Standard Quality Characteristics and Efficacy of a New Third-Generation Antivenom Developed in Colombia Covering Micrurus spp. Venoms
Abstract
:1. Introduction
2. Results
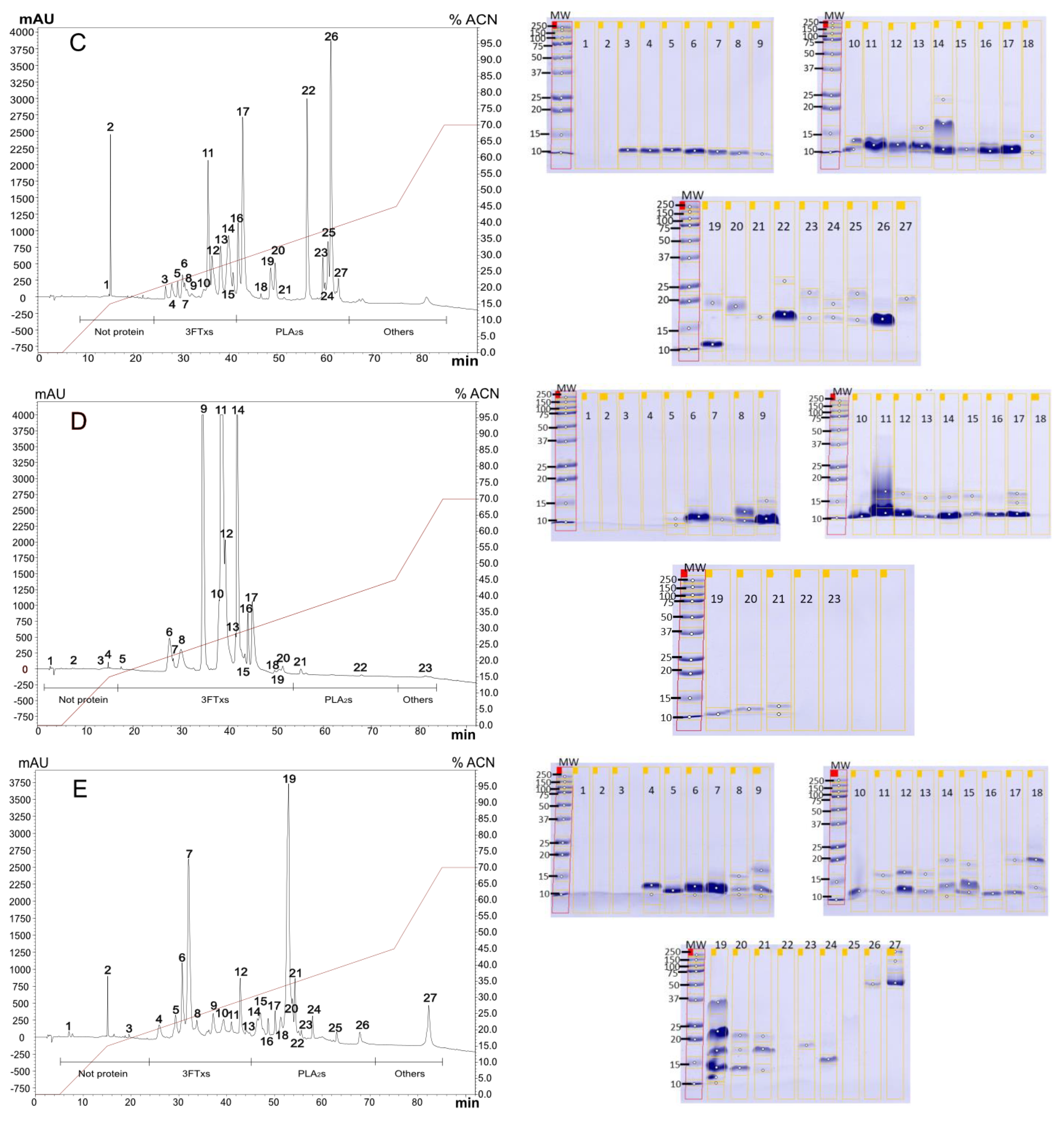
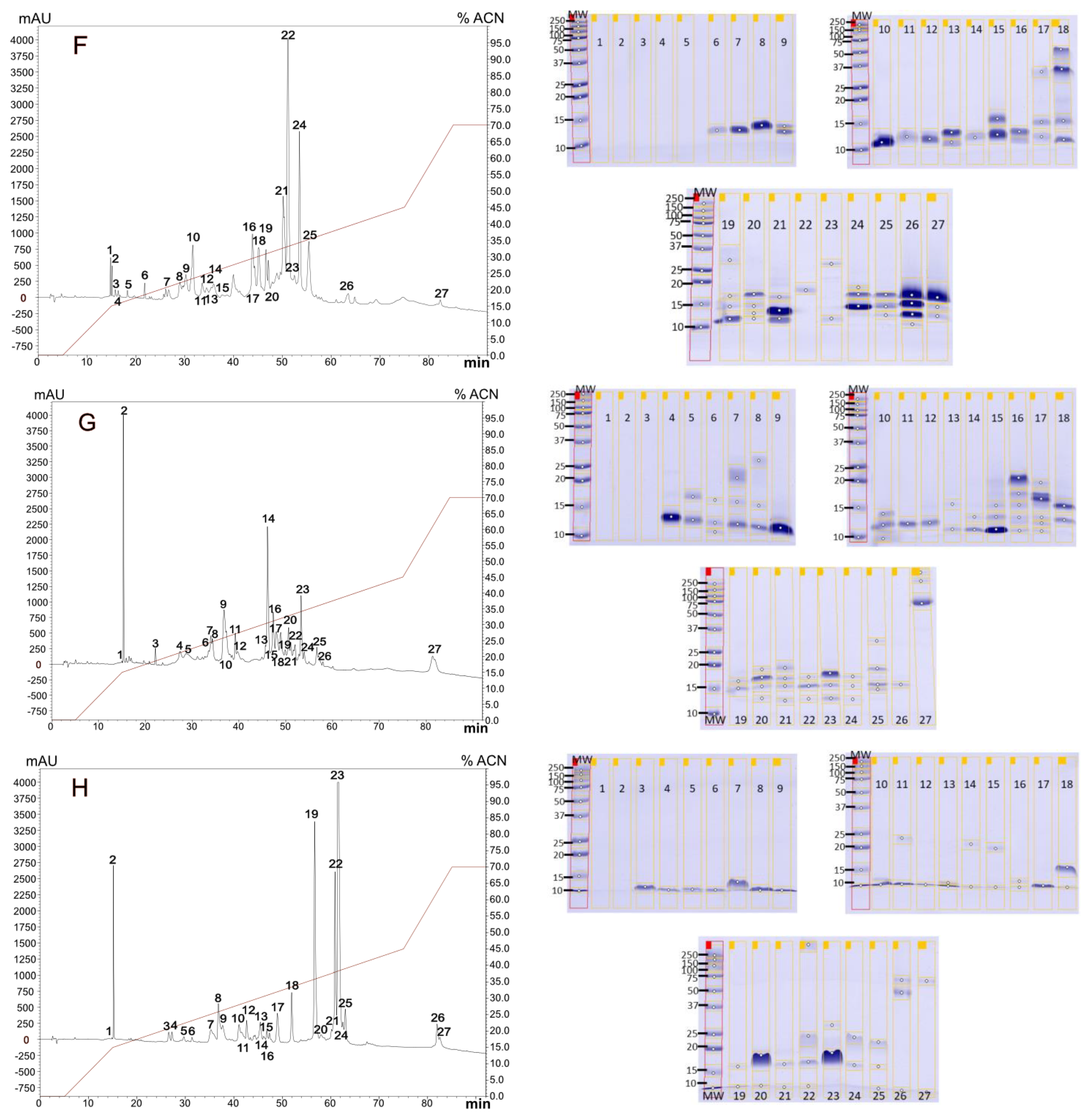
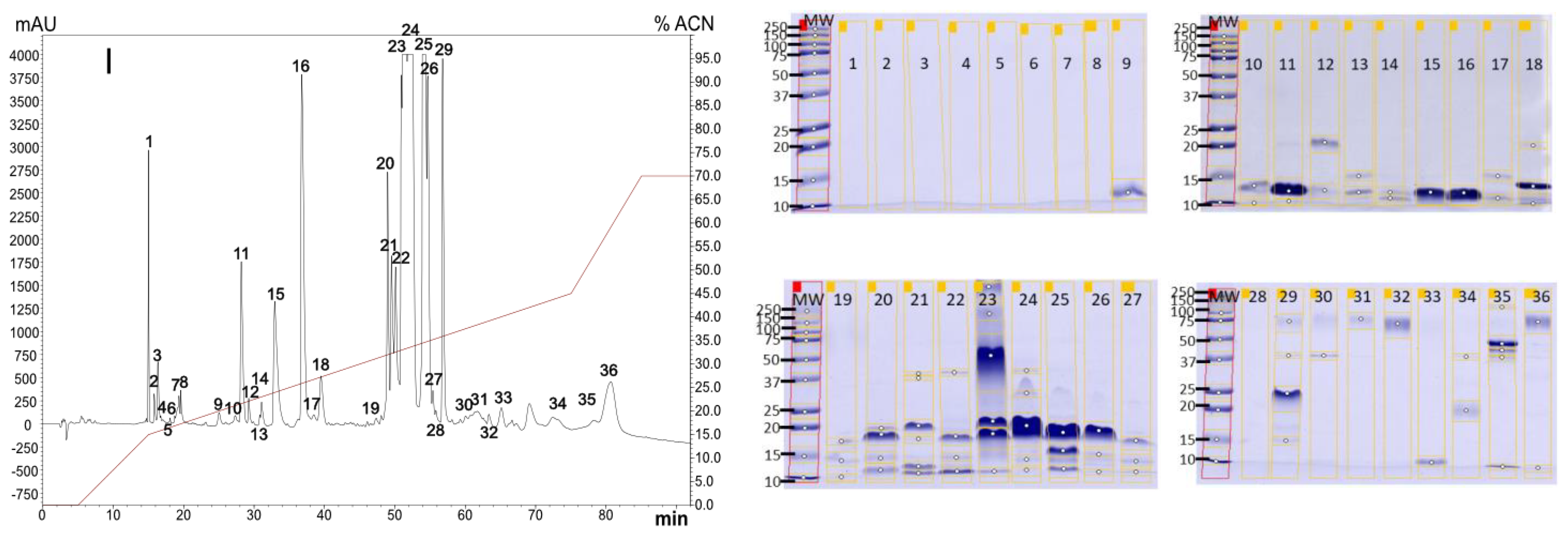
2.1. Venoms Analysis
2.1.1. Diversity in Venom Composition
2.1.2. Venoms Lethality
2.2. Antivenom Physicochemical Characterization
2.2.1. Antivenom Appearance, Solubility Time, Extractable Volume, and pH
2.2.2. Antivenom Total Protein and Albumin Concentration
2.2.3. Antivenom Purity, Integrity, and Molecular Size Distribution
2.2.4. Antivenom Identity (Ouchterlony)
2.3. Preclinical Tests
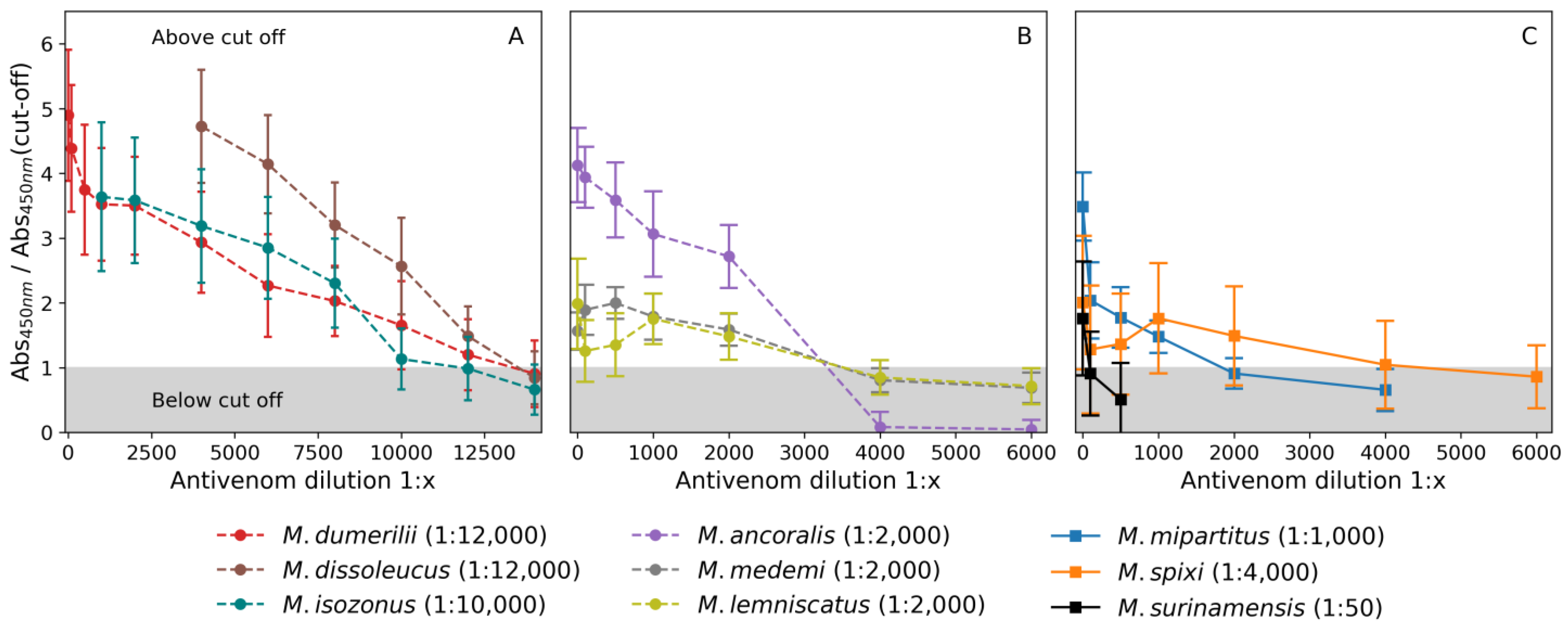
2.3.1. Antivenom Binding Titer
2.3.2. ED50
2.3.3. Acute Toxicity
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals
5.2. Antivenom
5.3. Venom Obtention
5.4. Venom Analysis
5.4.1. RP-HPLC and SDS-PAGE Analysis
5.4.2. Lethality Confirmation
5.5. Antivenom Physicochemical Characterization
5.5.1. Antivenom Appearance, Solubility, Extractable Volume, and pH
