Molecular Aspects Involved in the Mechanisms of Bothrops jararaca Venom-Induced Hyperalgesia: Participation of NK1 Receptor and Glial Cells
Abstract
:1. Introduction
2. Results
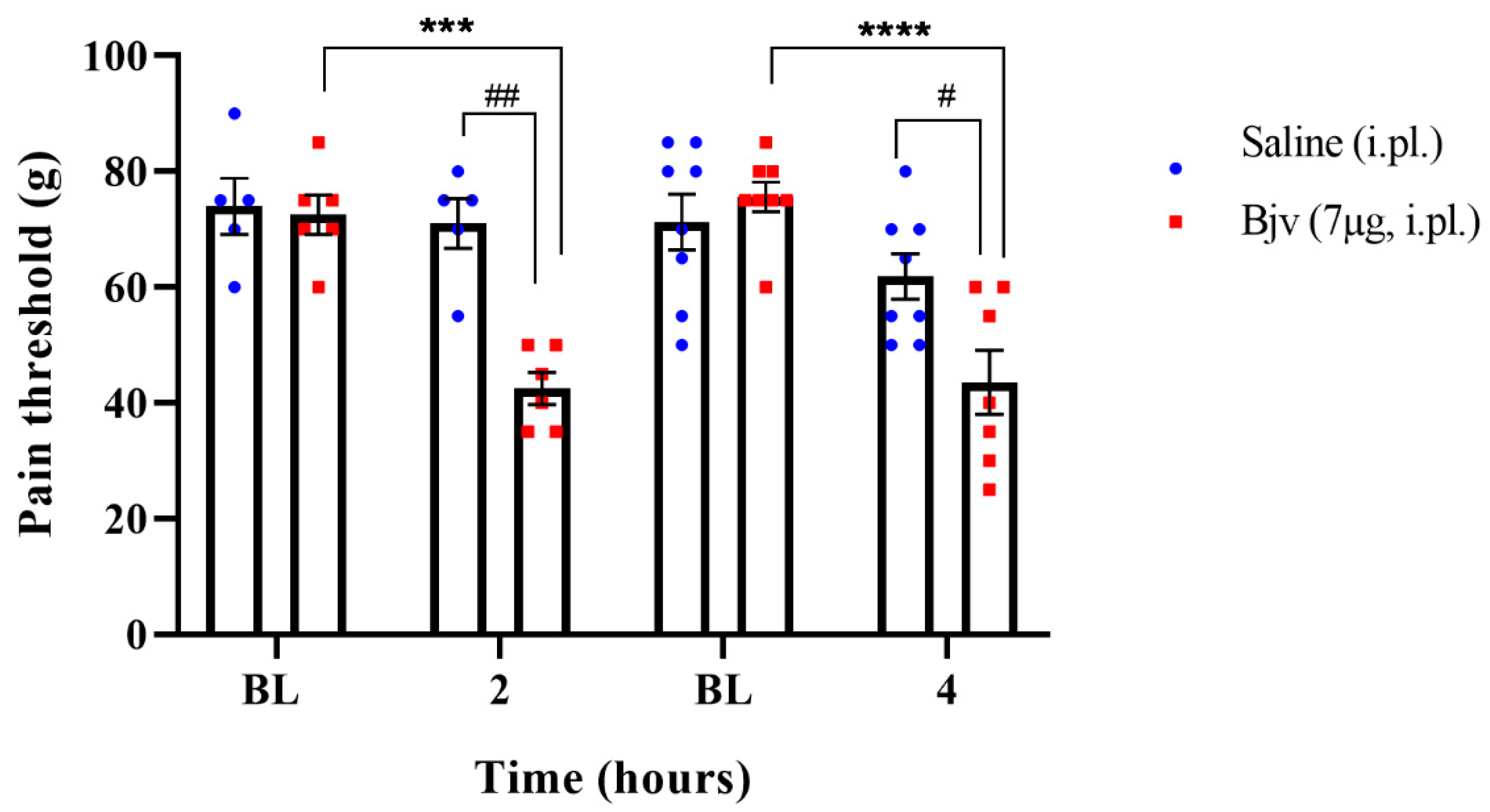
2.1. Hyperalgesia Induced by Bothrops jararaca Venom (Bjv)
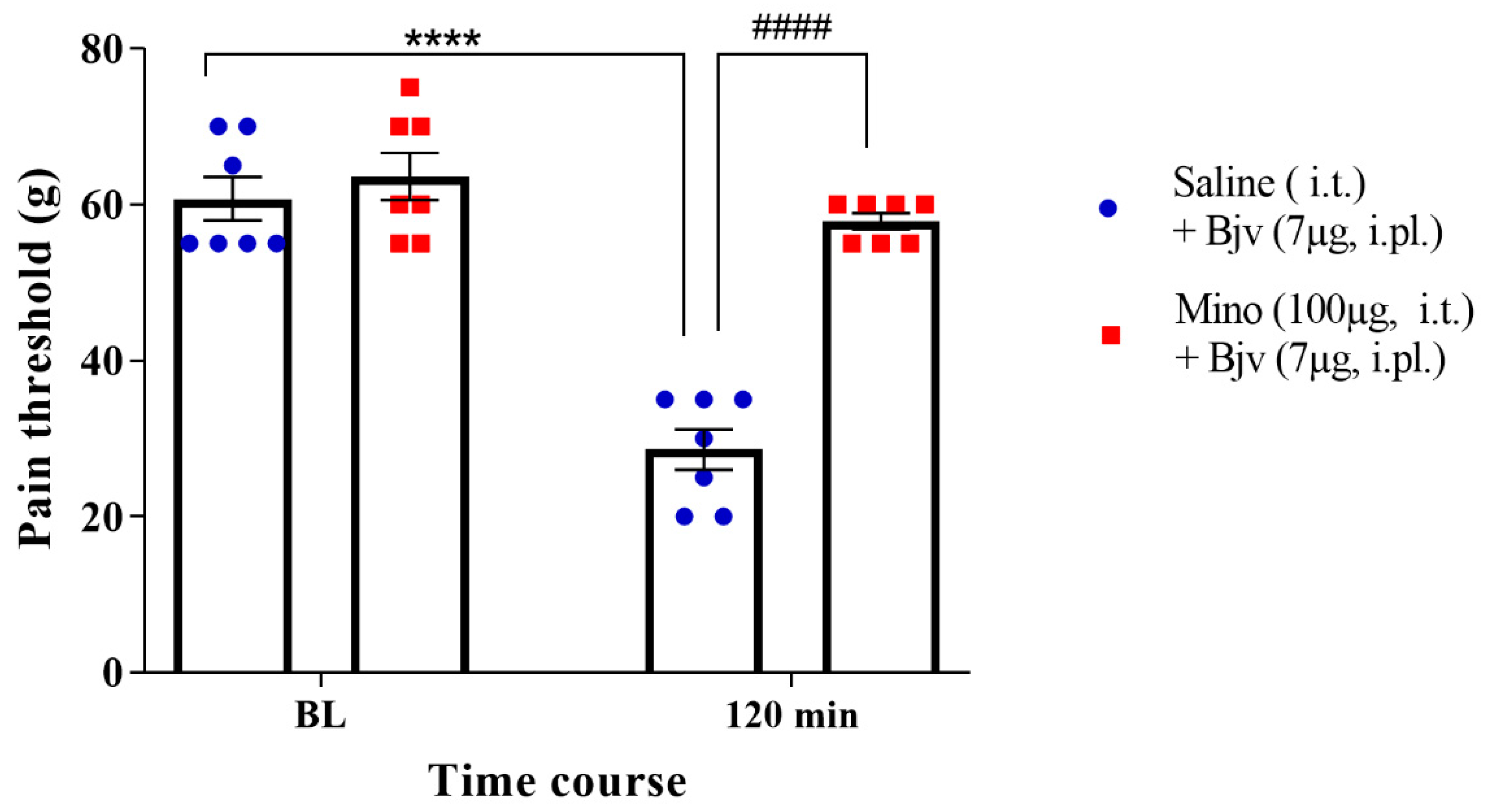
2.2. Spinal Microglia Are Involved in Bjv-Induced Hyperalgesia
2.3. Bjv Induces Increased Glial Cells and Neurons Marker Expression
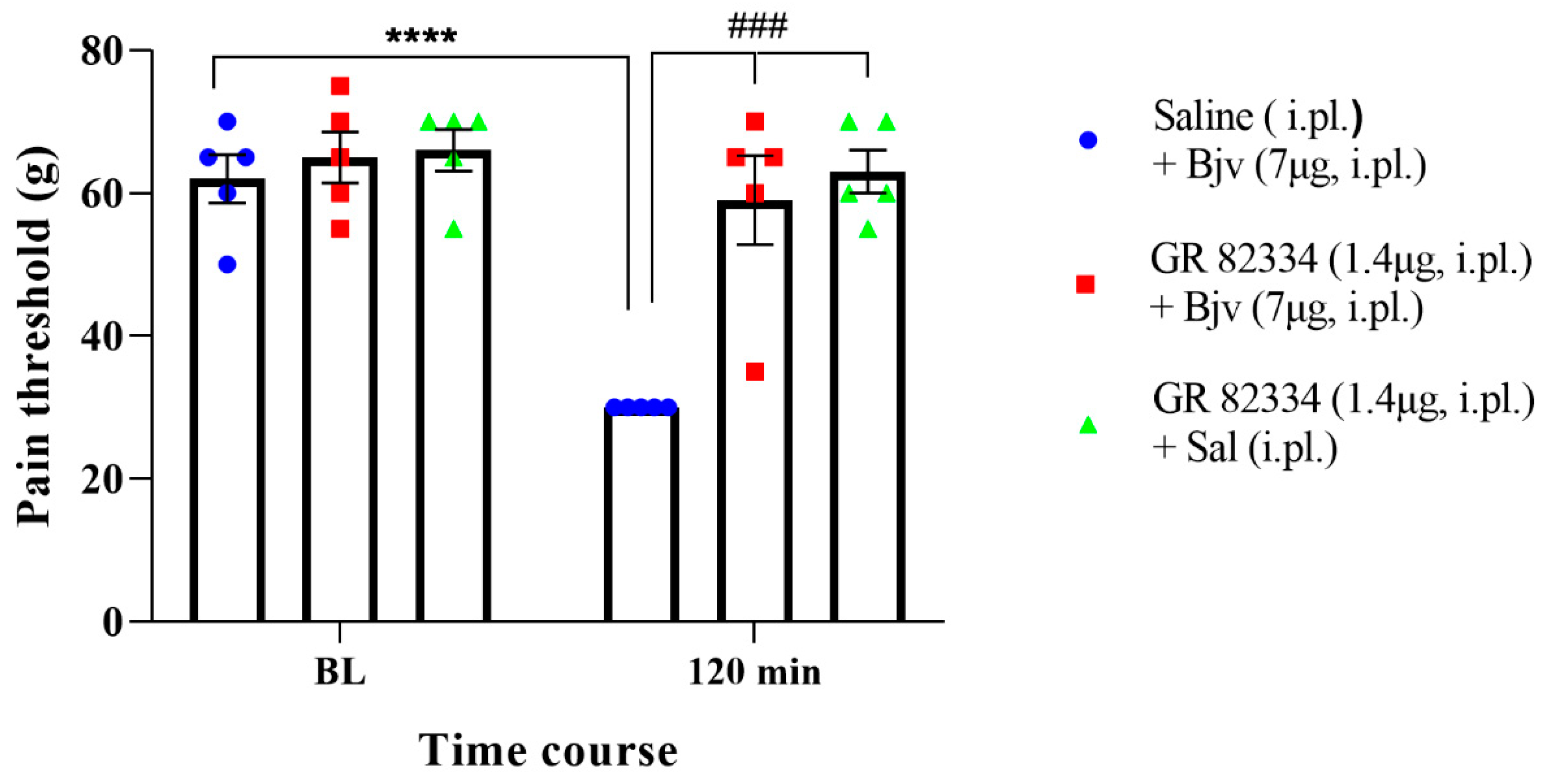
2.4. Participation of the Peripheral NK1 Tachykinin Receptors in the Hyperalgesic Effect of Bjv
2.5. Expression of NK1 Receptors in the Spinal Cord Is Increased after Bjv-Induced Hyperalgesia
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals
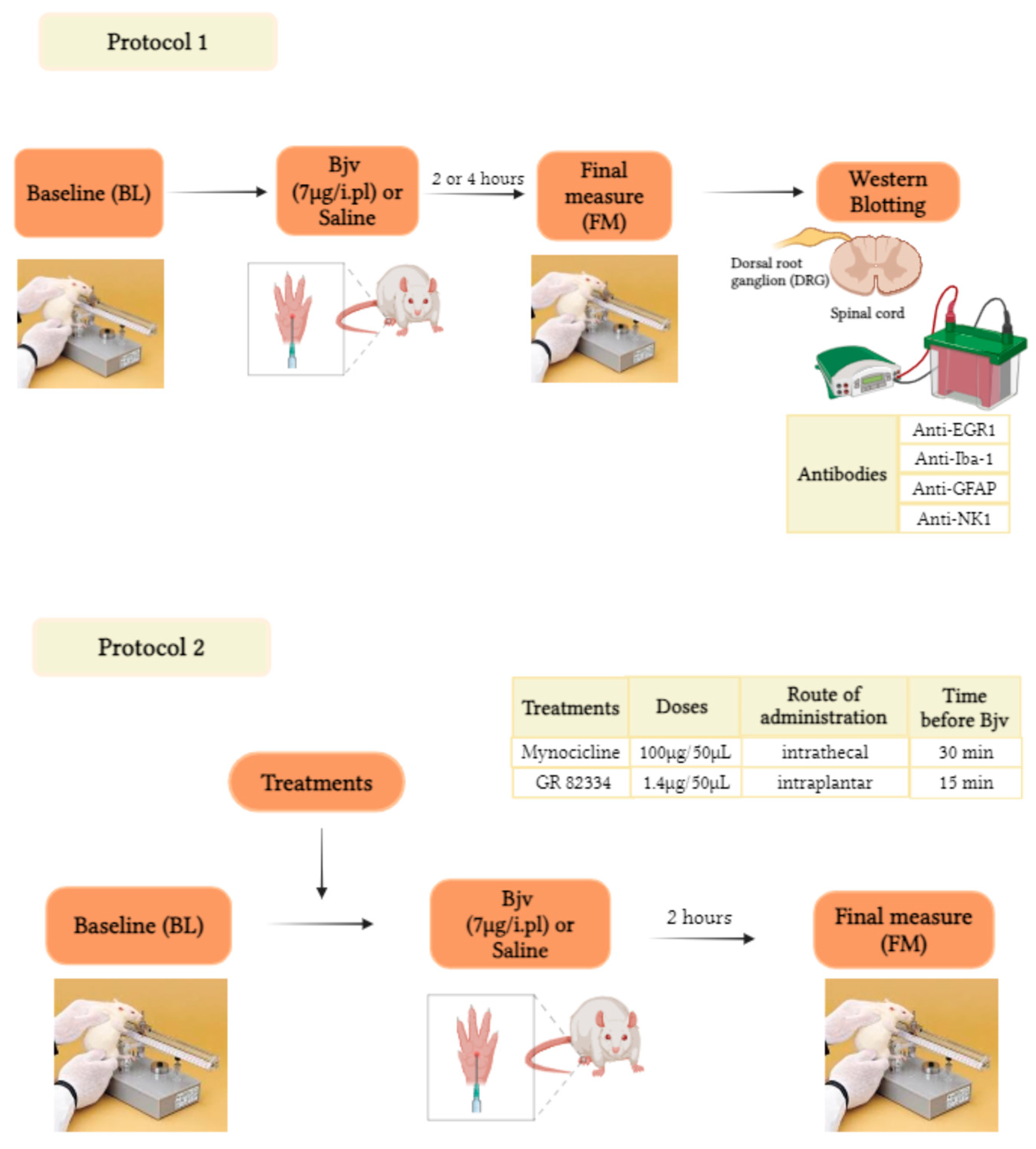
5.2. Experimental Design
5.3. Venom
5.4. Evaluation of Mechanical Hyperalgesia
5.5. Drugs
5.6. DRG and Spinal Cord Samples
5.7. Western Blot
5.8. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- dos Cavalcante, J.S.; Nogueira Júnior, F.A.; Bezerra Jorge, R.J.; Almeida, C. Pain Modulated by Bothrops Snake Venoms: Mechanisms of Nociceptive Signaling and Therapeutic Perspectives. Toxicon 2021, 201, 105–114. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Snakebite Envenoming. Available online: https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming#cms (accessed on 28 February 2024).
- MINISTÉRIO DA SAÚDE (Brasil). Available online: https://bvsms.saude.gov.br/bvs/publicacoes/guia_vigilancia_saude_3ed.pdf (accessed on 28 February 2024).
- SINAN. Available online: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sinannet/cnv/animaisbr.def%20 (accessed on 28 February 2024).
- Mamede, C.C.N.; de Sousa Simamoto, B.B.; da Cunha Pereira, D.F.; de Oliveira Costa, J.; Ribeiro, M.S.M.; de Oliveira, F. Edema, Hyperalgesia and Myonecrosis Induced by Brazilian Bothropic Venoms: Overview of the Last Decade. Toxicon 2020, 187, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Picolo, G.; Chacur, M.; Gutiérrez, J.M.; Teixeira, C.F.P.; Cury, Y. Evaluation of Antivenoms in the Neutralization of Hyperalgesia and Edema Induced by Bothrops Jararaca and Bothrops Asper Snake Venoms. Braz. J. Med. Biol. Res. 2002, 35, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, É.S.; Oliveira, I.; Nascimento, T.P.; da Silva Neto, A.V.; Leal, B.A.S.; Araújo, F.Q.; Julião, B.F.V.; Souza, A.R.N.; Abrahim, A.W.; Macedo, B.B.O.; et al. Prospecting Local Treatments Used in Conjunction with Antivenom Administration following Envenomation Caused by Animals: A Systematic Review. Toxins 2023, 15, 313. [Google Scholar] [CrossRef] [PubMed]
