Allo-Hemodialysis, a Novel Dialytic Treatment Option for Patients with Kidney Failure: Outcomes of Mathematical Modelling, Prototyping, and Ex Vivo Testing
Abstract
:1. Introduction
2. Results
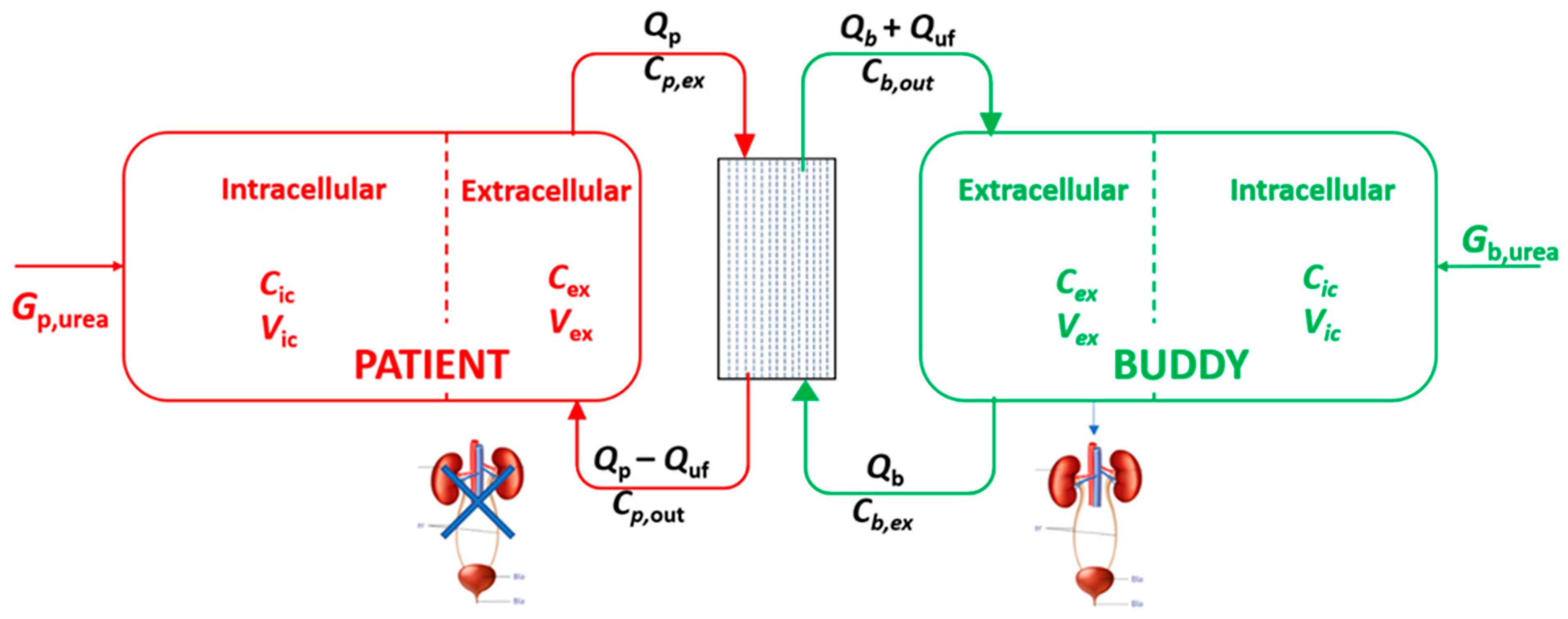
2.1. Mathematical Modeling
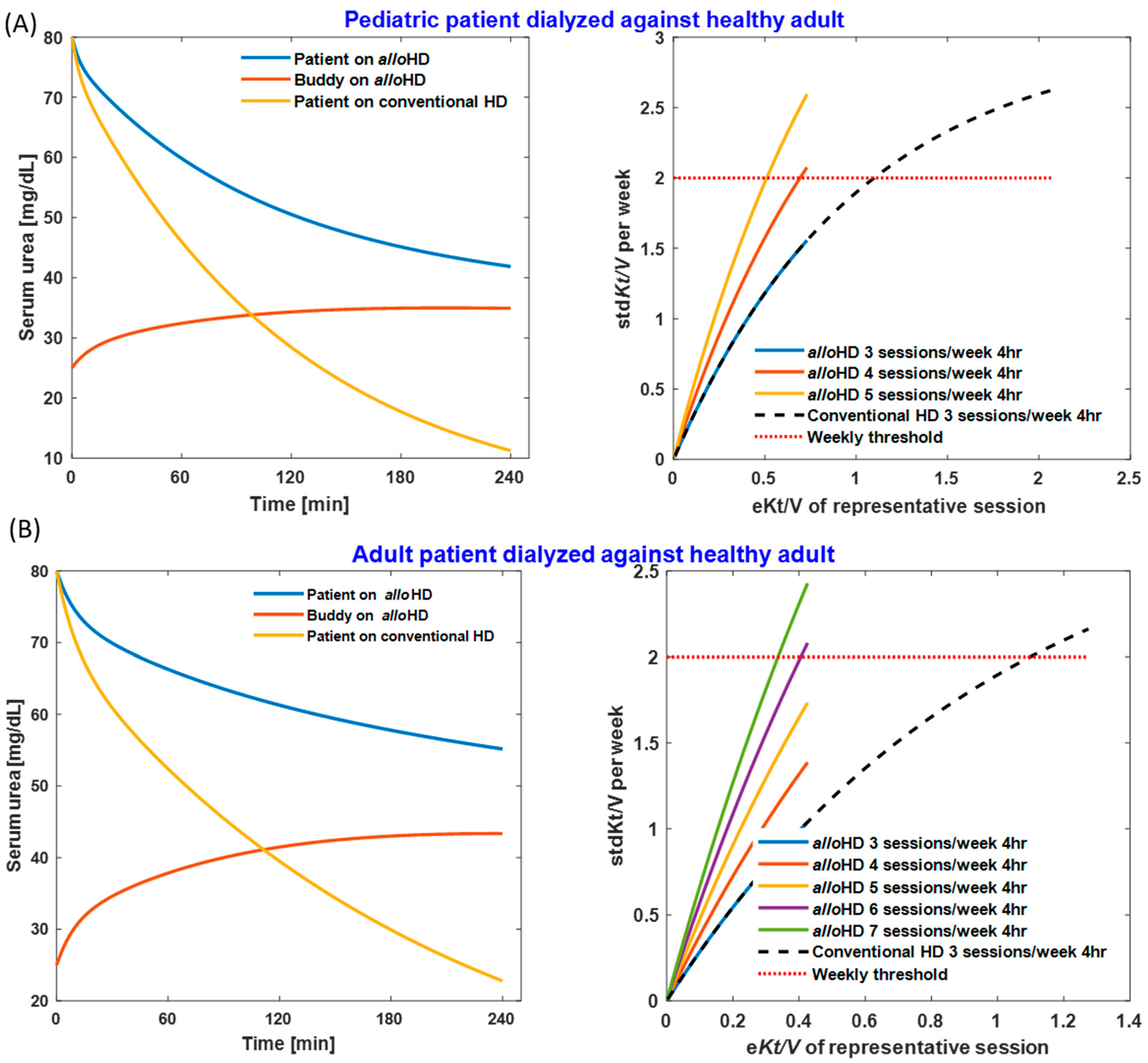
2.1.1. Urea Removal and Kt/Vurea
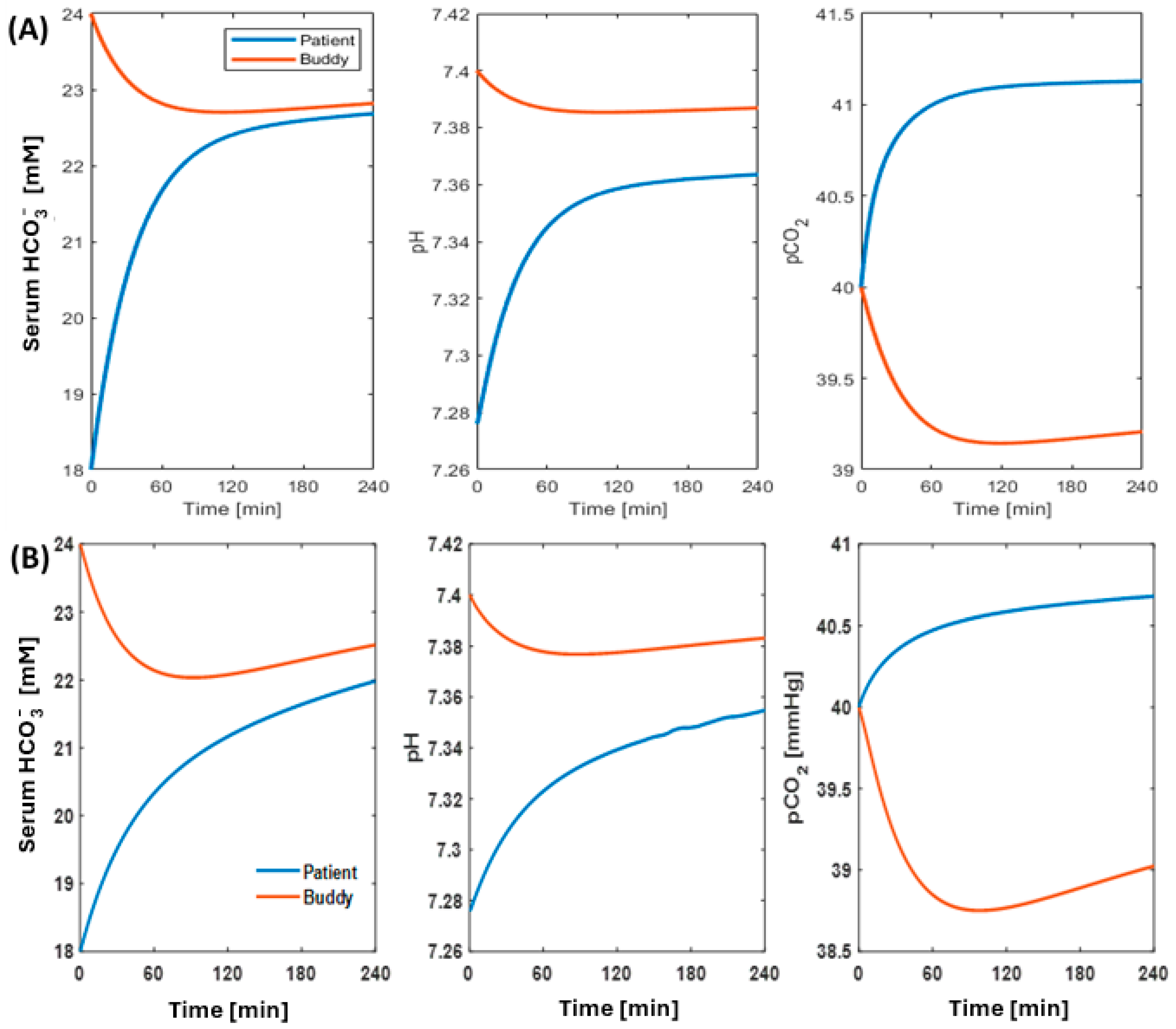
2.1.2. Correction of Metabolic Acidosis
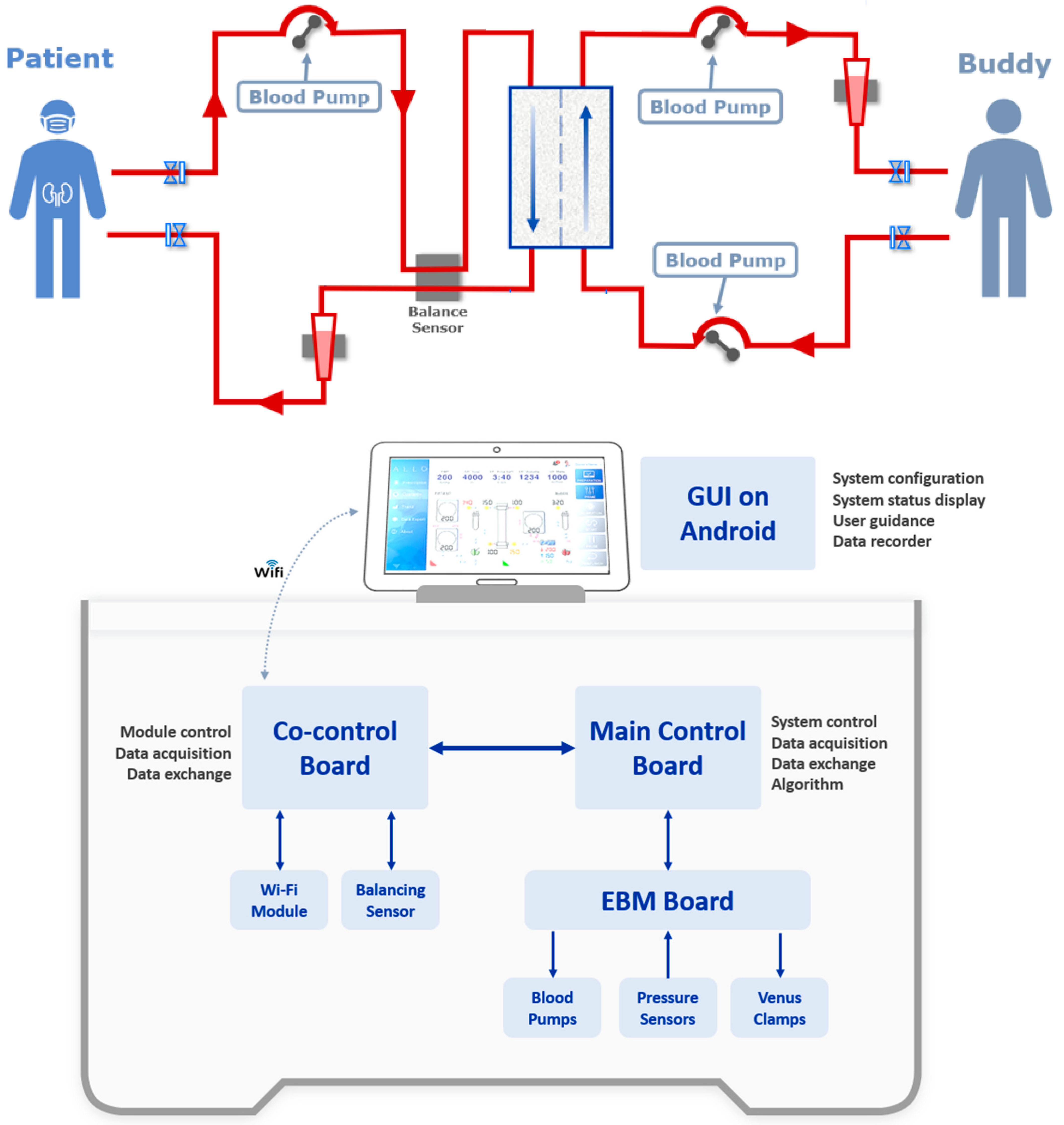
2.2. AlloHD Prototyping
2.2.1. AlloHD System Design
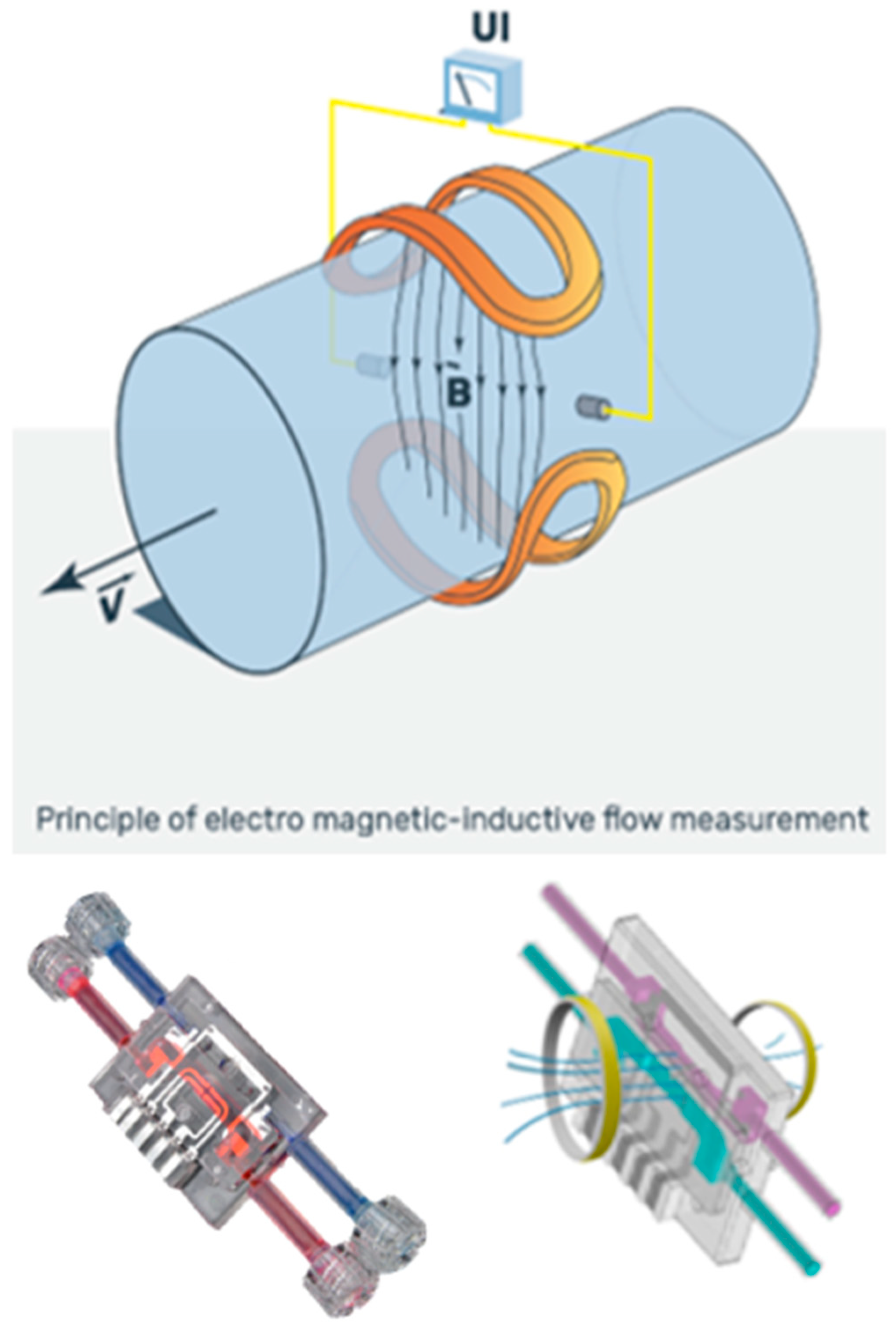
2.2.2. AlloHD Balancing Technology
2.2.3. AlloHD Machine Prototype
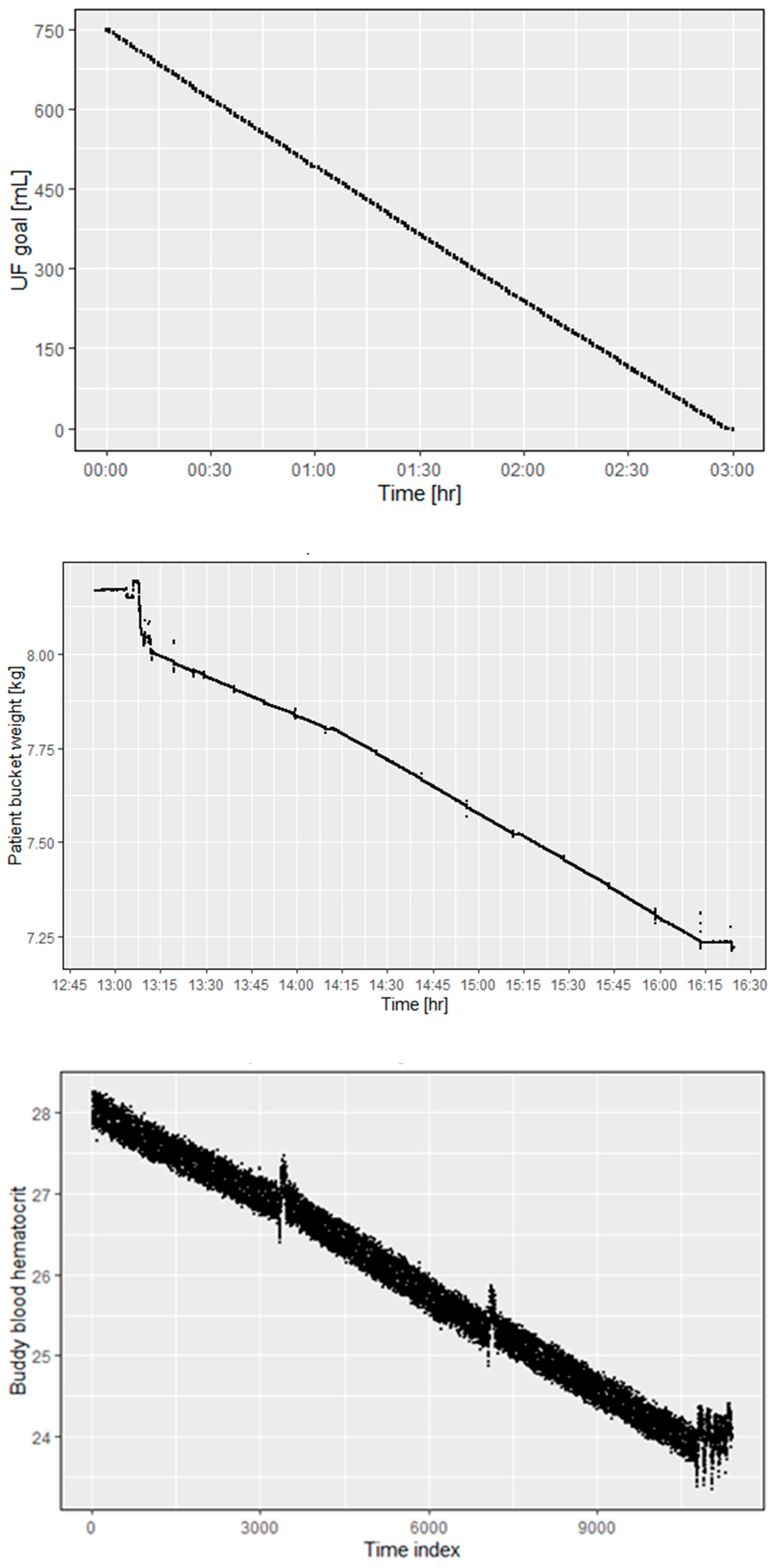
2.3. Bench Tests
3. Discussion
4. Materials and Methods
4.1. Mathematical Modeling of Solute Transport
4.1.1. Urea Kinetic Modeling (UKM)
4.1.2. Acid–Base Dynamics
4.2. Prototype Development
AlloHD Balancing Technology
4.3. Ex Vivo Experiments
4.3.1. Urea Kinetics
4.3.2. Removal of Creatinine and Protein-Bound Uremic Solutes
4.3.3. Ultrafiltration Performance
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.H.; Lv, J.; Garg, A.X.; Knight, J.; et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.K.L.A.; Lunney, M.; Osman, M.A.; Ye, F.; Ashuntantang, G.; Bellorin-Font, E.; Benghanem