Impact of Environmental Conditions on the Concentrations of Trichothecenes, Their Glucosides, and Emerging Fusarium Toxins in Naturally Contaminated, Irradiated, and Fusarium langsethiae Inoculated Oats
Abstract
:1. Introduction
2. Results
2.1. Method Validation Performance in the Oat Matrix
2.2. Initial Mycotoxin Concentrations of Oats
2.3. Impact of aw and Temperature on the Concentrations of T-2, HT-2, and HT-2-Glc in All Oat Treatments
2.4. The Effect of the Interactions of Water Activity, Temperature (T), and Treatments on the Percentage Concentration Ratios of HT-2-Glucoside
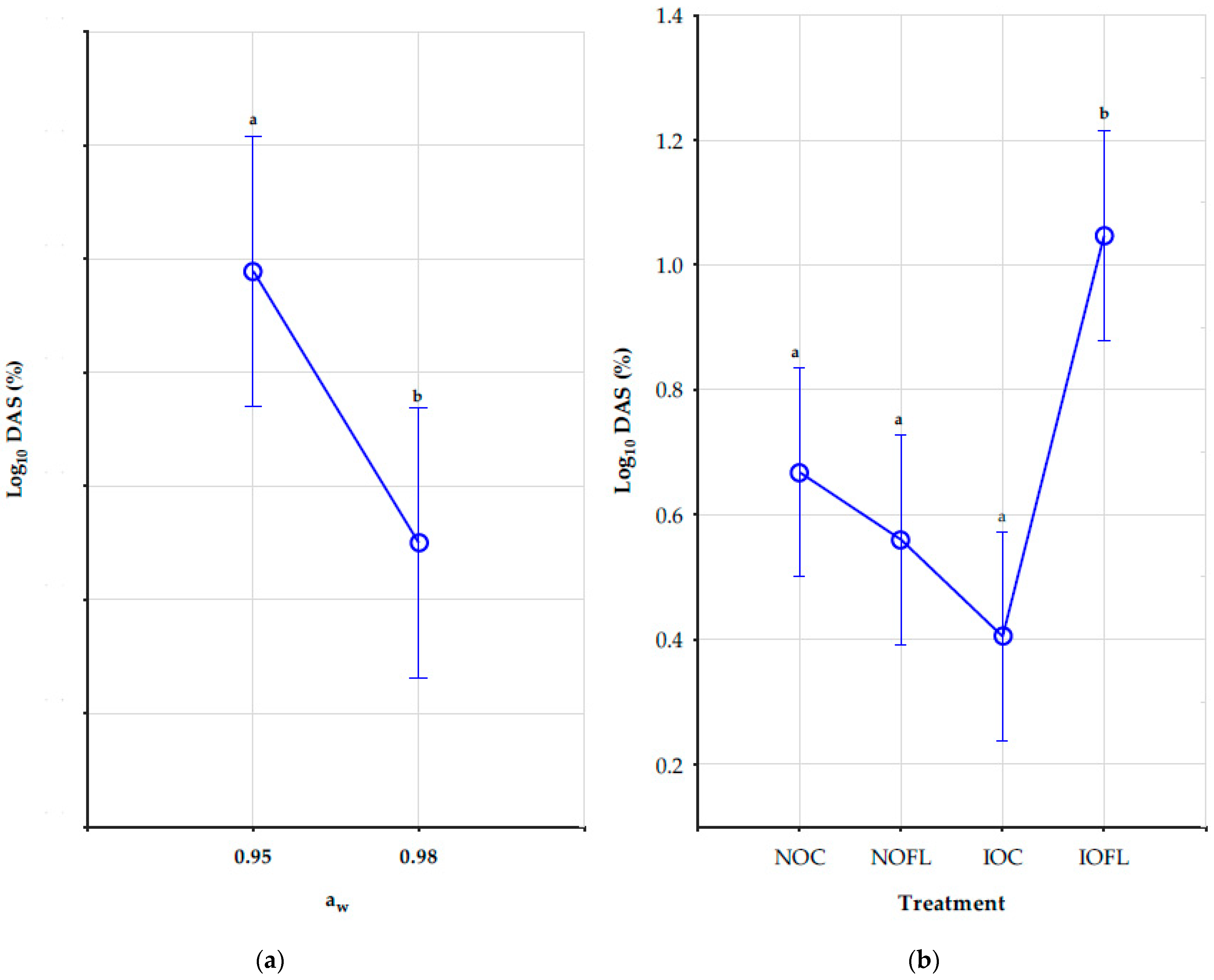
2.5. Impact of Water Activity, Temperature, and Treatments on DAS Concentrations
2.6. Impact of aw and Temperature on DON and DON-3-G in Both Natural and Irradiated Oat Grains with and without Fusarium langsethiae Inoculation
2.7. Impact of aw and Temperature on the Concentrations of Other Secondary Metabolites and Emerging Mycotoxins in Natural and Irradiated Oat Grains with and without Fusarium langsethiae Inoculation
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Fungal Isolates
5.2. Oat Grains and Moisture Adsorption Curve Analysis
5.3. Grain Inoculation
5.4. Mycotoxin Analysis
5.4.1. Chemical Reagents
5.4.2. Sample Preparation and Extraction
5.4.3. LC-MS/MS Parameters
5.4.4. Optimised LC-MS/MS Method Validation
5.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kosiak, B.; Torp, M.; Thrane, U.L.F. The occurrence of Fusarium spp. in Norwegian grain-A survey. Cereal Res. Commun. 1997, 25, 595–596. [Google Scholar] [CrossRef]
- Langseth, W.; Rundberget, T. The occurrence of HT-2 toxin and other trichothecenes in Norwegian cereals. Mycopathologia 1999, 147, 157–165. [Google Scholar] [CrossRef]
- Imathiu, S.M.; Edwards, S.G.; Ray, R.V.; Back, M.A. Fusarium langsethiae—A HT-2 and T-2 toxins producer that needs more attention. J. Phytopathol. 2013, 161, 1–10. [Google Scholar] [CrossRef]
- Edwards, S.G. Investigation of Fusarium Mycotoxins in UK Barley and Oat Production; HGCA Project Report No. 415; HGCA: London, UK, 2007. [Google Scholar]