- Zambelli, V.; Picolo, G.; Fernandes, C.; Fontes, M.; Cury, Y. Secreted Phospholipases A2 from Animal Venoms in Pain and Analgesia. Toxins 2017, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Chacur, M.; Picolo, G.; Teixeira, C.F.P.; Cury, Y. Bradykinin Is Involved in Hyperalgesia Induced by Bothrops Jararaca Venom. Toxicon 2002, 40, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.R.; Calixto-Campos, C.; Manchope, M.F.; Casagrande, R.; Clissa, P.B.; Baldo, C.; Verri, W.A. Jararhagin-Induced Mechanical Hyperalgesia Depends on TNF-α, IL-1β and NFκB in Mice. Toxicon 2015, 103, 119–128. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Cardos, F.C.; Clissa, P.B.; Vetter, I.; Verri, W.A., Jr.; Lewis, R.J. Jararhagin, a Metalloproteinase from Bothrops Jararaca Venom, Induces Mechanical and Thermal Hyperalgesia through TRPV1 Channel Activation. In Proceedings of Brisbane Pain Research: Multidisciplinary Perspectives & Therapeutics, Brisbane, QLD, Australia, 7 December 2018; The University of Queensland: Brisbane, QLD, Australia, 2018. [Google Scholar]
- Ferraz, C.R.; Carvalho, T.T.; Fattori, V.; Saraiva-Santos, T.; Pinho-Ribeiro, F.A.; Borghi, S.M.; Manchope, M.F.; Zaninelli, T.H.; Cunha, T.M.; Casagrande, R.; et al. Jararhagin, a Snake Venom Metalloproteinase, Induces Mechanical Hyperalgesia in Mice with the Neuroinflammatory Contribution of Spinal Cord Microglia and Astrocytes. Int. J. Biol. Macromol. 2021, 179, 610–619. [Google Scholar] [CrossRef]
- Trebien, H.A.; Calixto, J.B. Pharmacological Evaluation of Rat Paw Oedema Induced by Bothrops Jararaca Venom. Agents Actions 1989, 26, 292–300. [Google Scholar] [CrossRef]
- Teixeira, C.F.P.; Cury, Y.; Oga, S.; Jancar, S. Hyperalgesia Induced by Bothrops Jararaca Venom in Rats: Role of Eicosanoids and Platelet Activating Factor (PAF). Toxicon 1994, 32, 419–426. [Google Scholar] [CrossRef]
- Farsky, S.H.; Walber, J.; Costa-Cruz, M.; Cury, Y.; Teixeira, C.F.; Curry, Y. Leukocyte Response Induced by Bothrops Jararaca Crude Venom: In Vivo and in Vitro Studies. Toxicon Off. J. Int. Soc. Toxinology 1997, 35, 185–193. [Google Scholar] [CrossRef]
