, G.P.; Patnaik, R.; Iyer, S. Efficacy and Safety of Botulinum Toxin Type A in the Treatment of Palmar Hyperhidrosis: A Double-Blind, Randomized, Placebo-Controlled Study. Dermatol. Surg. 2002, 28, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Rystedt, A.; Karlqvist, M.; Bertilsson, M.; Naver, H.; Swartling, C. Effect of Botulinum Toxin Concentration on Reduction in Sweating: A Randomized, Double-Blind Study. Acta Derm. Venereol. 2013, 93, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Simonetta Moreau, M.; Cauhepe, C.; Magues, J.P.; Senard, J.M. A Double-Blind, Randomized, Comparative Study of DysportR vs. BotoxR in Primary Palmar Hyperhidrosis. Br. J. Dermatol. 2003, 149, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Saadia, D.; Voustianiouk, A.; Wang, A.K.; Kaufmann, H. Botulinum Toxin Type A in Primary Palmar Hyperhidrosis: Randomized, Single-Blind, Two-Dose Study. Neurology 2001, 57, 2095–2099. [Google Scholar] [CrossRef] [PubMed]
- Glaser, D.A.; Galperin, T.A. Botulinum Toxin for Hyperhidrosis of Areas Other than the Axillae and Palms/Soles. Dermatol. Clin. 2014, 32, 517–525. [Google Scholar] [CrossRef] [PubMed]
- George, S.M.C.; Atkinson, L.R.; Farrant, P.B.J.; Shergill, B.S. Botulinum Toxin for Focal Hyperhidrosis of the Face. Br. J. Dermatol. 2014, 170, 211–213. [Google Scholar] [CrossRef]
- Anders, D.; Moosbauer, S.; Naumann, M.K.; Hamm, H. Craniofacial Hyperhidrosis Successfully Treated with Botulinum Toxin Type A. Eur. J. Dermatol. EJD 2008, 18, 87–88. [Google Scholar] [CrossRef]
- Glaser, D.A.; Hebert, A.A.; Pariser, D.M.; Solish, N. Facial Hyperhidrosis: Best Practice Recommendations and Special Considerations. Cutis 2007, 79, 29–32. [Google Scholar]
- Alsharqi, A.; Wilson, N.J. Craniofacial Hyperhidrosis in Post-menopausal Women. Australas. J. Dermatol. 2012, 53, 158–159. [Google Scholar] [CrossRef]
- Cabreus, P.; Swartling, C.; Rystedt, A. Postmenopausal Craniofacial Hyperhidrosis Treated with Botulinum Toxin Type B. J. Dermatol. 2019, 46, 874–878. [Google Scholar] [CrossRef]
- Eustace, K.; Wilson, N.J. Postmenopausal Craniofacial Hyperhidrosis. Clin. Exp. Dermatol. 2018, 43, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, T.; Solish, N.; Murray, C. Botulinum Neurotoxin Treatment of Palmar and Plantar Hyperhidrosis. Dermatol. Clin. 2014, 32, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Segal, K.; Lisnyansky, I.; Nageris, B.; Feinmesser, R. Parasympathetic Innervation of the Salivary Glands. Oper. Tech. Otolaryngol.-Head Neck Surg. 1996, 7, 333–338. [Google Scholar] [CrossRef]
- Lakraj, A.; Moghimi, N.; Jabbari, B. Sialorrhea: Anatomy, Pathophysiology and Treatment with Emphasis on the Role of Botulinum Toxins. Toxins 2013, 5, 1010–1031. [Google Scholar] [CrossRef] [PubMed]
- Holsinger, F.C.; Bui, D.T. Anatomy, Function, and Evaluation of the Salivary Glands. In Salivary Gland Disorders; Myers, E.N., Ferris, R.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–16. ISBN 978-3-540-47070-0. [Google Scholar]
- Glickman, S.; Deaney, C.N. Treatment of Relative Sialorrhoea with Botulinum Toxin Type A: Description and Rationale for an Injection Procedure with Case Report. Eur. J. Neurol. 2001, 8, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Hockstein, N.G.; Samadi, D.S.; Gendron, K.; Handler, S.D. Sialorrhea: A Management Challenge. Am. Fam. Phys. 2004, 69, 2628–2634. [Google Scholar]