- Opoku, N.; Back, M.; Edwards, S.G. Development of Fusarium langsethiae in commercial cereal production. Eur. J. Plant Pathol. 2013, 136, 159–170. [Google Scholar] [CrossRef]
- Hofgaard, I.S.; Aamot, H.U.; Torp, T.; Jestoi, M.; Lattanzio, V.M.T.; Klemsdal, S.S.; Waalwijk, C.; Van der Lee, T.; Brodal, G. Associations between Fusarium species and mycotoxins in oats and spring wheat from farmers’ fields in Norway over a six-year period. World Mycotoxin J. 2016, 9, 365–378. [Google Scholar] [CrossRef]
- Morcia, C.; Tumino, G.; Ghizzoni, R.; Badeck, F.W.; Lattanzio, V.M.; Pascale, M.; Terzi, V. Occurrence of Fusarium langsethiae and T-2 and HT-2 toxins in Italian malting barley. Toxins 2016, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, A.; Wang, J.; Leifert, C. Effect of Organic and Conventional Cereal Production Methods on Fusarium Head Blight and Mycotoxin Contamination Levels. Agronomy 2022, 12, 797. [Google Scholar] [CrossRef]
- Venslovas, E.; Mankevičienė, A.; Kochiieru, Y.; Janavičienė, S.; Dabkevičius, Z.; Bartkevičs, V.; Bērziņa, Z.; Pavlenko, R. The Effect of Ustilago maydis and Delayed Harvesting on A- and B-Type Trichothecene Concentrations in Maize Grain. J. Fungi 2023, 9, 794. [Google Scholar] [CrossRef] [PubMed]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; et al. Risk to human and animal health related to the presence of 4, 15-diacetoxyscirpenol in food and feed. Efsa J. 2018, 16, e05367. [Google Scholar] [PubMed]
- Hoerr, F.J.; Carlton, W.W.; Yagen, B. Mycotoxicosis caused by a single dose of T-2 toxin or diacetoxyscirpenol in broiler chickens. Vet. Pathol. 1981, 18, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Haschek, W.M.; Beasley, V.R. Trichothecene mycotoxins. In Handbook of Toxicology of Chemical Warfare Agents; Academic Press: Cambridge, MA, USA, 2009; pp. 353–369. [Google Scholar]
- CDC. Centers for Disease Control and Prevention United States. 2002; Select Agents and Toxins List. Available online: https://www.selectagents.gov/sat/list.htm (accessed on 30 December 2022).