- Zychar, B.C.; Dale, C.S.; Demarchi, D.S.; Gonçalves, L.R.C. Contribution of Metalloproteases, Serine Proteases and Phospholipases A2 to the Inflammatory Reaction Induced by Bothrops Jararaca Crude Venom in Mice. Toxicon Off. J. Int. Soc. Toxinology 2010, 55, 227–234. [Google Scholar] [CrossRef]
- Yamashita, K.M.; Nogueira, T.O.; Senise, L.V.; Cirillo, M.C.; Gonçalves, L.R.C.; Sano-Martins, I.S.; Giorgi, R.; Santoro, M.L. Involvement of Circulating Platelets on the Hyperalgesic Response Evoked by Carrageenan and Bothrops Jararaca Snake Venom. J. Thromb. Haemost. JTH 2011, 9, 2057–2066. [Google Scholar] [CrossRef]
- Nielsen, V.G.; Wagner, M.T. Review of the Mechanisms of Snake Venom Induced Pain: It’s All about Location, Location, Location. Int. J. Mol. Sci. 2022, 23, 2128. [Google Scholar] [CrossRef]
- Chacur, M.; Gutiérrez, J.M.; Milligan, E.D.; Wieseler-Frank, J.; Britto, L.R.G.; Maier, S.F.; Watkins, L.R.; Cury, Y. Snake Venom Components Enhance Pain upon Subcutaneous Injection: An Initial Examination of Spinal Cord Mediators. Pain 2004, 111, 65–76. [Google Scholar] [CrossRef]
- Old, E.A.; Clark, A.K.; Malcangio, M. The Role of Glia in the Spinal Cord in Neuropathic and Inflammatory Pain. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 145–170. [Google Scholar] [CrossRef]
- Watkins, R.L.; Martin, D.; Ulrich, P.; Tracey, J.K.; Maier, F.S. Evidence for the Involvement of Spinal Cord Glia in Subcutaneous Formalin Induced Hyperalgesia in the Rat. Pain 1997, 71, 225–235. [Google Scholar] [CrossRef]
- Gu, N.; Yi, M.-H.; Murugan, M.; Xie, M.; Parusel, S.; Peng, J.; Eyo, U.B.; Hunt, C.L.; Dong, H.; Wu, L.-J. Spinal Microglia Contribute to Sustained Inflammatory Pain via Amplifying Neuronal Activity. Mol. Brain 2022, 15, 86. [Google Scholar] [CrossRef]
- Wang, H.; Xu, C. A Novel Progress: Glial Cells and Inflammatory Pain. ACS Chem. Neurosci. 2022, 13, 288–295. [Google Scholar] [CrossRef]
- Lu, J.; Wang, D.; Xu, J.; Zhang, H.; Yu, W. New Insights on the Role of Satellite Glial Cells. Stem Cell Rev. Rep. 2023, 19, 358–367. [Google Scholar] [CrossRef]
- Gonçalves, L.R.C.; Mariano, M. Local Haemorrhage Induced by Bothrops Jararaca Venom: Relationship to Neurogenic Inflammation. Mediat. Inflamm. 2000, 9, 101–107. [Google Scholar] [CrossRef]
- Snijdelaar, D.G.; Dirksen, R.; Slappendel, R.; Crul, B.J.P. Substance P. Eur. J. Pain 2000, 4, 121–135. [Google Scholar] [CrossRef]
- O’Connor, T.M.; O’Connell, J.; O’Brien, D.I.; Goode, T.; Bredin, C.P.; Shanahan, F. The Role of Substance P in Inflammatory Disease. J. Cell. Physiol. 2004, 201, 167–180. [Google Scholar] [CrossRef]