- Arbouw, M.E.L.; Movig, K.L.L.; Koopmann, M.; Poels, P.J.E.; Guchelaar, H.J.; Egberts, T.C.G.; Neef, C.; Van Vugt, J.P.P. Glycopyrrolate for Sialorrhea in Parkinson Disease: A Randomized, Double-Blind, Crossover Trial. Neurology 2010, 74, 1203–1207. [Google Scholar] [CrossRef]
- Thomsen, T.R.; Galpern, W.R.; Asante, A.; Arenovich, T.; Fox, S.H. Ipratropium Bromide Spray as Treatment for Sialorrhea in Parkinson’s Disease. Mov. Disord. 2007, 22, 2268–2273. [Google Scholar] [CrossRef]
- Bhatia, K.; Munchau, A.; Brown, P. Botulinum Toxin Is a Useful Treatment in Excessive Drooling of Saliva. J. Neurol. Neurosurg. Psychiatry 1999, 67, 697. [Google Scholar] [CrossRef]
- Lagalla, G.; Millevolte, M.; Capecci, M.; Provinciali, L.; Ceravolo, M.G. Botulinum Toxin Type A for Drooling in Parkinson’s Disease: A Double-Blind, Randomized, Placebo-Controlled Study. Mov. Disord. 2006, 21, 704–707. [Google Scholar] [CrossRef]
- Mazlan, M.; Rajasegaran, S.; Engkasan, J.; Nawawi, O.; Goh, K.-J.; Freddy, S. A Double-Blind Randomized Controlled Trial Investigating the Most Efficacious Dose of Botulinum Toxin-A for Sialorrhea Treatment in Asian Adults with Neurological Diseases. Toxins 2015, 7, 3758–3770. [Google Scholar] [CrossRef]
- Restivo, D.; Panebianco, M.; Casabona, A.; Lanza, S.; Marchese-Ragona, R.; Patti, F.; Masiero, S.; Biondi, A.; Quartarone, A. Botulinum Toxin A for Sialorrhoea Associated with Neurological Disorders: Evaluation of the Relationship between Effect of Treatment and the Number of Glands Treated. Toxins 2018, 10, 55. [Google Scholar] [CrossRef]
- Jost, W.H.; Friedman, A.; Michel, O.; Oehlwein, C.; Slawek, J.; Bogucki, A.; Ochudlo, S.; Banach, M.; Pagan, F.; Flatau-Baqué, B.; et al. SIAXI: Placebo-Controlled, Randomized, Double-Blind Study of incobotulinumtoxinA for Sialorrhea. Neurology 2019, 92, e1982–e1991. [Google Scholar] [CrossRef]
- Jost, W.H.; Friedman, A.; Michel, O.; Oehlwein, C.; Slawek, J.; Bogucki, A.; Ochudlo, S.; Banach, M.; Pagan, F.; Flatau-Baqué, B.; et al. Long-Term incobotulinumtoxinA Treatment for Chronic Sialorrhea: Efficacy and Safety over 64 Weeks. Park. Relat. Disord. 2020, 70, 23–30. [Google Scholar] [CrossRef]
- Costa, J.; Rocha, M.L.; Ferreira, J.; Evangelista, T.; Coelho, M.; De Carvalho, M. Botulinum Toxin Type-B Improves Sialorrhea and Quality of Life in Bulbaronset Amyotrophic Lateral Sclerosis. J. Neurol. 2008, 255, 545–550. [Google Scholar] [CrossRef]
- Isaacson, S.H.; Ondo, W.; Jackson, C.E.; Trosch, R.M.; Molho, E.; Pagan, F.; Lew, M.; Dashtipour, K.; Clinch, T.; Espay, A.J.; et al. Safety and Efficacy of RimabotulinumtoxinB for Treatment of Sialorrhea in Adults: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 461. [Google Scholar] [CrossRef] [PubMed]
- Guidubaldi, A.; Fasano, A.; Ialongo, T.; Piano, C.; Pompili, M.; Mascianà, R.; Siciliani, L.; Sabatelli, M.; Bentivoglio, A.R. Botulinum Toxin A versus B in Sialorrhea: A Prospective, Randomized, Double-Blind, Crossover Pilot Study in Patients with Amyotrophic Lateral Sclerosis or Parkinson’s Disease: Botulinum Toxin A and B for Sialorrhea. Mov. Disord. 2011, 26, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Hornby, P.J.; Abrahams, T.P. Central Control of Lower Esophageal Sphincter Relaxation. Am. J. Med. 2000, 108, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Cassella, R.R.; Ellis, F.H.; Brown, A.L. Fine-structure changes in achalasia of the esophagus: I. Vagus nerves. Am. J. Pathol. 1965, 46, 279–288. [Google Scholar] [PubMed]