Gharbi, M.; Ghnaimat, M.; Harden, P.; Jha, V.; et al. Global Kidney Health Atlas: A Report by the International Society of Nephrology on the Global Burden of End-stage Kidney Disease and Capacity for Kidney Replacement Therapy and Conservative Care across World Countries and Regions; International Society of Nephrology: Brussels, Belgium, 2019. [Google Scholar]
- Delville, M.; Manceau, S.; Ait Abdallah, N.; Stolba, J.; Awad, S.; Damy, T.; Gellen, B.; Sabbah, L.; Debbache, K.; Audard, V.; et al. Arterio-venous fistula for automated red blood cells exchange in patients with sickle cell disease: Complications and outcomes. Am. J. Hematol. 2017, 92, 136–140. [Google Scholar] [CrossRef]
- McCarthy, W.J.; Valentino, L.A.; Bonilla, A.S.; Goncharova, I.; Taylor, A.; Pooley, T.A.; Jacobs, C.E. Arteriovenous fistula for long-term venous access for boys with hemophilia. J. Vasc. Surg. 2007, 45, 986–991. [Google Scholar] [CrossRef]
- Al-Amin, A.; Wood, J.; Atturu, G.; Gouda, M.R.; Donnellan, C.F.; Burke, D.A. Use of Arteriovenous Fistulae for Home Parenteral Nutrition—A Review of the Literature. J. Vasc. Access 2013, 14, 99–103. [Google Scholar] [CrossRef]