- Brake, J.; Hamilton, P.B.; Kittrell, R.S. Effects of the trichothecene mycotoxin diacetoxyscirpenol on feed consumption, body weight, and oral lesions of broiler breeders. Poult. Sci. 2000, 79, 856–863. [Google Scholar] [CrossRef]
- Parikka, P.; Hakala, K.; Tiilikkala, K. Expected shifts in Fusarium species’ composition on cereal grain in Northern Europe due to climatic change. Food Addit. Contam. 2012, 29, 1543–1555. [Google Scholar] [CrossRef]
- Fredlund, E.; Gidlund, A.; Sulyok, M.; Börjesson, T.; Krska, R.; Olsen, M.; Lindblad, M. Deoxynivalenol and other selected Fusarium toxins in Swedish oats—Occurrence and correlation to specific Fusarium species. Int. J. Food Microbiol. 2013, 167, 276–283. [Google Scholar] [CrossRef]
- Imathiu, S.M.; Ray, R.V.; Back, M.; Hare, M.C.; Edwards, S.G. Fusarium langsethiae pathogenicity and aggressiveness towards oats and wheat in wounded and unwounded in vitro detached leaf assays. Eur. J. Plant Pathol. 2009, 124, 117–126. [Google Scholar] [CrossRef]
- Boenisch, M.J.; Schäfer, W. Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant Biol. 2011, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- De Boevre, M.; Di Mavungu, J.D.; Maene, P.; Audenaert, K.; Deforce, D.; Haesaert, G.; Eeckhout, M.; Callebaut, A.; Berthiller, F.; Van Peteghem, C.; et al. Development and validation of an LC-MS/MS method for the simultaneous determination of deoxynivalenol, zearalenone, T-2-toxin and some masked metabolites in different cereals and cereal-derived food. Food Addit. Contam. 2012, 29, 819–835. [Google Scholar] [CrossRef]
- Rasmussen, P.H.; Nielsen, K.F.; Ghorbani, F.; Spliid, N.H.; Nielsen, G.C.; Jørgensen, L.N. Occurrence of different trichothecenes and deoxynivalenol-3-β-D-glucoside in naturally and artificially contaminated Danish cereal grains and whole maize plants. Mycotoxin Res. 2012, 28, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Bryła, M.; Waśkiewicz, A.; Podolska, G.; Szymczyk, K.; Jędrzejczak, R.; Damaziak, K.; Sułek, A. Occurrence of 26 mycotoxins in the grain of cereals cultivated in Poland. Toxins 2016, 8, 160. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Canady, R.A.; Coker, R.D.; Egan, S.K.; Krska, R.; Olsen, M.; Resnik, S.; Schlatter, J. T-2 and HT-2 toxins. Safety evaluation of certain mycotoxins in food. WHO Food Addit. Ser. 2001, 47, 557–597. [Google Scholar]
- Meneely, J.; Greer, B.; Kolawole, O.; Elliott, C. T-2 and HT-2 Toxins: Toxicity, occurrence and analysis: A review. Toxins 2023, 15, 481. [Google Scholar] [CrossRef]
- Venturini, M.C.; Quiroga, M.A.; Risso, M.A.; Di Lorenzo, C.; Omata, Y.; Venturini, L.; Godoy, H. Mycotoxin T-2 and aflatoxin B1 as immunosuppressors in mice chronically infected with Toxoplasma gondii. J. Comp. Pathol. 1996, 115, 229–237. [Google Scholar] [CrossRef]
- Arcella, D.; Gergelova, P.; Innocenti, M.L.; Steinkellner, H. Human and animal dietary exposure to T-2 and HT-2 toxin. EFSA J. 2017, 15, e04972. [Google Scholar]
- Lattanzio, V.M.; Visconti, A.; Haidukowski, M.; Pascale, M. Identification, and characterization of new Fusarium masked mycotoxins, T2 and HT2 glycosyl derivatives, in naturally contaminated wheat and oats by liquid chromatography–high-resolution mass spectrometry. J. Mass. Spectrom. 2012, 47, 466–475. [Google Scholar] [CrossRef]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; Saeger, S.D.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef]