5.5.2. Total Protein Concentration
5.5.3. Albumin Concentration
5.5.4. Identity
5.5.5. Immunoglobulin Fragments Purity and Integrity
5.5.6. Molecular-Size Distribution
5.6. Preclinical Tests
5.6.1. Venom Binding Capacity (Titers)
5.6.2. ED50
5.6.3. Acute Toxicity
5.7. Statistical Analisis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter Check | Changes Observed for Doses Tested | |||
|---|---|---|---|---|
| 9 mg | 12 mg | 15 mg | ||
| Central nervous system and somatomotor | 0/3 | 0/3 | 0/3 | |
| Behavior | Aggressive, sedation, unusual, and others. | 0/3 | 0/3 | 0/3 |
| Movement | Shaking, ataxia, catatonia, paralysis, convulsions, and others. | 0/3 | 0/3 | 0/3 |
| Reactivity to stimulus | Irritability, passivity, anesthesia, hyperesthesia, and others. | 0/3 | 0/3 | 0/3 |
| Brain and spinal reflexes | Absent reflexes, others. | 0/3 | 0/3 | 0/3 |
| Muscular tone | Rigidity, flaccidity, and others. | 0/3 | 0/3 | 0/3 |
| Autonomic nervous system | 0/3 | 0/3 | 0/3 | |
| Pupil measurement | Miosis, Mydriasis, and others. | 0/3 | 0/3 | 0/3 |
| Respiratory | 0/3 | 0/3 | 0/3 | |