- Dukhin, S.S.; Tabani, Y.; Lai, R.; Labib, O.A.; Zydney, A.L.; Labib, M.E. Outside-in hemofiltration for prolonged operation without clogging. Outside-in hemofiltration for prolonged operation without clogging. J. Membr. Sci. 2014, 464, 173–178. [Google Scholar] [CrossRef]
- Gelderblom, H.R. Structure and classification of viruses. In Medical Microbiology; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Campos, I.; Arellano, J.; Gomez, V.; Quiroz, J.; Mariscal, L.A. Renal Replacement Therapy Preferences Survey: Is Allo-Hemodialysis an Acceptable Option for Patient Caregivers and Health Care Professionals? Blood Purif 2020, 49, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Gotch, F. A quantitative evaluation of small and middle molecule toxicity in therapy of uremia. Dial. Transplant. 1980, 9, 183–192. [Google Scholar]
- Maheshwari, V.; Thijssen, S.; Tao, X.; Fuertinger, D.; Kappel, F.; Kotanko, P. A novel mathematical model of protein-bound uremic toxin kinetics during hemodialysis. Sci. Rep. 2017, 7, 10371. [Google Scholar] [CrossRef] [PubMed]
- Villarroel, F.; Klein, E.; Holland, F. Solute flux in hemodialysis and hemofiltration membranes. Trans. Am. Soc. Artif. Intern. Organs. 1977, 23, 225–232. [Google Scholar] [CrossRef]