- Stenglein, S.A. Fusarium poae: A pathogen that needs more attention. J. Plant Pathol. 2009, 91, 25–36. [Google Scholar]
- Imathiu, S.M.; Hare, M.C.; Ray, R.V.; Back, M.; Edwards, S.G. Evaluation of pathogenicity and aggressiveness of F. langsethiae on oat and wheat seedlings relative to known seedling blight pathogens. Eur. J. Plant Pathol. 2010, 126, 203–216. [Google Scholar] [CrossRef]
- Medina, A.; Magan, N. Comparisons of water activity and temperature impacts on growth of Fusarium langsethiae strains from northern Europe on oat-based media. Int. J. Food Microbiol. 2010, 142, 365–369. [Google Scholar] [CrossRef]
- Mylona, K.; Magan, N. Fusarium langsethiae: Storage environment influences dry matter losses and T2 and HT-2 toxin contamination of oats. J. Stored Prod. Res. 2011, 47, 321–327. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2021/808 of 22 March 2021 on the performance of analytical methods for residues of pharmacologically active substances used in food-producing animals and on the interpretation of results as well as on the methods. Off. J. Eur. Union 2021, 180, 84–109. [Google Scholar]
- European Union. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 49, 5–24. [Google Scholar]
- European Food Safety Authority (EFSA). Arecella, D.; Gómez Ruiz, J.A. Use of cut-off values on the limits of quantification reported in datasets used to estimate dietary exposure to chemical contaminants. Efsa J. 2018, 15, 1452E. [Google Scholar]
- Aldred, D.; Magan, N. Prevention strategies for trichothecenes. Toxicol. Lett. 2004, 153, 165–171. [Google Scholar] [CrossRef]
- Hjelkrem, A.G.R.; Aamot, H.U.; Brodal, G.; Strand, E.C.; Torp, T.; Edwards, S.G.; Dill-Macky, R.; Hofgaard, I.S. HT-2 and T-2 toxins in Norwegian oat grains related to weather conditions at different growth stages. Eur. J. Plant Pathol. 2018, 151, 501–514. [Google Scholar] [CrossRef]
- Cairns-Fuller, V.; Aldred, D.; Magan, N. Water, temperature and gas composition interactions affect growth and ochratoxin A production by isolates of Penicillium verrucosum on wheat grain. J. Appl. Microbiol. 2005, 99, 1215–1221. [Google Scholar] [CrossRef]
- Chulze, S.N.; Palazzini, J.M.; Lullien-Pellerin, V.; Ramirez, M.L.; Cuniberti, M.; Magan, N. Fusarium species infection in wheat: Impact on quality and mycotoxin accumulation. Wheat Qual. Improv. Process. Hum. Health 2020, 421–452. [Google Scholar] [CrossRef]
- Daud, N.; Currie, V.; Duncan, G.; Filipe, J.A.N.; Yoshinari, T.; Stoddart, G.; Roberts, D.; Gratz, S.W. Free and Modified Mycotoxins in Organic and Conventional Oats (Avena sativa L.) Grown in Scotland. Toxins 2023, 15, 247. [Google Scholar] [CrossRef]
- Medina, A.; Magan, N. Temperature, and water activity effects on production of T-2 and HT-2 by Fusarium langsethiae strains from north European countries. Food Microbiol. 2011, 28, 392–398. [Google Scholar] [CrossRef]
- Lattanzio, V.M.; Solfrizzo, M.; Visconti, A. Enzymatic hydrolysis of T-2 toxin for the quantitative determination of total T-2 and HT-2 toxins in cereals. Anal. Bioanal. Chem. 2009, 395, 1325–1334. [Google Scholar] [CrossRef]