- Laird, J.M.A.; Roza, C.; De Felipe, C.; Hunt, S.P.; Cervero, F. Role of Central and Peripheral Tachykinin NK1 Receptors in Capsaicin-Induced Pain and Hyperalgesia in Mice. Pain 2001, 90, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Recio, S.; Gascón, P. Biological and Pharmacological Aspects of the NK1-Receptor. BioMed Res. Int. 2015, 2015, 495704. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.J.; Nimmo, A.J. Evidence for the Involvement of the Tachykinin NK1 Receptor in Acute Inflammation of the Central Nervous System. Receptors 2023, 2, 232–250. [Google Scholar] [CrossRef]
- Gautam, M.; Prasoon, P.; Kumar, R.; Reeta, K.H.; Kaler, S.; Ray, S.B. Role of Neurokinin Type 1 Receptor in Nociception at the Periphery and the Spinal Level in the Rat. Spinal Cord 2016, 54, 172–182. [Google Scholar] [CrossRef]
- Chippaux, J.-P. Snakebite Envenomation Turns Again into a Neglected Tropical Disease! J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 1–2. [Google Scholar] [CrossRef]
- Johnson, M.B.; Young, A.D.; Marriott, I. The Therapeutic Potential of Targeting Substance P/NK-1R Interactions in Inflammatory CNS Disorders. Front. Cell. Neurosci. 2017, 10, 1–14. [Google Scholar] [CrossRef]
- McGreevy, K.; Bottros, M.M.; Raja, S.N. Preventing Chronic Pain following Acute Pain: Risk Factors, Preventive Strategies, and Their Efficacy. Eur. J. Pain Suppl. 2011, 5, 365–376. [Google Scholar] [CrossRef]
- Hua, X.-Y.; Svensson, C.I.; Matsui, T.; Fitzsimmons, B.; Yaksh, T.L.; Webb, M. Intrathecal Minocycline Attenuates Peripheral Inflammation-Induced Hyperalgesia by Inhibiting P38 MAPK in Spinal Microglia. Eur. J. Neurosci. 2005, 22, 2431–2440. [Google Scholar] [CrossRef]
- Ledeboer, A.; Sloane, E.M.; Milligan, E.D.; Frank, M.G.; Mahony, J.H.; Maier, S.F.; Watkins, L.R. Minocycline Attenuates Mechanical Allodynia and Proinflammatory Cytokine Expression in Rat Models of Pain Facilitation. Pain 2005, 115, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, V.; Tanga, F.Y.; DeLeo, J.A. Complete Freunds Adjuvant-Induced Peripheral Inflammation Evokes Glial Activation and Proinflammatory Cytokine Expression in the CNS. Eur. J. Neurosci. 