- Waniewski, J. Mathematical modeling of fluid and solute transport in hemodialysis and peritoneal dialysis. J. Membr. Sci. 2006, 274, 24–37. [Google Scholar] [CrossRef]
- Brulles, A.; Gras, J.; Magrina, N.; Torres, N.; Caralps, A. Relation between urea clearance and glomerular filtration rate according to urine flow/minute. Clin. Chim. Acta 1969, 24, 261–265. [Google Scholar] [CrossRef]
- Bankir, L.; Bouby, N.; Trinh-Trang-Tan, M.-M.; Ahloulay, M.; Promeneur, D. Direct and indirect cost of urea excretion. Kidney Int. 1996, 49, 1598–1607. [Google Scholar] [CrossRef]
- Reid, F.; Lobo, D.N.; Williams, R.N.; Rowlands, B.J.; Allison, S.P. (Ab) normal saline and physiological Hartmann’s solution: A randomized double-blind crossover study. Clin. Sci. 2003, 104, 17–24. [Google Scholar] [CrossRef]
- Decaux, G.; Unger, J.; Brimioulle, S.; Mockel, J. Hyponatremia in the syndrome of inappropriate secretion of antidiuretic hormone: Rapid correction with urea, sodium chloride, and water restriction therapy. JAMA 1982, 247, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.; Brown, E.; Daley, C. Oxford Handbook of Dialysis; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Daugirdas, J.T. Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J. Am. Soc. Nephrol. 1993, 4, 1205–1213. [Google Scholar] [CrossRef]
- Leypoldt, J.K. Urea standard Kt/Vurea for assessing dialysis treatment adequacy. Hemodial. Int. 2004, 8, 193–197. [Google Scholar] [CrossRef]
- Cherif, A.; Maheshwari, V.; Fuertinger, D.; Schappacher-Tilp, G.; Preciado, P.; Bushinsky, D.; Thijssen, S.; Kotanko, P. A mathematical model of the four cardinal acid-base disorders. Math. Biosci. Eng. 2020, 17, 4457–4476. [Google Scholar] [CrossRef] [PubMed]
- Cherif, A.; Maheshwari, V.; Preciado, P.; Fuertinger, D.; Schappacher-tilp, G.; Thijssen, S.; Bushinsky, D.; Kotanko, P. Predicting the safety and efficacy of buffer therapy to control acidemia in uremic patients. Nephrol. Dial. Transplant. 2018, 33. [Google Scholar] [CrossRef]
- Cherif, A.; Maheshwari, V.; Fuertinger, D.H.; Gagel, A.; Bushinsky, D.A.; Kotanko, P. Acid-Base dynamics during hemodialysis: Quantitative insights from a novel physiology-based mathematical model. Nephrol. Dial. Transplant. 2019, 34 (Suppl. 1), SuO001. [Google Scholar] [CrossRef]