- Kokkonen, M.; Ojala, L.; Parikka, P.; Jestoi, M. Mycotoxin production of selected Fusarium species at different culture conditions. Int. J. Food Microbiol. 2010, 143, 17–25. [Google Scholar] [CrossRef]
- Scudamore, K.; Patel, S.; Edwards, S. HT-2 toxin and T-2 toxin in commercial cereal processing in the United Kingdom, 2004–2007. World Mycotoxin J. 2009, 2, 357–365. [Google Scholar] [CrossRef]
- Bryła, M.; Jędrzejczak, R.; Szymczyk, K.; Roszko, M.; Obiedziński, M.W. An LC-IT-MS/MS-based method to determine trichothecenes in grain products. Food Anal. Methods 2014, 7, 1056–1065. [Google Scholar] [CrossRef]
- Nathanail, A.V.; Syvahuoko, J.; Malachova, A.; Jestoi, M.; Varga, E.; Michlmayr, H.; Adam, G.; Sievilainen, E.; Berthiller, F.; Peltonen, K. Simultaneous Determination of Major Type A and B Trichothecenes, Zearalenone and Certain Modified Metabolites in Finnish Cereal Grains with a Novel Liquid Chromatography-Tandem Mass Spectrometric Method. Anal. Bioanal. Chem. 2015, 407, 4745–4755. [Google Scholar] [CrossRef]
- Schöneberg, T.; Jenny, E.; Wettstein, F.E.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Musa, T.; Seifert, K.; Gräfenhan, T.; Keller, B.; et al. Occurrence of Fusarium species and mycotoxins in Swiss oats—Impact of cropping factors. Eur. J. Agron. 2018, 92, 123–132. [Google Scholar] [CrossRef]
- Uhlig, S.; Torp, M.; Heier, B.T. Beauvericin and enniatins A, A1, B and B1 in Norwegian grain: A survey. Food Chem. 2006, 94, 193–201. [Google Scholar] [CrossRef]
- WHO/FAO. Safety Evaluation of Certain Mycotoxins in Food. Prepared by the Fifty-Sixth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); WHO Food Additives Series 47; FAO food and nutrition paper 74; International Programme on Chemical Safety; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Schuhmacher-Wolz, U.; Heine, K.; Schneider, K. Report on toxicity data on trichothecene mycotoxins HT-2 and T-2 toxins. EFSA Support. Publ. 2010, 7, 65. [Google Scholar] [CrossRef]
- Michlmayr, H.; Varga, E.; Malachová, A.; Fruhmann, P.; Piątkowska, M.; Hametner, C.; Šofrová, J.; Jaunecker, G.; Häubl, G.; Lemmens, M.; et al. UDP-glucosyltransferases from rice, Brachypodium, and barley: Substrate specificities and synthesis of type A and B trichothecene-3-O-β-D-glucosides. Toxins 2018, 10, 111. [Google Scholar] [CrossRef]
- Malachová, A.; Sulyok, M.; Beltrán, E.; Berthiller, F.; Krska, R. Optimization and validation of a quantitative liquid chromatography–tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J. Chromatogr. 2014, 1362, 145–156. [Google Scholar] [CrossRef]
- Siri-Anusornsak, W.; Kolawole, O.; Mahakarnchanakul, W.; Greer, B.; Petchkongkaew, A.; Meneely, J.; Elliott, C.; Vangnai, K. The occurrence and co-occurrence of regulated, emerging, and masked mycotoxins in rice bran and maize from Southeast Asia. Toxins 2022, 14, 567. [Google Scholar] [CrossRef]

| Components | RSD (%) | RA (%) | SSE (%) | RE (%) | LOD (ng/g) | LOQ (ng/g) |
|---|---|---|---|---|---|---|
| Moniliformin | 6 | 87 | 116 | 74 | 2.0 | 6.7 |
| 15-Acetyldeoxynivalenol | 13 | 57 | 65 | 95 | 2.8 | 9.2 |
| 3-Acetyldeoxynivalenol | 3 | 95 | 94 | 99 | 2.2 | 7.3 |