2004, 20, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.K.; Gentry, C.; Bradbury, E.J.; McMahon, S.B.; Malcangio, M. Role of Spinal Microglia in Rat Models of Peripheral Nerve Injury and Inflammation. Eur. J. Pain 2007, 11, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Kang, D.H.; Jeon, J.; Lee, H.G.; Kim, W.M.; Yoon, M.H.; Choi, J.I. Imbalance in the Spinal Serotonergic Pathway Induces Aggravation of Mechanical Allodynia and Microglial Activation in Carrageenan Inflammation. Korean J. Pain 2023, 36, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, A.M.; Wilce, P.A. Egr Transcription Factors in the Nervous System. Neurochem. Int. 1997, 31, 477–510. [Google Scholar] [CrossRef]
- Herdegen, T.; Leah, J.D. Inducible and Constitutive Transcription Factors in the Mammalian Nervous System: Control of Gene Expression by Jun, Fos and Krox, and CREB/ATF Proteins. Brain Res. Rev. 1998, 28, 370–490. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, I.; Giaginis, C.; Klonaris, C.; Theocharis, S. Insight into Pain-Inducing and -Related Gene Expression: A Challenge for Development of Novel Targeted Therapeutic Approaches. Fundam. Clin. Pharmacol. 2011, 25, 48–62. [Google Scholar] [CrossRef]
- Bliss, T.V.P.; Collingridge, G.L.; Kaang, B.-K.; Zhuo, M. Synaptic Plasticity in the Anterior Cingulate Cortex in Acute and Chronic Pain. Nat. Rev. Neurosci. 2016, 17, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.W.; Vadakkan, K.I.; Ao, H.; Gallitano-Mendel, A.; Wei, F.; Milbrandt, J.; Zhuo, M. Selective Contribution of Egr1 (Zif/268) to Persistent Inflammatory Pain. J. Pain 2005, 6, 12–20. [Google Scholar] [CrossRef]
- Buritova, J.; Honoré, P.; Besson, J.M. Indomethacin Reduces Both Krox-24 Expression in the Rat Lumbar Spinal Cord and Inflammatory Signs following Intraplantar Carrageenan. Brain Res. 1995, 674, 211–220. [Google Scholar] [CrossRef]
- Rahman, O.I.F.; Terayama, R.; Ikeda, T.; Koganemaru, M.; Nakamura, T.; Shiba, R.; Nishimori, T. Differential Effects of NMDA and AMPA/KA Receptor Antagonists on C-Fos or Zif/268 Expression in the Rat Spinal Dorsal Horn Induced by Noxious Thermal or Mechanical Stimulation, or Formalin Injection. Neurosci. Res. 2002, 43, 389–399. [Google Scholar] [CrossRef]
- Bojovic, O.; Bramham, C.R.; Tjølsen, A. Stimulation-Induced Expression of Immediate Early Gene Proteins in the Dorsal Horn Is Increased in Neuropathy. Scand. J. Pain 2016, 10, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Dale, C.S.; Cenac, N.; Britto, L.R.G.; Juliano, M.A.; Juliano, L.; Vergnolle, N.; Giorgi, R. The C-Terminus of Murine S100A9 Protein Inhibits Hyperalgesia Induced by the Agonist Peptide of Protease-Activated Receptor 2. Br. J. Pharmacol. 2006, 149, 374–384. [Google Scholar] [CrossRef]