- Cassella, R.R.; Ellis, F.H.; Brown, A.L. Fine-structure changes in achalasia of esophagus: II. Esophageal smooth muscle. Am. J. Pathol. 1965, 46, 467–475. [Google Scholar]
- Katzka, D.A.; Farrugia, G.; Arora, A.S. Achalasia Secondary to Neoplasia: A Disease with a Changing Differential Diagnosis: Achalasia Secondary to Neoplasia. Dis. Esophagus 2012, 25, 331–336. [Google Scholar] [CrossRef]
- Portale, G.; Costantini, M.; Zaninotto, G.; Ruol, A.; Guirroli, E.; Rampado, S.; Ancona, E. Pseudoachalasia: Not Only Esophago-Gastric Cancer. Dis. Esophagus 2007, 20, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.L.; Katzka, D.A. Achalasia: Update on the Disease and Its Treatment. Gastroenterology 2010, 139, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Pohl, D.; Tutuian, R. Achalasia: An Overview of Diagnosis and Treatment. J. Gastrointest. Liver Dis. JGLD 2007, 16, 297–303. [Google Scholar]
- Wei, P. Botulinum Toxin Injection for the Treatment of Upper Esophageal Sphincter Dysfunction. Toxins 2022, 14, 321. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, P.J.; Ravich, W.J.; Hendrix, T.R.; Sostre, S.; Jones, B.; Kalloo, A.N. Intrasphincteric Botulinum Toxin for the Treatment of Achalasia. N. Engl. J. Med. 1995, 332, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Annese; Bassotti; Coccia; D’Onofrio; Gatto; Repici; Andriulli; The Gismad Achalasia Study Group. Comparison of Two Different Formulations of Botulinum Toxin A for the Treatment of Oesophageal Achalasia. Aliment. Pharmacol. Ther. 1999, 13, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
- Martínek, J.; Široký, M.; Plottová, Z.; Bureš, J.; Hep, A.; Špičák, J. Treatment of Patients with Achalasia with Botulinum Toxin: A Multicenter Prospective Cohort Study. Dis. Esophagus 2003, 16, 204–209. [Google Scholar] [CrossRef]
- Muehldorfer, S.M.; Schneider, T.H.; Hochberger, J.; Martus, P.; Hahn, E.G.; Ell, C. Esophageal Achalasia: Intrasphincteric Injection of Botulinum Toxin A Versus Balloon Dilation. Endoscopy 1999, 31, 517–521. [Google Scholar] [CrossRef]
- Pasricha, P.; Rai, R.; Ravich, W.; Hendrix, T.; Kalloo, A. Botulinum Toxin for Achalasia: Long-Term Outcome and Predictors of Response. Gastroenterology 1996, 110, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jung, H.-Y. The Long-Term Outcome of Balloon Dilation versus Botulinum Toxin Injection in Patients with Primary Achalasia. Korean J. Intern. Med. 2014, 29, 727. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.E.; Lee, J.S.; Lee, T.H.; Kim, J.N.; Hong, S.J.; Kim, J.O.; Kim, H.G.; Jeon, S.R.; Cho, J.Y. Long-Term Outcomes of Balloon Dilation versus Botulinum Toxin Injection in Patients with Primary Achalasia. Korean J. Intern. Med. 2014, 29, 738. [Google Scholar] [CrossRef]
- Leyden, J.E.; Moss, A.C.; MacMathuna, P. Endoscopic Pneumatic Dilation versus Botulinum Toxin Injection in the Management of Primary Achalasia. Cochrane Database Syst. Rev. 2014, 12, CD005046. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Liu, J.; Yang, C. Clinical Study on Combined Therapy of Botulinum Toxin Injection and Small Balloon Dilation in Patients with Esophageal Achalasia. Dig. Surg. 2009, 26, 493–498. [Google Scholar] [CrossRef]