| Beauvericin | 5 | 94 | 92 | 101 | 2.6 | 8.6 |
| Diacetoxyscirpenol | 5 | 93 | 89 | 100 | 1.9 | 6.4 |
| Deoxynivalenol | 3 | 96 | 96 | 101 | 3.7 | 12.4 |
| DON-3-Glucoside | 5 | 69 | 88 | 77 | 1.7 | 5.5 |
| Enniatin A | 7 | 86 | 84 | 103 | 1.0 | 3.4 |
| Enniatin A1 | 5 | 89 | 91 | 98 | 1.6 | 5.4 |
| Enniatin B | 9 | 97 | 101 | 98 | 5.1 | 17.0 |
| Enniatin B1 | 7 | 94 | 96 | 98 | 2.8 | 9.2 |
| HT-2 toxin | 3 | 94 | 96 | 98 | 4.1 | 13.6 |
| HT-2-Glc | 5 | 93 | 93 | 95 | 5.6 | 15.8 |
| Nivalenol | 22 | 80 | 85 | 92 | 19.0 | 63.2 |
| T-2-toxin | 2 | 94 | 94 | 99 | 3.4 | 11.4 |
| Mycotoxins (ng/g of Grains) a | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20 °C | 25 °C | ||||||||
| Treatments | aw | T-2 1 | HT-2 2 | HT-2-Glc 3 | Sum | T-2 1 | HT-2 2 | HT-2-Glc 3 | Sum |
| Naturally contaminated oat control | 0.95 | <LOQ | 26 | 41 | 67 | <LOD | <LOD | <LOD | - |
| 0.98 | <LOD | <LOD | <LOQ | - | <LOD | <LOD | <LOQ | - | |
| Naturally contaminated oat + F. langsethiae | 0.95 | <LOQ | <LOQ | <LOQ | - | <LOD | <LOQ | <LOD | - |
| 0.98 | <LOQ | <LOQ | 37 | 37 | <LOD | <LOQ | <LOQ | - | |
| Irradiated oat control | 0.95 | <LOQ | 20 | <LOD | 20 | <LOQ | <LOQ | <LOQ | - |
| 0.98 | <LOQ | <LOQ | <LOD | - | <LOQ | <LOQ | <LOD | - | |
| Irradiated oat + F. langsethiae | 0.95 | 4488 * | 1655 * | 704 * | 6847 | 1822 * | 645 * | 226 * | 2693 |
| 0.98 | 7152 | 6037 | 5925 | 19,114 | 4117 | 7167 | 3690 | 14,974 | |
| Mycotoxins (ng/g of Grains) a | |||||||
|---|---|---|---|---|---|---|---|
| 20 °C | 25 °C | ||||||
| Treatments | aw | DON 1 | DON-3-Glc 2 | Sum | DON 1 | DON-3-Glc 2 | Sum |
| Naturally contaminated oat control | 0.95 | 72 | 18 | 90 | 27 | 6 | 33 |
| 0.98 | 5137 | 1869 | 7006 | 2212 * | 818 * | 3030 | |
| Naturally contaminated oat + F. langsethiae | 0.95 | 1141 * | 58 * | 1199 | 1636 * | 64 * | 1700 |
| 0.98 | 3995 * | 1061 * | 5056 | 5052 * | 2297 * | 7349 | |
| Irradiated oat control | 0.95 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 0.98 | 21 * | 7 * | 28 | 33 * | 8 * | 41 | |
| Irradiated oat + F. langsethiae | 0.95 | 20 | <LOQ | 20 | 14 | <LOQ | 14 |
| 0.98 | 55 * | 7 * | 62 | 16 * | 7 * | 23 | |
| Mycotoxins (ng/g of Grains) a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | T (°C) | aW | 3-AcDON | 15-AcDON | NIV | ENN A | ENN A1 | ENN B | ENN B1 | MON | BEA |
| Naturally contaminated oat control | 20 | 0.95 | 27 | 29 | 137 | <LOQ | <LOQ | 19 | 13 | 9 | 29 |
| 0.98 | 89 | 126 | 138 | 8 | 56 | 251 | 192 | 205 | 142 | ||
| 25 | 0.95 | 10 | 29 | 230 | <LOQ | 6 | 19 | 17 | 63 | 51 | |
| 0.98 | 26 | 89 | 90 | 25 | 190 | 539 | 461 | 3050 | 834 | ||
| Naturally contaminated oat + F. langsethiae | 20 | 0.95 | 9 | 62 | 237 | <LOQ | <LOQ | <LOQ | <LOQ | 13 | 23 |
| 0.98 | 26 | 192 | 860 | <LOQ | 9 | 45 | 29 | <LOQ | 141 | ||
| 25 | 0.95 | 21 | 93 | 206 | <LOQ | <LOQ | <LOQ | <LOQ | 19 | 22 | |
| 0.98 | 92 | 172 | 610 | <LOQ | 20 | 88 | 62 | 21 | 142 | ||
| Irradiated oat control | 20 | 0.95 | <LOD | 12 | 38 | <LOD | <LOD | <LOD | 13 | <LOD | 4 |
| 0.98 | <LOD | 15 | 42 | <LOD | <LOD | <LOD | 13 | <LOD | 12 | ||
| 25 | 0.95 | <LOD | 27 | 55 | <LOD | <LOD | <LOD | 24 | <LOD | 15 | |
| 0.98 | <LOD | 33 | 60 | <LOD | <LOD | <LOD | 21 | <LOD | 22 | ||
