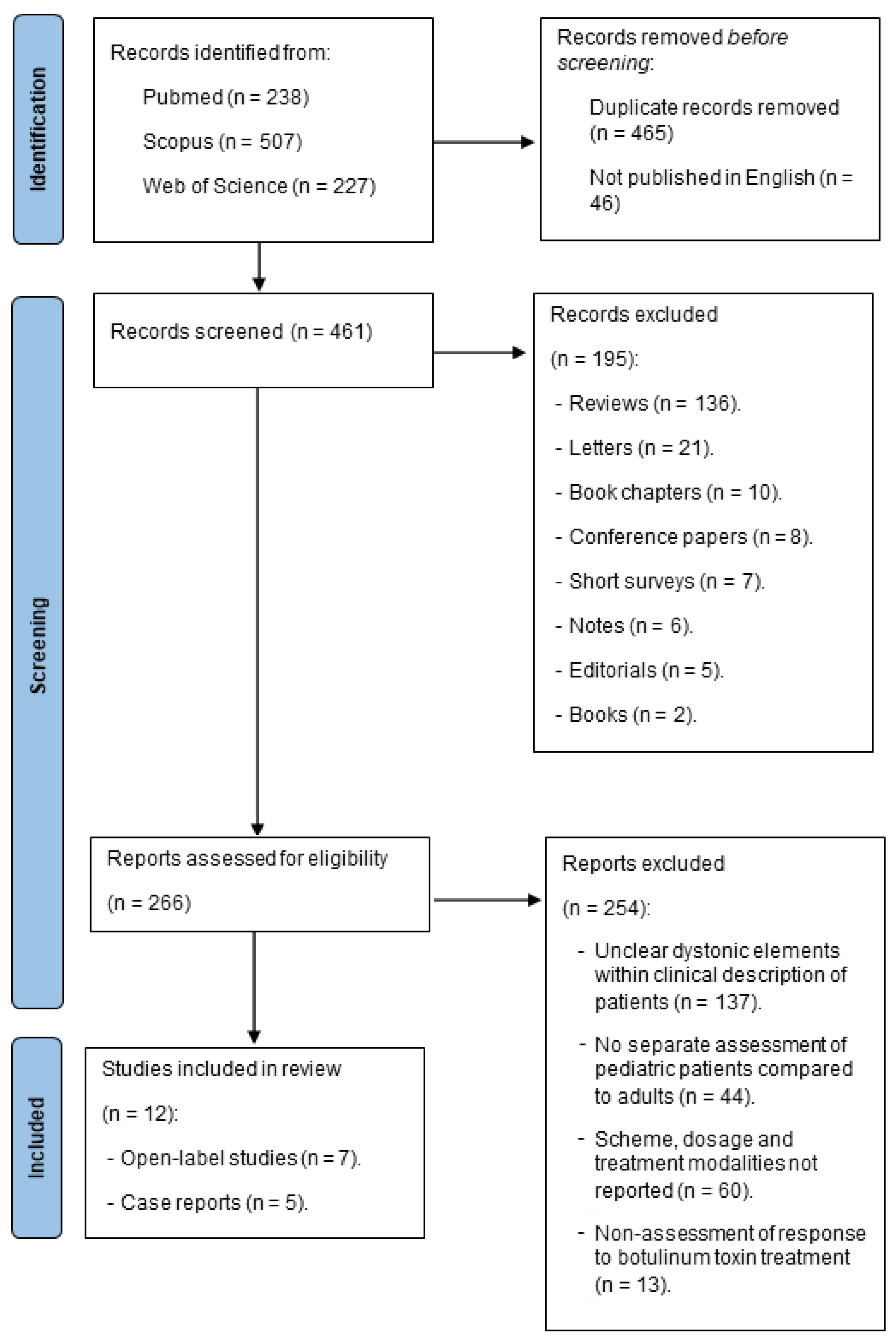
A Systematic Review of Botulinum Toxin Injection in Pediatric Dystonia
Abstract
1. Introduction
2. Results
3. Discussion
4. Conclusions
- Childhood dystonia rarely occurs in idiopathic forms with focal or segmental involvement, limiting BT use in clinical practice.