| Nostrils | Colored, not colored, others. | 0/3 | 0/3 | 0/3 |
| Character | Dyspnoea, bradypnea, and others. | 0/3 | 0/3 | 0/3 |
| Cardiovascular | 0/3 | 0/3 | 0/3 | |
| Palpitations in the cardiac region | Bradycardia, tachycardia, arrhythmia, heartbeat or pulsations, and others. | 0/3 | 0/3 | 0/3 |
| Gastrointestinal | 0/3 | 0/3 | 0/3 | |
| Events | Diarrhea, constipation, and others. | 0/3 | 0/3 | 0/3 |
| Belly shape | Flatulence, contractions, others. | 0/3 | 0/3 | 0/3 |
| Stool consistency and color | Formless, black, clay color, and others. | 0/3 | 0/3 | 0/3 |
| Genitourinary | 0/3 | 0/3 | 0/3 | |
| Vulva and mammary glands | Swelling, others. | 0/3 | 0/3 | 0/3 |
| Penis | Collapsed, others. | 0/3 | 0/3 | 0/3 |
| Perineal region | Dirty, others. | 0/3 | 0/3 | 0/3 |
| Skin and hair | 0/3 | 0/3 | 0/3 | |
| Color, turgidities, and integrity | Eruptions, redness, flaccidity, piloerection, and others. | 0/3 | 0/3 | 0/3 |
| Membranes and mucose | 0/3 | 0/3 | 0/3 | |
| Conjunctive | Congestion, hemorrhage, cyanosis, and others. | 0/3 | 0/3 | 0/3 |
| Eyes | 0/3 | 0/3 | 0/3 | |