- Jamshidi, R. Anorectal Complaints: Hemorrhoids, Fissures, Abscesses, Fistulae. Clin. Colon Rectal Surg. 2018, 31, 117–120. [Google Scholar] [CrossRef]
- Villalba, H.; Villalba, S.; Abbas, M.A. Anal Fissure: A Common Cause of Anal Pain. Perm. J. 2007, 11, 62–65. [Google Scholar] [CrossRef]
- Lindsey, I.; Jones, O.M.; Cunningham, C. A Contraction Response of the Internal Anal Sphincter to Botulinum Toxin: Does Low-Pressure Chronic Anal Fissure Have a Different Pathophysiology? Response of the Anal Sphincter to Botulinum. Color. Dis. 2011, 13, 1014–1018. [Google Scholar] [CrossRef]
- Amorim, H.; Santoalha, J.; Cadilha, R.; Festas, M.-J.; Barbosa, P.; Gomes, A. Botulinum Toxin Improves Pain in Chronic Anal Fissure. Porto Biomed. J. 2017, 2, 273–276. [Google Scholar] [CrossRef]
- Carter, D.; Dickman, R. The Role of Botox in Colorectal Disorders. Curr. Treat. Options Gastroenterol. 2018, 16, 541–547. [Google Scholar] [CrossRef]
- Gui, D.; Anastasio, G.; Maria, G.; Cassetta, E.; Bentivoglio, A.R.; Albanese, A. Botulinum Toxin for Chronic Anal Fissure. Lancet 1994, 344, 1127–1128. [Google Scholar] [CrossRef]
- Brisinda, G.; Maria, G.; Sganga, G.; Bentivoglio, A.R.; Albanese, A.; Castagneto, M. Effectiveness of Higher Doses of Botulinum Toxin to Induce Healing in Patients with Chronic Anal Fissures. Surgery 2002, 131, 179–184. [Google Scholar] [CrossRef]
- Maria, G.; Cassetta, E.; Gui, D.; Brisinda, G.; Bentivoglio, A.R.; Albanese, A. A Comparison of Botulinum Toxin and Saline for the Treatment of Chronic Anal Fissure. N. Engl. J. Med. 1998, 338, 217–220. [Google Scholar] [CrossRef]
- Maria, G.; Brisinda, G.; Bentivoglio, A.R.; Cassetta, E.; Gui, D.; Albanese, A. Botulinum Toxin Injections in the Internal Anal Sphincter for the Treatment of Chronic Anal Fissure: Long-Term Results after Two Different Dosage Regimens. Ann. Surg. 1998, 228, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Brisinda, G.; Maria, G.; Bentivoglio, A.R.; Cassetta, E.; Gui, D.; Albanese, A. A Comparison of Injections of Botulinum Toxin and Topical Nitroglycerin Ointment for the Treatment of Chronic Anal Fissure. N. Engl. J. Med. 1999, 341, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Berkel, A.E.M.; Rosman, C.; Koop, R.; Van Duijvendijk, P.; Van Der Palen, J.; Klaase, J.M. Isosorbide Dinitrate Ointment vs Botulinum Toxin A ( D Ysport ® ) as the Primary Treatment for Chronic Anal Fissure: A Randomized Multicentre Study. Color. Dis. 2014, 16, O360. [Google Scholar] [CrossRef] [PubMed]
- Brisinda, G.; Albanese, A.; Cadeddu, F.; Bentivoglio, A.R.; Mabisombi, A.; Marniga, G.; Maria, G. Botulinum Neurotoxin to Treat Chronic Anal Fissure: Results of a Randomized ‘Botox vs. Dysport’ Controlled Trial. Aliment. Pharmacol. Ther. 2004, 19, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, A.; Perez, F.; Serrano, P.; Candela, F.; Calpena, R. Long-Term Results of Botulinum Toxin for the Treatment of Chronic Anal Fissure: Prospective Clinical and Manometric Study. Int. J. Color. Dis. 2005, 20, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Rabchevsky, A.G. Autonomic Consequences of Spinal Cord Injury. Compr. Physiol. 2014, 4, 1419–1453. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.J.; Griffiths, D.; De Groat, W.C. The Neural Control of Micturition. Nat. Rev. Neurosci. 2008, 9, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.R. Not All Neurogenic Bladders Are the Same: A Proposal for a New Neurogenic Bladder Classification System. Transl. Androl. Urol. 2016, 5, 12–21. [Google Scholar] [CrossRef]