- Cherif, A.; Maheshwari, V.; Fuertinger, D.H.; Gagel, A.; Thijssen, S.; Bushinsky, D.A.; Kotanko, P. Predicting prescribed dialysis bicarbonate to correct acidemia: Application of a novel physiology-based mathematical model. Nephrol. Dial. Transplant. 2019, 34 (Suppl. 1), SuO005. [Google Scholar] [CrossRef]
- Maheshwari, V.; Cherif, A.; Fuertinger, D.; Schappacher-Tilp, G.; Preciado, P.; Thijssen, S.; Bushinsky, D.A.; Kotanko, P. An in silico method to predict net calcium transfer during hemodialysis. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Republic of Korea, 11–15 July 2017; pp. 2740–2743. [Google Scholar]
- Boron, W.F. Acid-base transport by the renal proximal tubule. J. Am. Soc. Nephrol. 2006, 17, 2368–2382. [Google Scholar] [CrossRef]
- Atkinson, K.F.; Nauli, S.M. pH sensors and ion Transporters: Potential therapeutic targets for acid-base disorders. Int. J. Pharma Res. Rev. 2016, 5, 51. [Google Scholar] [PubMed]
- Davenport, H. The ABC of Acid-Base Chemistry; The University of Chicago: Chicago, IL, USA, 1974. [Google Scholar]
- Hainsworth, R. Acid-Base Balance; Manchester University: Manchester, UK, 1986. [Google Scholar]