| Eyelids | Ptosis, others. | 0/3 | 0/3 | 0/3 |
| Eyeball | Exophthalmos, others. | 0/3 | 0/3 | 0/3 |
| Interferences | Nystagmus, opacity, and others. | 0/3 | 0/3 | 0/3 |
| Others | 0/3 | 0/3 | 0/3 | |
| Temperature | Abnormal, others. | 0/3 | 0/3 | 0/3 |
| Injection point | Swelling, others. | 0/3 | 0/3 | 0/3 |
| General conditions | Abnormal posture, emancipation, others. | 0/3 | 0/3 | 0/3 |
| Weight | 20.8 ± 0.65 g | 21.5 ± 0.81 | 20.4 ± 0.74 | |
| Water consumption | 14.42 g | 13.5 g | 17.2 g | |
| Food consumption | 19.9 g | 20.4 g | 16.3 g | |
References
- Lynch, J.; Angarita, T.; Ruiz, F. Programa Nacional Para La Conservación De Las Serpientes Presentes En Colombia; Ministerio de Ambiente y Desarrollo Sostenible: Bogotá, Colombia, 2014; ISBN 9789588901183.
- Uetz, P.; Freed, P.; Hošek, J. The Reptile Database. Choice Rev. Online 2012, 49, 49–6294. [Google Scholar] [CrossRef]
- Instituto Nacional de Salud. Accidente Ofídico Periodo Epidemiológico 2022. Available online: https://www.ins.gov.co/buscador-eventos/Informesdeevento/ACCIDENTEOFIDICOPEXIII2022.pdf (accessed on 18 April 2023).