- Rating scales to assess isolated or combined dystonia are lacking in the pediatric population, making outcome definitions and evaluations often arduous.
5. Materials and Methods
- Class I. A randomized, controlled trial (RCT) with a masked or objective outcome assessment in a representative population. The relevant baseline characteristics are presented and substantially equivalent among treatment groups or there is appropriate statistical adjustment for differences. The following are required: (a) concealed allocation; (b) primary outcome(s) clearly defined; (c) exclusion/inclusion criteria clearly defined; and (d) adequate accounting for dropouts (with at least 80% of enrolled subjects completing the study) and crossovers with numbers sufficiently low to have minimal potential for bias.
- Class II. A prospective matched group cohort study in a representative population with a masked outcome assessment that meets criteria b–d above or an RCT in a representative population that lacks one criterion a–d.
- Class III. All the other controlled trials (including well-defined natural history controls or patients serving as own controls) in a representative population, where the outcome is independently assessed or independently derived by an objective outcome measurement.
- Class IV. Studies not meeting Class I, II or III criteria, including consensus, expert opinion or a case report.
- A.
- Established as effective, ineffective or harmful (or established as useful/predictive or not useful/predictive) for the given condition in the specified population. (Level A rating requires at least two consistent Class I studies.)
- B.
- Probably effective, ineffective or harmful (or probably useful/predictive or not useful/predictive) for the given condition in the specified population. (Level B rating requires at least one Class I study or two consistent Class II studies.)
- C.
- Possibly effective, ineffective or harmful (or possibly useful/predictive or not useful/predictive) for the given condition in the specified population. (Level C rating requires at least one Class II study or two consistent Class III studies.)
- U.
- Data inadequate or conflicting; given current knowledge, treatment (test and predictor) is unproven.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Albanese, A.; Bhatia, K.; Bressman, S.B.; Delong, M.R.; Fahn, S.; Fung, V.S.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Klein, C.; et al. Phenomenology and classification of dystonia: A consensus update. Mov. Disord. Off. J. Mov. Disord. Soc. 2013, 28, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Sanger, T.D.; Chen, D.; Fehlings, D.L.; Hallett, M.; Lang, A.E.; Mink, J.W.; Singer, H.S.; Alter, K.; Ben-Pazi, H.; Butler, E.E.; et al. Definition and classification of hyperkinetic movements in childhood. Mov. Disord. Off. J. Mov. Disord. Soc. 2010, 25, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Mink, J.W. Special concerns in defining, studying, and treating dystonia in children. Mov. Disord. Off. J. Mov. Disord. Soc. 2013, 28, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Luc, Q.N.; Querubin, J. Clinical Management of Dystonia in Childhood. Paediatr. Drugs 2017, 19, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. Suppl. 2007, 109, 8–14. [Google Scholar] [PubMed]
- Monbaliu, E.; Himmelmann, K.; Lin, J.P.; Ortibus, E.; Bonouvrié, L.; Feys, H.; Vermeulen, R.J.; Dan, B. Clinical presentation and management of dyskinetic cerebral palsy. Lancet Neurol. 2017, 16, 741–749. [Google Scholar] [CrossRef]