- Kennelly, M.; Thiruchelvam, N.; Averbeck, M.A.; Konstatinidis, C.; Chartier-Kastler, E.; Trøjgaard, P.; Vaabengaard, R.; Krassioukov, A.; Jakobsen, B.P. Adult Neurogenic Lower Urinary Tract Dysfunction and Intermittent Catheterisation in a Community Setting: Risk Factors Model for Urinary Tract Infections. Adv. Urol. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Walter, M.; Kran, S.L.; Ramirez, A.L.; Rapoport, D.; Nigro, M.K.; Stothers, L.; Kavanagh, A.; Krassioukov, A.V. Intradetrusor OnabotulinumtoxinA Injections Ameliorate Autonomic Dysreflexia While Improving Lower Urinary Tract Function and Urinary Incontinence-Related Quality of Life in Individuals with Cervical and Upper Thoracic Spinal Cord Injury. J. Neurotrauma 2020, 37, 2023–2027. [Google Scholar] [CrossRef]
- Cardarelli, W.J. Managed Care Aspects of Managing Neurogenic Bladder/Neurogenic Detrusor Overactivity. Am. J. Manag. Care 2013, 19, s205–s208. [Google Scholar]
- Kuo, H.-C.; Liao, C.-H.; Chung, S.-D. Adverse Events of Intravesical Botulinum Toxin A Injections for Idiopathic Detrusor Overactivity: Risk Factors and Influence on Treatment Outcome. Eur. Urol. 2010, 58, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Fougere, R.J.; Currie, K.D.; Nigro, M.K.; Stothers, L.; Rapoport, D.; Krassioukov, A.V. Reduction in Bladder-Related Autonomic Dysreflexia after OnabotulinumtoxinA Treatment in Spinal Cord Injury. J. Neurotrauma 2016, 33, 1651–1657. [Google Scholar] [CrossRef]
- Schurch, B.; Stöhrer, M.; Kramer, G.; Schmid, D.M.; Gaul, G.; Hauri, D. Botulinum-a toxin for treating detrusor hyperreflexia in spinal cord injured patients: A new alternative to anticholinergic drugs? Preliminary results. J. Urol. 2000, 164, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Herschorn, S.; Gajewski, J.; Ethans, K.; Corcos, J.; Carlson, K.; Bailly, G.; Bard, R.; Valiquette, L.; Baverstock, R.; Carr, L.; et al. Efficacy of Botulinum Toxin A Injection for Neurogenic Detrusor Overactivity and Urinary Incontinence: A Randomized, Double-Blind Trial. J. Urol. 2011, 185, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kuo, H. The Therapeutic Effects of Repeated Detrusor Injections Between 200 or 300 Units of OnabotulinumtoxinA in Chronic Spinal Cord Injured Patients. Neurourol. Urodyn. 2014, 33, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Grise, P.; Ruffion, A.; Denys, P.; Egon, G.; Chartier Kastler, E. Efficacy and Tolerability of Botulinum Toxin Type A in Patients with Neurogenic Detrusor Overactivity and Without Concomitant Anticholinergic Therapy: Comparison of Two Doses. Eur. Urol. 2010, 58, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Asafu-Adjei, D.; Small, A.; McWilliams, G.; Galea, G.; Chung, D.; Pak, J. The Intravesical Injection of Highly Purified Botulinum Toxin for the Treatment of Neurogenic Detrusor Overactivity. Can. Urol. Assoc. J. 2019, 14, E520. [Google Scholar] [CrossRef] [PubMed]
- Lightner, D.J.; Gomelsky, A.; Souter, L.; Vasavada, S.P. Diagnosis and Treatment of Overactive Bladder (Non-Neurogenic) in Adults: AUA/SUFU Guideline Amendment 2019. J. Urol. 2019, 202, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Dmochowski, R.; Chapple, C.; Nitti, V.W.; Chancellor, M.; Everaert, K.; Thompson, C.; Daniell, G.; Zhou, J.; Haag-Molkenteller, C. Efficacy and Safety of OnabotulinumtoxinA for Idiopathic Overactive Bladder: A Double-Blind, Placebo Controlled, Randomized, Dose Ranging Trial. J. Urol. 2010, 184, 2416–2422. [Google Scholar] [CrossRef]