- Lomonte, B.; Rey-Suárez, P.; Fernández, J.; Sasa, M.; Pla, D.; Vargas, N.; Bénard-Valle, M.; Sanz, L.; Corrêa-Netto, C.; Núñez, V.; et al. Venoms of Micrurus Coral Snakes: Evolutionary Trends in Compositional Patterns Emerging from Proteomic Analyses. Toxicon 2016, 122, 7–25. [Google Scholar] [CrossRef]
- Rey-Suárez, P.; Núñez, V.; Gutiérrez, J.M.; Lomonte, B. Proteomic and Biological Characterization of the Venom of the Redtail Coral Snake, Micrurus Mipartitus (Elapidae), from Colombia and Costa Rica. J. Proteom. 2011, 75, 655–667. [Google Scholar] [CrossRef]
- Rey-Suárez, P.; Núñez, V.; Fernández, J.; Lomonte, B. Integrative Characterization of the Venom of the Coral Snake Micrurus Dumerilii (Elapidae) from Colombia: Proteome, Toxicity, and Cross-Neutralization by Antivenom. J. Proteom. 2016, 136, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Naturalista Comisión Nacional Para El Conocimiento y Uso de La Biodiversidad. Available online: https://colombia.inaturalist.org/ (accessed on 7 May 2023).
- Campbell, J.; Lamar, W.; Brodie, E. The Venomous Reptiles of the Western Hemisphere; Comstock, Cornell University Press: Ithaca, NY, USA, 2004. [Google Scholar]
- Pardal, P.P.d.O.; Pardal, J.S.d.O.; Gadelha, M.A.d.C.; Rodrigues, L.d.S.; Feitosa, D.T.; Prudente, A.L.d.C.; Fan, H.W. Envenenamento Por Coral Do Gênero Micrurus Na Amazônia Brasileira: Relato de Dois Casos. Rev. Inst. Med. Trop. Sao Paulo 2010, 52, 333–337. [Google Scholar] [CrossRef]
- Cañas, C.A.; Castro-Herrera, F.; Castaño-Valencia, S. Envenomation by the Red-Tailed Coral Snake (Micrurus mipartitus) in Colombia. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.L.; Gutmann, L. Snake Venoms and the Neuromuscular Junction. Semin Neurol. 2004, 24, 175–179. [Google Scholar] [CrossRef]
- Estrada-Gómez, S.; Vargas-Muñoz, L.J.; Higuita-Gutiérrez, L.F. Epidemiology of Snake Bites Linked with the Antivenoms Production in Colombia 2008–2020: Produced Vials Do Not Meet the Needs. Drug Healthc. Patient. Saf. 2022, 14, 171–184. [Google Scholar] [CrossRef]
- Temprano, G.; Aprea, P.; Christian Dokmetjian, J. La Producción Pública de Antivenenos En La Región de Las Américas Como Factor Clave En Su Accesibilidad. Rev. Panam. De Salud Pública 2017, 41, 1. [Google Scholar] [CrossRef]
- Mendes, G.F.; Stuginski, D.R.; Loibel, S.M.C.; De Morais-Zani, K.; Da Rocha, M.M.T.; Fernandes, W.; Sant’anna, S.S.; Grego, K.F. Factors That Can Influence the Survival Rates of Coral Snakes (Micrurus coralnus) for Antivenom Production. J. Anim. Sci. 2019, 97, 972–980. [Google Scholar] [CrossRef]
- INVIMA Medicamentos Vitales No Disponibles. Available online: https://www.invima.gov.co/medicamentos-vitales-no-disponibles (accessed on 13 November 2021).
- Chippaux, J.P.; Goyffon, M. Venoms, Antivenoms and Immunotherapy. Toxicon 1998, 36, 823–846. [Google Scholar] [CrossRef]
- Sarmiento, K.; Rodríguez, A.; Quevedo-Buitrago, W.; Torres, I.; Ríos, C.; Ruíz, L.; Salazar, J.; Hidalgo-Martínez, P.; Diez, H. Comparación de La Eficacia, La Seguridad y La Farmacocinética de Los Antivenenos Antiofídicos: Revisión de Literatura. Univ. Médica 2019, 61, 30–51. [Google Scholar] [CrossRef]
- Vázquez, H.; Olvera, F.; Alagón, A.; Sevcik, C. Production of Anti-Horse Antibodies Induced by IgG, F(Ab’)2 and Fab Applied Repeatedly to Rabbits. Effect on Antivenom Pharmacokinetics. Toxicon 2013, 76, 362–369. [Google Scholar] [CrossRef]
- World Health Organization. Annex 5: Guidelines for the production, control and regulation of snake antivenom immunoglobulins. In WHO Expert Committee on Biological Standardization; World Health Organization: Geneva, Switzerland, 2017; pp. 197–388. Available online: http://www.jstor.org/stable/resrep44225.16 (accessed on 1 May 2023).
- Sanz, L.; de Freitas-Lima, L.N.; Quesada-Bernat, S.; Graça-de-Souza, V.K.; Soares, A.M.; Calderón, L.d.A.; Calvete, J.J.; Caldeira, C.A.S. Comparative Venomics of Brazilian Coral Snakes: Micrurus Frontalis, Micrurus Spixii Spixii, and Micrurus Surinamensis. Toxicon 2019, 166, 39–45. [Google Scholar] [CrossRef]
- Sanz, L.; Quesada-Bernat, S.; Ramos, T.; Casais-e-Silva, L.L.; Corrêa-Netto, C.; Silva-Haad, J.J.; Sasa, M.; Lomonte, B.; Calvete, J.J. New Insights into the Phylogeographic Distribution of the 3FTx/PLA 2 Venom Dichotomy across Genus Micrurus in South America. J. Proteom. 2019, 200, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Otero, R.; Osorio, R.G.; Valderrama, R.; Giraldo, C.A. Efectos Farmacologicos y Enzimaticos de Los Venenos de Serpientes de Antioquia y Choco (Colombia). Toxicon 1992, 30, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Istvan Lazar GelAnalyzer. Available online: http://www.gelanalyzer.com/index.html (accessed on 1 May 2023).