- Fehlings, D.; Brown, L.; Harvey, A.; Himmelmann, K.; Lin, J.P.; Macintosh, A.; Mink, J.W.; Monbaliu, E.; Rice, J.; Silver, J.; et al. Pharmacological and neurosurgical interventions for managing dystonia in cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2018, 60, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Mathur, A.M.; Aravamuthan, B.R. Spasticity and Dystonia are Underidentified in Young Children at High Risk for Cerebral Palsy. J. Child Neurol. 2022, 37, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Di Giovanni, M.; Lalli, S. Dystonia: Diagnosis and management. Eur. J. Neurol. 2019, 26, 5–17. [Google Scholar] [CrossRef]
- Dolly, J.O.; Aoki, K.R. The structure and mode of action of different botulinum toxins. Eur. J. Neurol. 2006, 13 (Suppl. S4), 1–9. [Google Scholar] [CrossRef]
- Tabbal, S.D. Childhood dystonias. Curr. Treat. Options Neurol. 2015, 17, 339. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Alvarez, E.; Nardocci, N. Update on pediatric dystonias: Etiology, epidemiology, and management. Degener. Neurol. Neuromuscul. Dis. 2012, 2, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Arens, L.J.; Leary, P.M.; Goldschmidt, R.B. Experience with botulinum toxin in the treatment of cerebral palsy. S. Afr. Med. J. Suid-Afr. Tydskr. Vir Geneeskd. 1997, 87, 1001–1003. [Google Scholar]
- Sanger, T.D.; Kukke, S.N.; Sherman-Levine, S. Botulinum toxin type B improves the speed of reaching in children with cerebral palsy and arm dystonia: An open-label, dose-escalation pilot study. J. Child Neurol. 2007, 22, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Lundy, C.T.; Doherty, G.M.; Fairhurst, C.B. Botulinum toxin type A injections can be an effective treatment for pain in children with hip spasms and cerebral palsy. Dev. Med. Child Neurol. 2009, 51, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.; Davidson, S.A.; Bear, N.; Blair, E.; Paterson, L.; Ward, R.; Forbes, D.; Elliott, C. A prospective study investigating gross motor function of children with cerebral palsy and GMFCS level II after long-term Botulinum toxin type A use. BMC Pediatr. 2020, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Heinen, F.; Wissel, J.; Philipsen, A.; Mall, V.; Leititis, J.U.; Schenkel, A.; Stücker, R.; Korinthenberg, R. Interventional neuropediatrics: Treatment of dystonic and spastic muscular hyperactivity with botulinum toxin A. Neuropediatrics 1997, 28, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Schwerin, A.; Berweck, S.; Fietzek, U.M.; Heinen, F. Botulinum toxin B treatment in children with spastic movement disorders: A pilot study. Pediatr. Neurol. 2004, 31, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.; Parnes, M.; Jankovic, J. Botulinum Neurotoxin Injections in Childhood Opisthotonus. Toxins 2021, 13, 137. [Google Scholar] [CrossRef]
- Crisci, C.; Esposito, M. Efficacy of botulinum toxin A treatment in a case of pantothenate kinase associated neurodegeneration (PKAN). BMJ Case Rep. 2011, 2011, bcr0720114514. [Google Scholar] [CrossRef]
- Lin, C.I.; Chen, K.L.; Kuan, T.S.; Lin, S.H.; Lin, W.P.; Lin, Y.C. Botulinum toxin injection to improve functional independence and to alleviate parenting stress in a child with advanced pantothenate kinase-associated neurodegeneration: A case report and literature review. Medicine 2018, 97, e10709. [Google Scholar] [CrossRef] [PubMed]
- Dressler, D.; Wittstock, M.; Benecke, R. Botulinum toxin for treatment of jaw opening dystonia in Hallervorden-Spatz syndrome. Eur. Neurol. 2001, 45, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Heinen, F.; Korinthenberg, R.; Stücker, R.; Deuschl, G. Dystonic posture of lower extremities associated with myelomeningocele: Successful treatment with botulinum A toxin in a six-month-old child. Neuropediatrics 1995, 26, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Seiff, S.R.; Freeman, L.N.; Bluestone, D.L.; Berg, B.O. Use of botulinum toxin to treat blepharospasm in a 16-year-old with a dystonic syndrome. Pediatr. Neurol. 