- Hamm, L.L.; Nakhoul, N.; Hering-Smith, K.S. Acid-base homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Masco, N.A. Acid-Base Homeostasis. J. Infus. Nurs. 2016, 39, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Nagami, G.T.; Hamm, L.L. Regulation of acid-base balance in chronic kidney disease. Adv. Chronic Kidney Dis. 2017, 24, 274–279. [Google Scholar] [CrossRef]
- Quigley, R. Acid-base homeostasis. In Clinical Pediatric Nephrology; CRC Press: Boca Raton, FL, USA, 2016; p. 235. [Google Scholar]
- Schwartz, W.B.; Cohen, J.J. The nature of the renal response to chronic disorders of acid-base equilibrium. Am. J. Med. 1978, 64, 417–428. [Google Scholar] [CrossRef]
- Skelton, L.A.; Boron, W.F.; Zhou, Y. Acid-base transport by the renal proximal tubule. J. Nephrol. 2010, 23, S4. [Google Scholar]
- West, J.B. Respiratory Physiology, 9th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Widdicombe, J.; Davies, A. Respiratory Physiology; Edward Arnold Publishers: London, UK, 1983. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maheshwari, V.; Grobe, N.; Wang, X.; Patel, A.; Cherif, A.; Tao, X.; Chao, J.; Heide, A.; Nikolic, D.; Dong, J.; et al. Allo-Hemodialysis, a Novel Dialytic Treatment Option for Patients with Kidney Failure: Outcomes of Mathematical Modelling, Prototyping, and Ex Vivo Testing. Toxins 2024, 16, 292. https://doi.org/10.3390/toxins16070292