- Diehl, L.; Morse, M. A Comparison of Selected Organ Weights and Clinical Pathology Parameters in Male and Female CD-1 and CByB6F1 Hybrid Mice 12–14 Weeks in Age; Charles River: Spencerville, OH, USA, 2015. [Google Scholar]
- Rey-Suárez, P.; Floriano, R.S.; Rostelato-Ferreira, S.; Saldarriaga-Córdoba, M.; Núñez, V.; Rodrigues-Simioni, L.; Lomonte, B. Mipartoxin-I, a Novel Three-Finger Toxin, Is the Major Neurotoxic Component in the Venom of the Redtail Coral Snake Micrurus Mipartitus (Elapidae). Toxicon 2012, 60, 851–863. [Google Scholar] [CrossRef]
- Rodríguez-Vargas, A.; Franco-Vásquez, A.M.; Bolívar-Barbosa, J.A.; Vega, N.; Reyes-Montaño, E.; Arreguín-Espinosa, R.; Carbajal-Saucedo, A.; Angarita-Sierra, T.; Ruiz-Gómez, F. Unveiling the Venom Composition of the Colombian Coral Snakes Micrurus Helleri, M. Medemi, and M. Sangilensis. Toxins 2023, 15, 622. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; León, G.; Lomonte, B.; Angulo, Y. Antivenoms for Snakebite Envenomings. Inflamm. Allergy Drug Targets 2011, 10, 369–380. [Google Scholar] [CrossRef]
- Castillo-Beltrán, M.C.; Hurtado-Gómez, J.P.; Corredor-Espinel, V.; Ruiz-Gómez, F.J. A Polyvalent Coral Snake Antivenom with Broad Neutralization Capacity. PLoS Negl. Trop Dis. 2018, 13, e0007250. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.; Laing, G.; Smith, D.C.; Theakston, D.; Landon, J. A New Antivenom to Treat Eastern Coral Snake (Micrurus Fulvius Fulvius) Envenoming. Toxicon 1994, 32, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Solano, S.; Segura, Á.; León, G.; Gutiérrez, J.M.; Burnouf, T. Low PH Formulation of Whole IgG Antivenom: Impact on Quality, Safety, Neutralizing Potency and Viral Inactivation. Biologicals 2012, 40, 129–133. [Google Scholar] [CrossRef]
- Cádiz, A.; Esther Castellano, M.; Hernández, J.; Castillo, J.; Mustelier, P.; Teresa Ramírez, A. Influencia Del PH Sobre La Estabilidad de Preparados de Inmunoglobulinas Intravenosas Para Uso Humano Durante El Almacenamiento. Vaccimonitor 2000, 9, 21–25. [Google Scholar]
- Zurbano, B.N.; Tavarone, E.; Viacava, B.G.; Dokmetjian, J.C.; Cascone, O.; Fingermann, M. Critical Aspects on Traditional Antivenom Production Processes and Their Optimization by Factorial Analysis. Biologicals 2020, 68, 65–73. [Google Scholar] [CrossRef]
- Gutiérrez, J.M. Envenenamientos Por Mordeduras de Serpientes En América Latina y El Caribe: Una Visión Integral de Carácter Regional. Bol. Malariol. Salud Ambient. 2011, 51, 1–16. [Google Scholar]