- Chen, S.-F.; Kuo, H.-C. Therapeutic Outcome and Patient Adherence to Repeated onabotulinumtoxinA Detrusor Injections in Chronic Spinal Cord-Injured Patients and Neurogenic Detrusor Overactivity. J. Formos. Med. Assoc. 2015, 114, 583–589. [Google Scholar] [CrossRef]
- Nitti, V.W.; Dmochowski, R.; Herschorn, S.; Sand, P.; Thompson, C.; Nardo, C.; Yan, X.; Haag-Molkenteller, C.; EMBARK Study Group. OnabotulinumtoxinA for the Treatment of Patients with Overactive Bladder and Urinary Incontinence: Results of a Phase 3, Randomized, Placebo Controlled Trial. J. Urol. 2013, 189, 2186–2193. [Google Scholar] [CrossRef]
- Wecht, J.M.; Krassioukov, A.V.; Alexander, M.; Handrakis, J.P.; McKenna, S.L.; Kennelly, M.; Trbovich, M.; Biering-Sorensen, F.; Burns, S.; Elliott, S.L.; et al. International Standards to Document Autonomic Function Following SCI (ISAFSCI). Top. Spinal Cord Inj. Rehabil. 2021, 27, 23–49. [Google Scholar] [CrossRef]
- Krassioukov, A. Autonomic Function Following Cervical Spinal Cord Injury. Respir. Physiol. Neurobiol. 2009, 169, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Krassioukov, A.; Linsenmeyer, T.A.; Beck, L.A.; Elliott, S.; Gorman, P.; Kirshblum, S.; Vogel, L.; Wecht, J.; Clay, S. Evaluation and Management of Autonomic Dysreflexia and Other Autonomic Dysfunctions: Preventing the Highs and Lows. Top. Spinal Cord Inj. Rehabil. 2021, 27, 225–290. [Google Scholar] [CrossRef]
- Wan, D.; Krassioukov, A.V. Life-Threatening Outcomes Associated with Autonomic Dysreflexia: A Clinical Review. J. Spinal Cord Med. 2014, 37, 2–10. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, M.; Biering-Sørensen, F.; Krassioukov, A.V. Iatrogenic Urological Triggers of Autonomic Dysreflexia: A Systematic Review. Spinal Cord 2015, 53, 500–509. [Google Scholar] [CrossRef]
- Inskip, J.A.; Lucci, V.-E.M.; McGrath, M.S.; Willms, R.; Claydon, V.E. A Community Perspective on Bowel Management and Quality of Life after Spinal Cord Injury: The Influence of Autonomic Dysreflexia. J. Neurotrauma 2018, 35, 1091–1105. [Google Scholar] [CrossRef]
- Li, G.-P.; Wang, X.-Y.; Zhang, Y. Efficacy and Safety of OnabotulinumtoxinA in Patients with Neurogenic Detrusor Overactivity Caused by Spinal Cord Injury: A Systematic Review and Meta-Analysis. Int. Neurourol. J. 2018, 22, 275–286. [Google Scholar] [CrossRef]
- Dorey, T.W.; Walter, M.; Krassioukov, A.V. Reduced Reflex Autonomic Responses Following Intradetrusor OnabotulinumtoxinA Injections: A Pre-/Post-Study in Individuals with Cervical and Upper Thoracic Spinal Cord Injury. Front. Physiol. 2021, 12, 796277. [Google Scholar] [CrossRef]
- Huang, M.; Zheng, H.; Huang, T.; Yang, X.; Liu, Q.; Li, Q.; Tang, P.; Xie, K.; Chen, H. Intravesical Injection of Botulinum Toxin Type a May Be an Effective Treatment Option for Autonomic Dysreflexia in Patients with High-Level Spinal Cord Injury. J. Spinal Cord Med. 2022, 47, 74–78. [Google Scholar] [CrossRef]
- Jung, I.-Y.; Mo, K.I.; Leigh, J.-H. Effect of Intravesical Botulinum Toxin Injection on Symptoms of Autonomic Dysreflexia in a Patient with Chronic Spinal Cord Injury: A Case Report. J. Spinal Cord Med. 2019, 42, 806–809. [Google Scholar] [CrossRef]
