Residual Kidney Function in Hemodialysis: Its Importance and Contribution to Improved Patient Outcomes
Abstract
:1. Residual Kidney Function: What It Is
2. Residual Kidney Function: Why It Is Important
2.1. Uremic Toxin and Acidic Metabolite Clearance
2.1.1. Small Water-Soluble (Non-Protein-Bound) Uremic Toxins
2.1.2. Middle-Molecule Uremic Toxins
2.1.3. Protein-Bound Uremic Toxins
2.1.4. Acidic Metabolites
2.2. Fluid Elimination
2.3. Immune Function
2.4. Lipid Regulation
2.5. Glucose Regulation
2.6. Protein Metabolism
2.7. Hormone Production
3. RKF Indices and Their Limitations
3.1. Glomerular Filtration Rate (GFR)
3.2. CLurea
3.3. Urine Volume
4. RKF Evaluation
4.1. Timed Urine Collection for GFR
4.2. Timed Urine Collection for CLurea
Limitations of Timed Urine Collection
4.3. GFR Measurement Using Exogenous Filtration Markers
4.4. RKF Estimating Equations without Timed Urine Collection
5. Potential Benefits of Targeted Interventions Aimed at Preserving RKF
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kong, J.; Davies, M.; Mount, P. The importance of residual kidney function in haemodialysis patients. Nephrology 2018, 23, 1073–1080. [Google Scholar] [CrossRef]
- Alrowiyti, I.M.; Bargman, J. A Review of Residual Kidney Function in Peritoneal Dialysis Patients. Indian J. Nephrol. 2023, 33, 239–246. [Google Scholar] [CrossRef]
- Murea, M.; Deira, J.; Kalantar-Zadeh, K.; Casino, F.G.; Basile, C. The spectrum of kidney dysfunction requiring chronic dialysis therapy: Implications for clinical practice and future clinical trials. Semin. Dial. 2022, 35, 107–116. [Google Scholar] [CrossRef]
- Said, N.; Lau, W.J.; Ho, Y.C.; Lim, S.K.; Zainol Abidin, M.N.; Ismail, A.F. A Review of Commercial Developments and Recent Laboratory Research of Dialyzers and Membranes for Hemodialysis Application. Membranes 2021, 11, 767. [Google Scholar] [CrossRef]
- Murea, M.; Flythe, J.E.; Anjay, R.; Emaad, A.M.; Gupta, N.; Kovach, C.; Vachharajani, T.J.; Kalantar-Zadeh, K.; Casino, F.G.; Basile, C. Kidney dysfunction requiring dialysis is a heterogeneous syndrome: We should treat it like one. Curr. Opin. Nephrol. Hypertens. 2022, 31, 92–99. [Google Scholar] [CrossRef]
- Meyer, T.W.; Hostetter, T.H. Uremia. N. Engl. J. Med. 2007, 357, 1316–1325. [Google Scholar] [CrossRef]
- Glorieux, G.; Tattersall, J. Uraemic toxins and new methods to control their accumulation: Game changers for the concept of dialysis adequacy. Clin. Kidney J. 2015, 8, 353–362. [Google Scholar] [CrossRef]
- Bello-Reuss, E.; Reuss, L. Homeostatic and Excretory Functions of the Kidney. In The Kidney and Body Fluids in Health and Disease; Klahr, S., Ed.; Springer: Boston, MA, USA, 1983; pp. 35–63. [Google Scholar] [CrossRef]
- Meyer, T.W.; Sirich, T.L.; Hostetter, T.H. Dialysis cannot be dosed. Semin. Dial. 2011, 24, 471–479. [Google Scholar] [CrossRef]
- Bohle, A.; Aeikens, B.; Eenboom, A.; Fronholt, L.; Plate, W.R.; Xiao, J.C.; Greschniok, A.; Wehrmann, M. Human glomerular structure under normal conditions and in isolated glomerular disease. Kidney Int. Suppl. 1998, 67, S186–S188. [Google Scholar] [CrossRef]