- Serna-Trejos, J.S.; Erazo-Zamora, A.M.; Ibarguen-Gómez, W.T.; Vergara-Portocarrero, M.L. Escenario Epidemiológico Del Accidente Ofídico En Colombia, 2023. Rev. Exp. En Med. Del Hosp. Reg. Lambayeque 2023, 9, 124–125. [Google Scholar] [CrossRef]
- Albus, U. Guide for the Care and Use of Laboratory Animals (8th Edn). Lab. Anim. 2012, 46, 267–268. [Google Scholar] [CrossRef]
- He, F. Laemmli-SDS-PAGE. Bio. Protoc. 2011, 1, e80. [Google Scholar] [CrossRef]
- Zheng, K.; Wu, L.; He, Z.; Yang, B.; Yang, Y. Measurement of the Total Protein in Serum by Biuret Method with Uncertainty Evaluation. Measurement 2017, 112, 16–21. [Google Scholar] [CrossRef]
- Doumas, B.T.; Bayse, D.D.; Carter, R.J.; Peters, T.; Schaffer, R. A Candidate Reference Method for Determination of Total Protein in Serum. I. Development and Validation. Clin. Chem. 1981, 27, 1642–1650. [Google Scholar] [CrossRef]
- Doumas, B.T.; Ard Watson, W.; Biggs, H.G. Albumin Standards and the Measurement of Serum Albumin with Bromcresol Green. Clin. Chim. Acta 1971, 31, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ouchterlony, O. Antigen-Antibody Reactions in Gels. Acta Pathol. Microbiol. Scand. 1949, 26, 507–515. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Otero-Patiño, R.; Segura, Á.; Herrera, M.; Angulo, Y.; León, G.; Gutiérrez, J.M.; Barona, J.; Estrada, S.; Pereañez, A.; Quintana, J.C.; et al. Comparative Study of the Efficacy and Safety of Two Polyvalent, Caprylic Acid Fractionated [IgG and F(Ab’)2] Antivenoms, in Bothrops Asper Bites InColombia. Toxicon 2012, 59, 344–355. [Google Scholar] [CrossRef]
- Weil, C.S. Tables for Convenient Calculation of Median-Effective Dose (LD 50 or ED 50 ) and Instructions in Their Use. Biometrics 1952, 8, 249. [Google Scholar] [CrossRef]


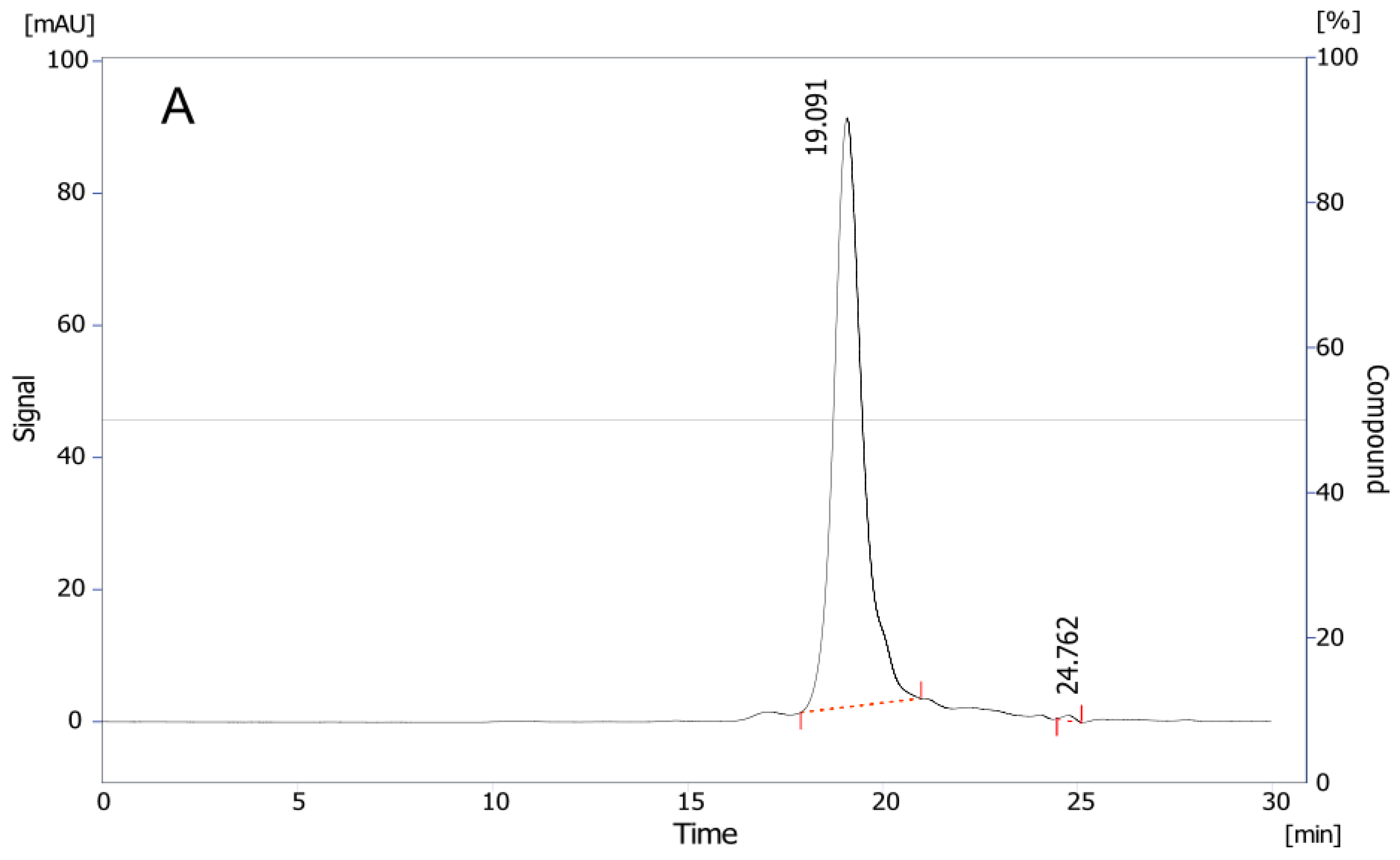

| Vial | pH | Dilution 100% (Minutes:Seconds) | Extractable Volume (mL) |
|---|---|---|---|
| 1 | 6.01 | 3:53 | 10.63 |
| 2 | 6.05 | 4:24 | 10.53 |
| 3 | 5.96 | 4:31 | 10.52 |
| 4 | 6.08 | 4:26 | 10.16 |
| 5 | 6.11 | 4:45 | 10.09 |
| 6 | 6.06 | 4:32 | 10.42 |
| Average ± standard deviation | 6.04 ± 0.05 | 4:25 ± 0:17 | 10.39 ± 0.19 |
| Vial | Total Protein mg/mL | Albumin mg/mL (%albumin/Protein) |
|---|---|---|
| 1 | 33.82 | 0.377 (1.115) |
| 2 | 33.57 | 0.377 (1.123) |
| 3 | 31.37 | 0.333 (1.062) |
| 4 | 28.74 | 0.421 (1.146) |
| 5 | 29.11 | 0.377 (1.295) |
| 6 | 29.18 | 0.377 (1.295) |
| Average ± standard deviation | 30.97 ± 2.3 | 0.377 ± 0.02 (1.22% ± 0.15) |
| INS antivenom control | 42.68 | 1.05 (2.46%) |
| Venom Used (3xLD50) | Venom to Be Neutralized for 1 mL of Antivenom (µg) | Mice Survivor |