- Kendall, G.; Ensor, E.; Brar-Rai, A.; Winter, J.; Latchman, D.S. Nerve Growth Factor Induces Expression of Immediate-Early Genes NGFI-A (Egr-1) and NGFI-B (Nur 77) in Adult Rat Dorsal Root Ganglion Neurons. Mol. Brain Res. 1994, 25, 73–79. [Google Scholar] [CrossRef]
- Hargett, G.; Carlton, S. Localization of EGR-1 in the Rat Dorsal Root Ganglia and Its Co-Localization with TRPV1 and IB4. J. Pain 2012, 13, S56. [Google Scholar] [CrossRef]
- Ohsawa, K.; Imai, Y.; Kanazawa, H.; Sasaki, Y.; Kohsaka, S. Involvement of Iba1 in Membrane Ruffling and Phagocytosis of Macrophages/Microglia. J. Cell Sci. 2000, 113 Pt 17, 3073–3084. [Google Scholar] [CrossRef]
- Yu, X.; Liu, H.; Hamel, K.A.; Morvan, M.G.; Yu, S.; Leff, J.; Guan, Z.; Braz, J.M.; Basbaum, A.I. Dorsal Root Ganglion Macrophages Contribute to Both the Initiation and Persistence of Neuropathic Pain. Nat. Commun. 2020, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Lier, J.; Streit, W.J.; Bechmann, I. Beyond Activation: Characterizing Microglial Functional Phenotypes. Cells 2021, 10, 2236. [Google Scholar] [CrossRef] [PubMed]
- Chen, O.; Luo, X.; Ji, R.-R. Macrophages and Microglia in Inflammation and Neuroinflammation Underlying Different Pain States. Med. Rev. 2023, 3, 381–407. [Google Scholar] [CrossRef]
- Silva, C.E.A.; Guimarães, R.M.; Cunha, T.M. Sensory Neuron–Associated Macrophages as Novel Modulators of Neuropathic Pain. PAIN Rep. 2021, 6, e873. [Google Scholar] [CrossRef]
- Von Banchet, G.S.; Boettger, M.K.; Fischer, N.; Gajda, M.; Bräuer, R.; Schaible, H.-G. Experimental Arthritis Causes Tumor Necrosis Factor-α-Dependent Infiltration of Macrophages into Rat Dorsal Root Ganglia Which Correlates with Pain-Related Behavior. Pain 2009, 145, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Domoto, R.; Sekiguchi, F.; Tsubota, M.; Kawabata, A. Macrophage as a Peripheral Pain Regulator. Cells 2021, 10, 1881. [Google Scholar] [CrossRef] [PubMed]
- Van der Vlist, M.; Raoof, R.; Willemen, H.L.D.M.; Prado, J.; Versteeg, S.; Martin Gil, C.; Vos, M.; Lokhorst, R.E.; Pasterkamp, R.J.; Kojima, T.; et al. Macrophages Transfer Mitochondria to Sensory Neurons to Resolve Inflammatory Pain. Neuron 2022, 110, 613–626.e9. [Google Scholar] [CrossRef]