- Toth-Manikowski, S.M.; Sirich, T.L.; Meyer, T.W.; Hostetter, T.H.; Hwang, S.; Plummer, N.S.; Hai, X.; Coresh, J.; Powe, N.R.; Shafi, T. Contribution of ‘clinically negligible’ residual kidney function to clearance of uremic solutes. Nephrol. Dial. Transplant. 2020, 35, 846–853. [Google Scholar] [CrossRef]
- Suchy-Dicey, A.M.; Laha, T.; Hoofnagle, A.; Newitt, R.; Sirich, T.L.; Meyer, T.W.; Thummel, K.E.; Yanez, N.D.; Himmelfarb, J.; Weiss, N.S.; et al. Tubular Secretion in CKD. J. Am. Soc. Nephrol. 2016, 27, 2148–2155. [Google Scholar] [CrossRef] [PubMed]
- Mair, R.D.; Sirich, T.L.; Meyer, T.W. Uremic Toxin Clearance and Cardiovascular Toxicities. Toxins 2018, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.J.; Hagge, W.W.; Wagoner, R.D.; Dinapoli, R.P.; Rosevear, J.W. Effects of urea loading in patients with far-advanced renal failure. Mayo Clin. Proc. 1972, 47, 21–29. [Google Scholar]
- Velasquez, M.T.; Ramezani, A.; Raj, D.S. Urea and protein carbamylation in ESRD: Surrogate markers or partners in crime? Kidney Int. 2015, 87, 1092–1094. [Google Scholar] [CrossRef]
- Jaworska, K.; Hering, D.; Mosieniak, G.; Bielak-Zmijewska, A.; Pilz, M.; Konwerski, M.; Gasecka, A.; Kapłon-Cieślicka, A.; Filipiak, K.; Sikora, E.; et al. TMA, A Forgotten Uremic Toxin, but Not TMAO, Is Involved in Cardiovascular Pathology. Toxins 2019, 11, 490. [Google Scholar] [CrossRef]
- Hsu, B.G.; Wang, C.H.; Lin, Y.L.; Lai, Y.H.; Tsai, J.P. Serum Trimethylamine N-Oxide Level Is Associated with Peripheral Arterial Stiffness in Advanced Non-Dialysis Chronic Kidney Disease Patients. Toxins 2022, 14, 526. [Google Scholar] [CrossRef] [PubMed]
- Poesen, R.; Claes, K.; Evenepoel, P.; de Loor, H.; Augustijns, P.; Kuypers, D.; Meijers, B. Microbiota-Derived Phenylacetylglutamine Associates with Overall Mortality and Cardiovascular Disease in Patients with CKD. J. Am. Soc. Nephrol. 2016, 27, 3479–3487. [Google Scholar] [CrossRef] [PubMed]
- Eknoyan, G.; Beck, G.J.; Cheung, A.K.; Daugirdas, J.T.; Greene, T.; Kusek, J.W.; Allon, M.; Bailey, J.; Delmez, J.A.; Depner, T.A.; et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N. Engl. J. Med. 2002, 347, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Iwasawa, H.; Nakao, T.; Matsumoto, H.; Okada, T.; Nagaoka, Y.; Wada, T. Phosphate handling by end-stage kidneys and benefits of residual renal function on phosphate removal in patients on haemodialysis. Nephrology 2013, 18, 285–291. [Google Scholar] [CrossRef]
- Wang, M.; You, L.; Li, H.; Lin, Y.; Zhang, Z.; Hao, C.; Chen, J. Association of circulating fibroblast growth factor-23 with renal phosphate excretion among hemodialysis patients with residual renal function. Clin. J. Am. Soc. Nephrol. 2013, 8, 116–125. [Google Scholar] [CrossRef]
- Rosner, M.H.; Reis, T.; Husain-Syed, F.; Vanholder, R.; Hutchison, C.; Stenvinkel, P.; Blankestijn, P.J.; Cozzolino, M.; Juillard, L.; Kashani, K.; et al. Classification of Uremic Toxins and Their Role in Kidney Failure. Clin. J. Am. Soc. Nephrol. 2021, 16, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Blankestijn, P.J.; Vernooij, R.W.M.; Hockham, C.; Strippoli, G.F.M.; Canaud, B.; Hegbrant, J.; Barth, C.; Covic, A.; Cromm, K.; Cucui, A.; et al. Effect of Hemodiafiltration or Hemodialysis on Mortality in Kidney Failure. N. Engl. J. Med. 2023, 389, 700–709. [Google Scholar] [CrossRef]