1989, 5, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Henschel, A.D.; Rothenberger, L.G.; Boos, J. Randomized clinical trials in children--ethical and methodological issues. Curr. Pharm. Des. 2010, 16, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Bertoncelli, C.M.; Latalski, M.; Bertoncelli, D.; Bagui, S.; Bagui, S.C.; Gautier, D.; Solla, F. Prediction Model for Identifying Computational Phenotypes of Children with Cerebral Palsy Needing Neurotoxin Treatments. Toxins 2022, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Bourseul, J.S.; Molina, A.; Lintanf, M.; Houx, L.; Chaléat-Valayer, E.; Pons, C.; Brochard, S. Early Botulinum Toxin Injections in Infants with Musculoskeletal Disorders: A Systematic Review of Safety and Effectiveness. Arch. Phys. Med. Rehabil. 2018, 99, 1160–1176.e5. [Google Scholar] [CrossRef] [PubMed]
- Marciniec, M.; Szczepańska-Szerej, A.; Kulczyński, M.; Sapko, K.; Popek-Marciniec, S.; Rejdak, K. Pain in cervical dystonia and the antinociceptive effects of botulinum toxin: What is currently known? Rev. Neurosci. 2019, 30, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Carraro, E.; Trevisi, E.; Martinuzzi, A. Safety profile of incobotulinum toxin A [Xeomin(®)] in gastrocnemious muscles injections in children with cerebral palsy: Randomized double-blind clinical trial. Eur. J. Paediatr. Neurol. 2016, 20, 532–537. [Google Scholar] [CrossRef]
- Heinen, F.; Desloovere, K.; Schroeder, A.S.; Berweck, S.; Borggraefe, I.; van Campenhout, A.; Andersen, G.L.; Aydin, R.; Becher, J.G.; Bernert, G.; et al. The updated European Consensus 2009 on the use of Botulinum toxin for children with cerebral palsy. Eur. J. Paediatr. Neurol. 2010, 14, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Gordon, N. The role of botulinus toxin type A in treatment--with special reference to children. Brain Dev. 1999, 21, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Dressler, D.; Adib Saberi, F.; Rosales, R.L. Botulinum toxin therapy of dystonia. J. Neural Transm. 2021, 128, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, L.L.; Ostrem, J.L.; Bledsoe, I.O. FDA Approvals and Consensus Guidelines for Botulinum Toxins in the Treatment of Dystonia. Toxins 2020, 12, 332. [Google Scholar] [CrossRef]
- Peck, J.; Urits, I.; Kassem, H.; Lee, C.; Robinson, W.; Cornett, E.M.; Berger, A.A.; Herman, J.; Jung, J.W.; Kaye, A.D.; et al. Interventional Approaches to Pain and Spasticity Related to Cerebral Palsy. Psychopharmacol. Bull. 2020, 50 (Suppl. S1), 108–120. [Google Scholar] [PubMed]
- Kuyper, D.J.; Parra, V.; Aerts, S.; Okun, M.S.; Kluger, B.M. Nonmotor manifestations of dystonia: A systematic review. Mov. Disord. Off. J. Mov. Disord. Soc. 2011, 26, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Weise, D.; Weise, C.M.; Naumann, M. Central Effects of Botulinum Neurotoxin-Evidence from Human Studies. Toxins 2019, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Appendix C: AAN Classification of Evidence for the Rating of a Therapeutic Study. Continuum 2015, 21, 1169.
- Getchius, T.S.; Moses, L.K.; French, J.; Gronseth, G.S.; England, J.D.; Miyasaki, J. AAN guidelines: A benefit to the neurologist. Neurology 2010, 75, 1126–1127. [Google Scholar] [CrossRef]
| Author | Age (Years) | Study Design (No. of Patients) | Disorder | Formulation, Total Dose | Injection Site | Outcome Assessment | Results | Adverse Events (%) | Level of Evidence | Recommendation |
|---|---|---|---|---|---|---|---|---|---|---|
| Seiff et al., 1989 [24] | 16 | Case report (n = 1) | Generalized and face dystonia | BTA, 80 U | OO | Clinical | Clinical improvement | None | IV | U |