| Irradiated oat + F. langsethiae | 20 | 0.95 | <LOQ | 44 | 70 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 25 |
| 0.98 | <LOQ | 68 | 90 | <LOQ | <LOQ | <LOQ | <LOQ | <LOD | 47 | ||
| 25 | 0.95 | <LOQ | 47 | 147 | <LOQ | <LOQ | <LOQ | 12 | <LOD | 36 | |
| 0.98 | <LOQ | 81 | <LOQ | <LOQ | <LOQ | <LOQ | 12 | <LOD | 39 | ||
| Mycotoxins | Precursor Ion (m/z) | Product Ion (m/z) | DP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|
| Regulated mycotoxins | |||||
| Deoxynivalenol | 297.1 | 249.1 | 91 | 21 | 20 |
| 297.1 | 203.2 | 91 | 21 | 20 | |
| T-2 toxin | 484.3 | 215.2 | 76 | 29 | 18 |
| 484.3 | 185.1 | 76 | 31 | 11 | |
| HT-2 toxin | 442.3 | 263.1 | 71 | 19 | 14 |
| 442.3 | 215.1 | 71 | 19 | 22 | |
| Masked Mycotoxins | |||||
| 3-Acetyldeoxynivalenol | 397.3 | 59.2 | −60 | −38 | −8 |
| 397.3 | 307.1 | −60 | −20 | −7 | |
| 15-Acetyldeoxynivalenol | 339.1 | 321.3 | 81 | 13 | 18 |
| 339.1 | 261.1 | 81 | 17 | 14 | |
| Deoxynivalenol-3-Glucoside | 517.2 | 427.1 | −115 | −30 | −11 |
| 517.2 | 457.2 | −115 | −20 | −19 | |
| HT-2-Glc | 604.3 | 323.1 | 101 | 17 | 16 |
| 604.3 | 263.1 | 101 | 23 | 14 | |
| Emerging mycotoxins | |||||
| Beauvericin | 801.3 | 784.3 | 141 | 27 | 14 |
| 801.3 | 244.1 | 141 | 43 | 12 | |
| Diacetoxyscirpenol | 384.2 | 307.3 | 86 | 17 | 16 |
| 384.2 | 247.3 | 86 | 21 | 14 | |
| Enniatin A | 699.4 | 682.4 | 10 | 27 | 24 |
| 699.4 | 210.2 | 10 | 39 | 22 | |
| Enniatin B | 657.3 | 640.3 | 10 | 27 | 22 |
| 657.3 | 196.1 | 10 | 39 | 10 | |
| Enniatin B1 | 671.3 | 654.4 | 6 | 27 | 22 |
| 671.3 | 196.1 | 6 | 41 | 22 | |
| Enniatin A1 | 685.4 | 668.5 | 11 | 27 | 12 |
| 685.4 | 210.1 | 11 | 39 | 10 | |
| Moniliformin | 96.9 | 41.2 | −5 | −38 | −14 |
| Nivalenol | 371.1 | 281.1 | −90 | −20 | −15 |
| 371.1 | 59.1 | −90 | −50 | −7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oluwakayode, A.; Greer, B.; Meneely, J.; Berthiller, F.; Krska, R.; Medina, A. Impact of Environmental Conditions on the Concentrations of Trichothecenes, Their Glucosides, and Emerging Fusarium Toxins in Naturally Contaminated, Irradiated, and Fusarium langsethiae Inoculated Oats. Toxins 2024, 16, 166. https://doi.org/10.3390/toxins16040166
Oluwakayode A, Greer B, Meneely J, Berthiller F, Krska R, Medina A. Impact of Environmental Conditions on the Concentrations of Trichothecenes, Their Glucosides, and Emerging Fusarium Toxins in Naturally Contaminated, Irradiated, and Fusarium langsethiae Inoculated Oats. Toxins. 2024; 16(4):166. https://doi.org/10.3390/toxins16040166
Chicago/Turabian StyleOluwakayode, Abimbola, Brett Greer, Julie Meneely, Franz Berthiller, Rudolf Krska, and Angel Medina. 2024. "Impact of Environmental Conditions on the Concentrations of Trichothecenes, Their Glucosides, and Emerging Fusarium Toxins in Naturally Contaminated, Irradiated, and Fusarium langsethiae Inoculated Oats" Toxins 16, no. 4: 166. https://doi.org/10.3390/toxins16040166
APA StyleOluwakayode, A., Greer, B., Meneely, J., Berthiller, F., Krska, R., & Medina, A. (2024). Impact of Environmental Conditions on the Concentrations of Trichothecenes, Their Glucosides, and Emerging Fusarium Toxins in Naturally Contaminated, Irradiated, and Fusarium langsethiae Inoculated Oats. Toxins, 16(4), 166. https://doi.org/10.3390/toxins16040166