- Cheung, A.K.; Levin, N.W.; Greene, T.; Agodoa, L.; Bailey, J.; Beck, G.; Clark, W.; Levey, A.S.; Leypoldt, J.K.; Ornt, D.B.; et al. Effects of high-flux hemodialysis on clinical outcomes: Results of the HEMO study. J. Am. Soc. Nephrol. 2003, 14, 3251–3263. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.C.; Singh, D.K.; Chandna, S.M.; Farrington, K. Relative importance of residual renal function and convection in determining beta-2-microglobulin levels in high-flux haemodialysis and on-line haemodiafiltration. Blood Purif. 2007, 25, 295–302. [Google Scholar] [CrossRef]
- Lowenstein, J.; Grantham, J.J. Residual renal function: A paradigm shift. Kidney Int. 2017, 91, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Sirich, T.L.; Funk, B.A.; Plummer, N.S.; Hostetter, T.H.; Meyer, T.W. Prominent accumulation in hemodialysis patients of solutes normally cleared by tubular secretion. J. Am. Soc. Nephrol. 2014, 25, 615–622. [Google Scholar] [CrossRef]
- Marquez, I.O.; Tambra, S.; Luo, F.Y.; Li, Y.; Plummer, N.S.; Hostetter, T.H.; Meyer, T.W. Contribution of residual function to removal of protein-bound solutes in hemodialysis. Clin. J. Am. Soc. Nephrol. 2011, 6, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.C.; Sao, J.N.; Taussig, A.; Plummer, N.S.; Meyer, T.W.; Sirich, T.L. Residual Function Effectively Controls Plasma Concentrations of Secreted Solutes in Patients on Twice Weekly Hemodialysis. J. Am. Soc. Nephrol. 2018, 29, 1992–1999. [Google Scholar] [CrossRef]
- Bammens, B.; Evenepoel, P.; Keuleers, H.; Verbeke, K.; Vanrenterghem, Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006, 69, 1081–1087. [Google Scholar] [CrossRef]
- Liabeuf, S.; Barreto, D.V.; Barreto, F.C.; Meert, N.; Glorieux, G.; Schepers, E.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol. Dial. Transplant. 2010, 25, 1183–1191. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef]
- Meijers, B.K.; Claes, K.; Bammens, B.; de Loor, H.; Viaene, L.; Verbeke, K.; Kuypers, D.; Vanrenterghem, Y.; Evenepoel, P. p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 1182–1189. [Google Scholar] [CrossRef]
- Meijers, B.K.; Bammens, B.; De Moor, B.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int. 2008, 73, 1174–1180. [Google Scholar] [CrossRef]
- Shafi, T.; Sirich, T.L.; Meyer, T.W.; Hostetter, T.H.; Plummer, N.S.; Hwang, S.; Melamed, M.L.; Banerjee, T.; Coresh, J.; Powe, N.R. Results of the HEMO Study suggest that p-cresol sulfate and indoxyl sulfate are not associated with cardiovascular outcomes. Kidney Int. 2017, 92, 1484–1492. [Google Scholar] [CrossRef]
- Suda, T.; Hiroshige, K.; Ohta, T.; Watanabe, Y.; Iwamoto, M.; Kanegae, K.; Ohtani, A.; Nakashima, Y. The contribution of residual renal function to overall nutritional status in chronic haemodialysis patients. Nephrol. Dial. Transplant. 2000, 15, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Sterns, R.H. Chapter 38—Water Homeostasis in Chronic Kidney Disease. In Chronic Renal Disease, 2nd ed.; Kimmel, P.L., Rosenberg, M.E., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 615–632. [Google Scholar] [CrossRef]