- Zigmond, R.E.; Echevarria, F.D. Macrophage Biology in the Peripheral Nervous System after Injury. Prog. Neurobiol. 2019, 173, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.A.L.; Moreira Neto, F.L. Satellite Glial Cells in Sensory Ganglia: Its Role in Pain. Rev. Bras. Anestesiol. 2015, 65, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Takahashi, M.; Matsumoto, S. Contribution of the Activation of Satellite Glia in Sensory Ganglia to Pathological Pain. Neurosci. Biobehav. Rev. 2009, 33, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.M.; Dos Santos, G.G.; Neves, A.F.; Athie, M.C.P.; Bonet, I.J.M.; Nishijima, C.M.; Farias, F.H.; Figueiredo, J.G.; Hernandez-Olmos, V.; Alshaibani, S.; et al. Diabetes-Induced Neuropathic Mechanical Hyperalgesia Depends on P2X4 Receptor Activation in Dorsal Root Ganglia. Neuroscience 2019, 398, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Tanimoto, T.; Kadoi, J.; Nasu, M.; Takahashi, M.; Kitagawa, J.; Matsumoto, S. Enhanced Excitability of Nociceptive Trigeminal Ganglion Neurons by Satellite Glial Cytokine following Peripheral Inflammation. Pain 2007, 129, 155–166. [Google Scholar] [CrossRef]
- Villa, G.; Ceruti, S.; Zanardelli, M.; Magni, G.; Jasmin, L.; Ohara, P.T.; Abbracchio, M.P. Temporomandibular Joint Inflammation Activates Glial and Immune Cells in Both the Trigeminal Ganglia and in the Spinal Trigeminal Nucleus. Mol. Pain 2010, 6, 1744–1789. [Google Scholar] [CrossRef]
- Zieglgänsberger, W. Substance P and Pain Chronicity. Cell Tissue Res. 2019, 375, 227–241. [Google Scholar] [CrossRef]
- Douglas, S.D.; Leeman, S.E. Neurokinin-1 Receptor: Functional Significance in the Immune System in Reference to Selected Infections and Inflammation. Ann. N. Y. Acad. Sci. 2010, 1217, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Zamuner, S.R.; Gutiérrez, J.M.; Muscará, M.N.; Teixeira, S.A.; Teixeira, C.F.P. Bothrops Asper and Bothrops Jararaca Snake Venoms Trigger Microbicidal Functions of Peritoneal Leukocytes in Vivo. Toxicon 2001, 39, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-S.; Zhao, Z.-Q. Small Sensory Neurons in the Rat Dorsal Root Ganglia Express Functional NK-1 Tachykinin Receptor. Eur. J. Neurosci. 1998, 10, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.J.; Spike, R.C.; Polgár, E. A Quantitative Study of Neurons Which Express Neurokinin-1 or Somatostatin Sst2a Receptor in Rat Spinal Dorsal Horn. Neuroscience 1998, 85, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Trafton, J.A.; Basbaum, A.I. The Contribution of Spinal Cord Neurokinin-1 Receptor Signaling to Pain. J. Pain 2000, 1, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Krause, J.E.; DiMaggio, D.A.; McCarson, K.E. Alterations in Neurokinin 1 Receptor Gene Expression in Models of Pain and Inflammation. Can. J. Physiol. Pharmacol. 1995, 73, 854–859. [Google Scholar] [CrossRef]