- Krassioukov, A.; Warburton, D.E.; Teasell, R.; Eng, J.J. A Systematic Review of the Management of Autonomic Dysreflexia after Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2009, 90, 682–695. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2014, 130, 2071–2104. [Google Scholar] [CrossRef] [PubMed]
- Keidar, N.; Elul, Y.; Schuster, A.; Yaniv, Y. Visualizing and Quantifying Irregular Heart Rate Irregularities to Identify Atrial Fibrillation Events. Front. Physiol. 2021, 12, 637680. [Google Scholar] [CrossRef] [PubMed]
- Romanov, A.; Pokushalov, E.; Ponomarev, D.; Bayramova, S.; Shabanov, V.; Losik, D.; Stenin, I.; Elesin, D.; Mikheenko, I.; Strelnikov, A.; et al. Long-Term Suppression of Atrial Fibrillation by Botulinum Toxin Injection into Epicardial Fat Pads in Patients Undergoing Cardiac Surgery: Three-Year Follow-up of a Randomized Study. Heart Rhythm 2019, 16, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Staerk, L.; Sherer, J.A.; Ko, D.; Benjamin, E.J.; Helm, R.H. Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes. Circ. Res. 2017, 120, 1501–1517. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P. Thromboembolic Complications in Atrial Fibrillation. Stroke 1990, 21, 4–13. [Google Scholar] [CrossRef]
- Arsenault, K.A.; Yusuf, A.M.; Crystal, E.; Healey, J.S.; Morillo, C.A.; Nair, G.M.; Whitlock, R.P. Interventions for Preventing Post-Operative Atrial Fibrillation in Patients Undergoing Heart Surgery. Cochrane Database Syst. Rev. 2013, 2013, CD003611. [Google Scholar] [CrossRef]
- Waldron, N.H.; Cooter, M.; Haney, J.C.; Schroder, J.N.; Gaca, J.G.; Lin, S.S.; Sigurdsson, M.I.; Fudim, M.; Podgoreanu, M.V.; Stafford-Smith, M.; et al. Temporary Autonomic Modulation with Botulinum Toxin Type A to Reduce Atrial Fibrillation after Cardiac Surgery. Heart Rhythm 2019, 16, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Schwartzman, R.J. Systemic Complications of Complex Regional Pain Syndrome. Neurosci. Med. 2012, 03, 225–242. [Google Scholar] [CrossRef]
- Yoo, Y.; Lee, C.-S.; Kim, J.; Jo, D.; Moon, J.Y. Botulinum Toxin Type A for Lumbar Sympathetic Ganglion Block in Complex Regional Pain Syndrome: A Randomized Trial. Anesthesiology 2022, 136, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Tereshko, Y.; Dalla Torre, C.; Lettieri, C.; Belgrado, E.; Gigli, G.L.; Valente, M. Subcutaneous BoNT/A Injection for Intractable Pain and Disability in Complex Regional Pain Syndrome: A Case Report. Toxins 2022, 14, 411. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.-S.; Noor, N.; Urits, I.; Paladini, A.; Sadhu, M.S.; Gibb, C.; Carlson, T.; Myrcik, D.; Varrassi, G.; Viswanath, O. Complex Regional Pain Syndrome: A Comprehensive Review. Pain Ther. 2021, 10, 875–892. [Google Scholar] [CrossRef] [PubMed]
- Carroll, I.; Clark, J.D.; Mackey, S. Sympathetic Block with Botulinum Toxin to Treat Complex Regional Pain Syndrome. Ann. Neurol. 2009, 65, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, C.; Choi, E.; Lee, P.; Lee, H.-J.; Nahm, F. Lumbar Sympathetic Block with Botulinum Toxin Type A and Type B for the Complex Regional Pain Syndrome. Toxins 2018, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.Y.; Park, E.S. Immunogenicity of Botulinum Toxin. Arch. Plast. Surg. 2022, 49, 12–18. [Google Scholar] [CrossRef]