| Heinen et al., 1995 [23] | 6 months | Case report (n = 1) | Lower limb dystonia and myelomeningocele | Abo BTA, 10–12 U/kg (total dose 140 U in 2 sessions) | Left RF | Clinical | Improved motor function and dystonic posture | None | IV | U |
| Arens et al., 1997 [13] | 5–17 | Open label (n = 15), pure dystonia (n = 5), mixed dystonia (n = 5) | Cerebral palsy | Ona BTA, 4–6 U/kg | Limbs muscles | Motor function scale (0–4) | Improvement in motor function scale scores | Transient post-injection weakness (N = 2/15, 13%) | IV | U |
| Heinen et al., 1997 [17] | 15–18 | Open label (n = 6/28) | Cervical dystonia | Abo BTA, 4.8–11.2 U/kg | SC, SCM, TRA | Joint mobility (ROM), Tsui index, global rating scale | Improvement in all outcomes | None | IV | U |
| Dressler et al., 2001 [22] | 20 | Case report (n = 1) | Mandibular dystonia in HSS | Abo BTA, 400 U | LP, M | Clinical | Improvement in dystonic symptoms and daily activities | None | IV | U |
| Schwerin et al., 2004 [18] | 3–19 | Open label (n = 28, lower leg dystonia, n = 1) | Lower limb dystonia | Rima BTB (large muscles 1000–5000 U, small muscles 250–1000 U) | Not specified | Improved motor function, care, hygiene, orthotic management; correction of cosmetically and functionally distressing limb positions | Goals met in all three categories | Not specified | IV | U |
| Sanger et al., 2007 [14] | 2–15 | Open label (n = 7) | Cerebral palsy and upper limb dystonia | Rima BTB, 50–200 U/kg | BB, BR | Primary. Maximal velocity of outward reaching Secondary. UDRS (upper limb components), UPDRS (upper limb motor subscale), BFMDS upper limb components, MAS, PQLQ | Increase in maximal velocity of outward reaching Improved BFMDS and UPDRS scores | Transient post-injection weakness (N = 2/7, 29%) | IV | U |
| Lundy et al., 2009 [15] | 2–19 | Open label (n = 26, 16 with mixed forms) | Cerebral palsy (mixed spasticity and superimposed dystonia) | Abo BTA, 30 U/kg (range, 400–1000 U) Ona BTA, 12 U/kg (range, 100–300 U) | ILIO, AM, medial HAM | PPPQ | Improvement | None | IV | U |
| Crisci and Esposito 2011 [20] | Not reported | Case report (n = 1) | Limb dystonia in PKAN | Abo BTA, 400 U (200 U per muscle) | TP, GM | Clinical | Improvement in motor (hypertonia, internal foot rotation) and social aspects (quality of life) | None | IV | U |
| Lin et al., 2018 [21] | 10 | Case report (n = 1) | Head and neck dystonia in PKAN | Ona BTA, 180 U (20/30 U per muscle) | SCM, SC, SSC, LS (right) TM, BB, FPL (left) | BADS CP QOL-Child WeeFIM PSI-SF | Improvement in all domains | None | IV | U |
| Valentine et al., 2020 [16] | 8–16 | Open label (n = 28, 5 with concomitant dystonia) | Cerebral palsy | Ona BTA, 4.5–11 U/kg | Lower limb muscles | GMFCS | Improvement in 21.4% and no change in 78.6%. | None | IV | U |
| Hull et al., 2021 [19] | 1–13 | Open label (n = 7) | Opisthotonus | Ona BTA, 16.7 to 23.8 U/kg (mean, 19.6) | PM (N = 7/7), SC (N = 5/7) + other muscles | Clinical | Complete resolution of opisthotonus | Transitory neck extensor weakness (14%) | IV | U |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasera, A.; Squintani, G.M.; Cerruto, M.A. A Systematic Review of Botulinum Toxin Injection in Pediatric Dystonia. Toxins 2024, 16, 289. https://doi.org/10.3390/toxins16070289
Rasera A, Squintani GM, Cerruto MA. A Systematic Review of Botulinum Toxin Injection in Pediatric Dystonia. Toxins. 2024; 16(7):289. https://doi.org/10.3390/toxins16070289
Chicago/Turabian StyleRasera, Andrea, Giovanna Maddalena Squintani, and Maria Angela Cerruto. 2024. "A Systematic Review of Botulinum Toxin Injection in Pediatric Dystonia" Toxins 16, no. 7: 289. https://doi.org/10.3390/toxins16070289
APA StyleRasera, A., Squintani, G. M., & Cerruto, M. A. (2024). A Systematic Review of Botulinum Toxin Injection in Pediatric Dystonia. Toxins, 16(7), 289. https://doi.org/10.3390/toxins16070289