- Burmeister, A.R.; Johnson, M.B.; Chauhan, V.S.; Moerdyk-Schauwecker, M.J.; Young, A.D.; Cooley, I.D.; Martinez, A.N.; Ramesh, G.; Philipp, M.T.; Marriott, I. Human Microglia and Astrocytes Constitutively Express the Neurokinin-1 Receptor and Functionally Respond to Substance P. J. Neuroinflammation 2017, 14, 245. [Google Scholar] [CrossRef] [PubMed]
- Miyano, K.; Morioka, N.; Sugimoto, T.; Shiraishi, S.; Uezono, Y.; Nakata, Y. Activation of the Neurokinin-1 Receptor in Rat Spinal Astrocytes Induces Ca2+ Release from IP3-Sensitive Ca2+ Stores and Extracellular Ca2+ Influx through TRPC3. Neurochem. Int. 2010, 57, 923–934. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Randall, L.O.; Selitto, J.J. A Method for Measurement of Analgesic Activity on Inflamed Tissue. Arch. Int. Pharmacodyn. Ther. 1957, 111, 409–419. [Google Scholar]
- Zanchet, E.M.; Cury, Y. Peripheral Tackykinin and Excitatory Amino Acid Receptors Mediate Hyperalgesia Induced by Phoneutria Nigriventer Venom. Eur. J. Pharmacol. 2003, 467, 111–118. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bom, A.d.O.P.; Dias-Soares, M.; Corrêa, R.C.D.; Neves, C.L.; Hosch, N.G.; Lucena, G.G.d.; Oliveira, C.G.; Pagano, R.L.; Chacur, M.; Giorgi, R. Molecular Aspects Involved in the Mechanisms of Bothrops jararaca Venom-Induced Hyperalgesia: Participation of NK1 Receptor and Glial Cells. Toxins 2024, 16, 187. https://doi.org/10.3390/toxins16040187
Bom AdOP, Dias-Soares M, Corrêa RCD, Neves CL, Hosch NG, Lucena GGd, Oliveira CG, Pagano RL, Chacur M, Giorgi R. Molecular Aspects Involved in the Mechanisms of Bothrops jararaca Venom-Induced Hyperalgesia: Participation of NK1 Receptor and Glial Cells. Toxins. 2024; 16(4):187. https://doi.org/10.3390/toxins16040187
Chicago/Turabian StyleBom, Ariela de Oliveira Pedro, Monique Dias-Soares, Raíssa Cristina Darroz Corrêa, Camila Lima Neves, Natalia Gabriele Hosch, Gabriela Gomes de Lucena, Camilla Garcia Oliveira, Rosana Lima Pagano, Marucia Chacur, and Renata Giorgi. 2024. "Molecular Aspects Involved in the Mechanisms of Bothrops jararaca Venom-Induced Hyperalgesia: Participation of NK1 Receptor and Glial Cells" Toxins 16, no. 4: 187. https://doi.org/10.3390/toxins16040187