- Su, W.; Cao, R.; Zhang, X.Y.; Guan, Y. Aquaporins in the kidney: Physiology and pathophysiology. Am. J. Physiol. Renal Physiol. 2020, 318, F193–F203. [Google Scholar] [CrossRef] [PubMed]
- Assimon, M.M.; Flythe, J.E. Rapid ultrafiltration rates and outcomes among hemodialysis patients: Re-examining the evidence base. Curr. Opin. Nephrol. Hypertens. 2015, 24, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.J.; Marcelli, D.; Canaud, B.J.; Carioni, P.; Wang, Y.; Grassmann, A.; Konings, C.J.; Kotanko, P.; Leunissen, K.M.; Levin, N.W.; et al. Impact of fluid status and inflammation and their interaction on survival: A study in an international hemodialysis patient cohort. Kidney Int. 2017, 91, 1214–1223. [Google Scholar] [CrossRef]
- Zoccali, C.; Moissl, U.; Chazot, C.; Mallamaci, F.; Tripepi, G.; Arkossy, O.; Wabel, P.; Stuard, S. Chronic Fluid Overload and Mortality in ESRD. J. Am. Soc. Nephrol. 2017, 28, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Moissl, U.; Fuentes, L.R.; Hakim, M.I.; Hassler, M.; Kothari, D.A.; Rosales, L.; Zhu, F.; Raimann, J.G.; Thijssen, S.; Kotanko, P. Prevalence of fluid overload in an urban US hemodialysis population: A cross-sectional study. Hemodial. Int. 2022, 26, 264–273. [Google Scholar] [CrossRef]
- Morduchowicz, G.; Winkler, J.; Zabludowski, J.R.; Boner, G. Effects of residual renal function in haemodialysis patients. Int. Urol. Nephrol. 1994, 26, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Vilar, E.; Wellsted, D.; Chandna, S.M.; Greenwood, R.N.; Farrington, K. Residual renal function improves outcome in incremental haemodialysis despite reduced dialysis dose. Nephrol. Dial. Transplant. 2009, 24, 2502–2510. [Google Scholar] [CrossRef]
- Ma, T.; Ding, G. Effects of residual renal function on left ventricle and analysis of related factors in patients with hemodialysis. Ren. Fail. 2013, 35, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Wang, M.; Woo, J.; Law, M.C.; Chow, K.M.; Li, P.K.; Lui, S.F.; Sanderson, J.E. A novel association between residual renal function and left ventricular hypertrophy in peritoneal dialysis patients. Kidney Int. 2002, 62, 639–647. [Google Scholar] [CrossRef]
- Kurts, C.; Ginhoux, F.; Panzer, U. Kidney dendritic cells: Fundamental biology and functional roles in health and disease. Nat. Rev. Nephrol. 2020, 16, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, H.; Liu, C.; Cheng, A.; Deng, Q.; Zhu, H.; Chen, J. Dendritic Cells: Versatile Players in Renal Transplantation. Front. Immunol. 2021, 12, 654540. [Google Scholar] [CrossRef] [PubMed]
- Rogers, N.M.; Ferenbach, D.A.; Isenberg, J.S.; Thomson, A.W.; Hughes, J. Dendritic cells and macrophages in the kidney: A spectrum of good and evil. Nat. Rev. Nephrol. 2014, 10, 625–643. [Google Scholar] [CrossRef]