Maheshwari V, Grobe N, Wang X, Patel A, Cherif A, Tao X, Chao J, Heide A, Nikolic D, Dong J, et al. Allo-Hemodialysis, a Novel Dialytic Treatment Option for Patients with Kidney Failure: Outcomes of Mathematical Modelling, Prototyping, and Ex Vivo Testing. Toxins. 2024; 16(7):292. https://doi.org/10.3390/toxins16070292
Chicago/Turabian StyleMaheshwari, Vaibhav, Nadja Grobe, Xin Wang, Amrish Patel, Alhaji Cherif, Xia Tao, Joshua Chao, Alexander Heide, Dejan Nikolic, Jiaming Dong, and et al. 2024. "Allo-Hemodialysis, a Novel Dialytic Treatment Option for Patients with Kidney Failure: Outcomes of Mathematical Modelling, Prototyping, and Ex Vivo Testing" Toxins 16, no. 7: 292. https://doi.org/10.3390/toxins16070292
APA StyleMaheshwari, V., Grobe, N., Wang, X., Patel, A., Cherif, A., Tao, X., Chao, J., Heide, A., Nikolic, D., Dong, J., & Kotanko, P. (2024). Allo-Hemodialysis, a Novel Dialytic Treatment Option for Patients with Kidney Failure: Outcomes of Mathematical Modelling, Prototyping, and Ex Vivo Testing. Toxins, 16(7), 292. https://doi.org/10.3390/toxins16070292