- Dressler, D.; Adib Saberi, F. New Formulation of Botox: Complete Antibody-Induced Treatment Failure in Cervical Dystonia. J. Neurol. Neurosurg. Psychiatry 2007, 78, 108–109. [Google Scholar] [CrossRef] [PubMed]
- Solish, N.; Carruthers, J.; Kaufman, J.; Rubio, R.G.; Gross, T.M.; Gallagher, C.J. Overview of DaxibotulinumtoxinA for Injection: A Novel Formulation of Botulinum Toxin Type A. Drugs 2021, 81, 2091–2101. [Google Scholar] [CrossRef] [PubMed]
- Dressler, D.; Adib Saberi, F. Botulinum Toxin: Mechanisms of Action. Eur. Neurol. 2005, 53, 3–9. [Google Scholar] [CrossRef] [PubMed]

| Chemical, Brand Name, and Company | Initial FDA Approval | Dosage | Approved General Indications | Approved Autonomic Indications | Contraindications |
|---|---|---|---|---|---|
| OnabotulinumtoxinA, Botox®, Allergan Inc. [15] | 1989 | 50 units (U), 100, U, 200 U vials Do not exceed the lesser of 400 U or 7 U/kg in a 3-month interval for adults Do not exceed the lesser of 340 U or 10 U/kg 3-month interval for pediatric patients | Chronic migraine, spasticity, cervical dystonia, blepharospasm, strabismus | Overactive bladder, neurogenic detrusor overactivity, severe axillary hyperhidrosis | Known hypersensitivity to botulinum toxin product or infection at proposed injection site, urinary tract infection, urinary retention for intradetrusor injections, or in patients not willing and able to have clean intermittent catheterization initiated |
| AbobotulinumtoxinA, Dysport®, Ipsen Pharmaceuticals [16] | 2009 | 300 U or 500 U vials | Adult cervical dystonia, spasticity, glabellar lines | Neurogenic detrusor overactivity incontinence (European Union) [21] | Known hypersensitivity to botulinum toxin product, cow’s milk protein, or infection at proposed injection site |
| IncobotulinumtoxinA, Xeomin®, Merz [17] | 2010 | 50 U, 100 U, or 200 U vials | Spasticity, adult cervical dystonia, blepharospasm, hemifacial spasm, glabellar lines | Chronic sialorrhea in adults | Known hypersensitivity to botulinum toxin product or infection at proposed injection site |
| RimabotulinumtoxinB, MyoblocTM or NeuroBlocTM, Solstice Neurosciences [18] | 2000 | 2500 U, 5000 U, 10,000 U vials | Cervical dystonia | Sialorrhea | Known hypersensitivity to botulinum toxin product or infection at proposed injection site |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rempel, L.; Malik, R.N.; Shackleton, C.; Calderón-Juárez, M.; Sachdeva, R.; Krassioukov, A.V. From Toxin to Treatment: A Narrative Review on the Use of Botulinum Toxin for Autonomic Dysfunction. Toxins 2024, 16, 96. https://doi.org/10.3390/toxins16020096
Rempel L, Malik RN, Shackleton C, Calderón-Juárez M, Sachdeva R, Krassioukov AV. From Toxin to Treatment: A Narrative Review on the Use of Botulinum Toxin for Autonomic Dysfunction. Toxins. 2024; 16(2):96. https://doi.org/10.3390/toxins16020096
Chicago/Turabian StyleRempel, Lucas, Raza N. Malik, Claire Shackleton, Martín Calderón-Juárez, Rahul Sachdeva, and Andrei V. Krassioukov. 2024. "From Toxin to Treatment: A Narrative Review on the Use of Botulinum Toxin for Autonomic Dysfunction" Toxins 16, no. 2: 96. https://doi.org/10.3390/toxins16020096
APA StyleRempel, L., Malik, R. N., Shackleton, C., Calderón-Juárez, M., Sachdeva, R., & Krassioukov, A. V. (2024). From Toxin to Treatment: A Narrative Review on the Use of Botulinum Toxin for Autonomic Dysfunction. Toxins, 16(2), 96. https://doi.org/10.3390/toxins16020096