|---|---|---|
| M. mipartitus | 100 | 3/3 |
| 200 | 2/3 | |
| 300 | 1/3 | |
| 400 | 0/3 | |
| 232 (Confirmation test) | 2/4 | |
| M. dumerilii | 250 | 3/3 |
| 350 | 2/3 | |
| 450 | 1/3 | |
| 600 | 3/3 | |
| 399 (Confirmation test) | 2/4 |
| Doses of Antivenom (mg) | Mice 1 (g) | Mice 1 (g) | Mice 1 (g) | Reference Value (g) | Value p in t-Student | |
|---|---|---|---|---|---|---|
| 9 | Heart | 0.130 | 0.154 | 0.120 | 0.155 | 0.198 |
| Spleen | 0.055 | 0.099 | 0.067 | 0.118 | 0.082 | |
| Kidneys | 0.294 | 0.403 | 0.518 | 0.413 | 0.998 | |
| Liver | 1.320 | 1.280 | 1.450 | 1.486 | 0.114 | |
| 12 | Heart | 0.124 | 0.124 | 0.159 | 0.155 | 0.240 |
| Spleen | 0.079 | 0.112 | 0.097 | 0.118 | 0.149 | |
| Kidneys | 0.395 | 0.402 | 0.427 | 0.413 | 0.610 | |
| Liver | 1.340 | 1.384 | 1.204 | 1.486 | 0.086 | |
| 15 | Heart | 0.153 | 0.138 | 0.166 | 0.155 | 0.785 |
| Spleen | 0.052 | 0.107 | 0.074 | 0.118 | 0.129 | |
| Kidneys | 0.270 | 0.327 | 0.421 | 0.413 | 0.239 | |
| Liver | 1.020 | 1.385 | 1.258 | 1.486 | 0.133 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabares Vélez, S.; Preciado, L.M.; Vargas Muñoz, L.J.; Madrid Bracamonte, C.A.; Zuluaga, A.; Gómez Robles, J.; Renjifo-Ibañez, C.; Estrada-Gómez, S. Standard Quality Characteristics and Efficacy of a New Third-Generation Antivenom Developed in Colombia Covering Micrurus spp. Venoms. Toxins 2024, 16, 183. https://doi.org/10.3390/toxins16040183
Tabares Vélez S, Preciado LM, Vargas Muñoz LJ, Madrid Bracamonte CA, Zuluaga A, Gómez Robles J, Renjifo-Ibañez C, Estrada-Gómez S. Standard Quality Characteristics and Efficacy of a New Third-Generation Antivenom Developed in Colombia Covering Micrurus spp. Venoms. Toxins. 2024; 16(4):183. https://doi.org/10.3390/toxins16040183
Chicago/Turabian StyleTabares Vélez, Santiago, Lina María Preciado, Leidy Johana Vargas Muñoz, Carlos Alberto Madrid Bracamonte, Angelica Zuluaga, Jeisson Gómez Robles, Camila Renjifo-Ibañez, and Sebastián Estrada-Gómez. 2024. "Standard Quality Characteristics and Efficacy of a New Third-Generation Antivenom Developed in Colombia Covering Micrurus spp. Venoms" Toxins 16, no. 4: 183. https://doi.org/10.3390/toxins16040183
APA StyleTabares Vélez, S., Preciado, L. M., Vargas Muñoz, L. J., Madrid Bracamonte, C. A., Zuluaga, A., Gómez Robles, J., Renjifo-Ibañez, C., & Estrada-Gómez, S. (2024). Standard Quality Characteristics and Efficacy of a New Third-Generation Antivenom Developed in Colombia Covering Micrurus spp. Venoms. Toxins, 16(4), 183. https://doi.org/10.3390/toxins16040183