- Weisheit, C.K.; Engel, D.R.; Kurts, C. Dendritic Cells and Macrophages: Sentinels in the Kidney. Clin. J. Am. Soc. Nephrol. 2015, 10, 1841–1851. [Google Scholar] [CrossRef]
- Jamaly, S.; Rakaee, M.; Abdi, R.; Tsokos, G.C.; Fenton, K.A. Interplay of immune and kidney resident cells in the formation of tertiary lymphoid structures in lupus nephritis. Autoimmun. Rev. 2021, 20, 102980. [Google Scholar] [CrossRef]
- Leavy, O. Macrophages: Early antifungal defence in kidneys. Nat. Rev. Immunol. 2014, 14, 6–7. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Swamydas, M.; Fischer, B.G.; Plantinga, T.S.; Johnson, M.D.; Jaeger, M.; Green, N.M.; Masedunskas, A.; Weigert, R.; Mikelis, C.; et al. CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. J. Clin. Investig. 2013, 123, 5035–5051. [Google Scholar] [CrossRef] [PubMed]
- Lukacs-Kornek, V.; Burgdorf, S.; Diehl, L.; Specht, S.; Kornek, M.; Kurts, C. The kidney-renal lymph node-system contributes to cross-tolerance against innocuous circulating antigen. J. Immunol. 2008, 180, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Teteris, S.A.; Engel, D.R.; Kurts, C. Homeostatic and pathogenic role of renal dendritic cells. Kidney Int. 2011, 80, 139–145. [Google Scholar] [CrossRef]
- Stamatiades, E.G.; Tremblay, M.E.; Bohm, M.; Crozet, L.; Bisht, K.; Kao, D.; Coelho, C.; Fan, X.; Yewdell, W.T.; Davidson, A.; et al. Immune Monitoring of Trans-endothelial Transport by Kidney-Resident Macrophages. Cell 2016, 166, 991–1003. [Google Scholar] [CrossRef]
- Cobo, G.; Lindholm, B.; Stenvinkel, P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol. Dial. Transplant. 2018, 33, iii35–iii40. [Google Scholar] [CrossRef] [PubMed]
- Campo, S.; Lacquaniti, A.; Trombetta, D.; Smeriglio, A.; Monardo, P. Immune System Dysfunction and Inflammation in Hemodialysis Patients: Two Sides of the Same Coin. J. Clin. Med. 2022, 11, 3759. [Google Scholar] [CrossRef]
- Wang, A.Y.; Wang, M.; Woo, J.; Lam, C.W.; Lui, S.F.; Li, P.K.; Sanderson, J.E. Inflammation, residual kidney function, and cardiac hypertrophy are interrelated and combine adversely to enhance mortality and cardiovascular death risk of peritoneal dialysis patients. J. Am. Soc. Nephrol. 2004, 15, 2186–2194. [Google Scholar] [CrossRef]
- de Sequera, P.; Corchete, E.; Bohorquez, L.; Albalate, M.; Perez-Garcia, R.; Alique, M.; Marques, M.; García-Menéndez, E.; Portolés, J.; Ramirez, R. Residual Renal Function in Hemodialysis and Inflammation. Ther. Apher. Dial. 2017, 21, 592–598. [Google Scholar] [CrossRef]
- Wildgruber, M.; Aschenbrenner, T.; Wendorff, H.; Czubba, M.; Glinzer, A.; Haller, B.; Schiemann, M.; Zimmermann, A.; Berger, H.; Eckstein, H.H.; et al. The “Intermediate” CD14(++)CD16(+) monocyte subset increases in severe peripheral artery disease in humans. Sci. Rep. 2016, 6, 39483. [Google Scholar] [CrossRef]
- Shafi, T.; Jaar, B.G.; Plantinga, L.C.; Fink, N.E.; Sadler, J.H.; Parekh, R.S.; Powe, N.R.; Coresh, J. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: The Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am. J. Kidney Dis. 2010, 56, 348–358. [Google Scholar] [CrossRef]
- Yang, P.Y.; Lin, J.L.; Lin-Tan, D.T.; Hsu, C.W.; Yen, T.H.; Chen, K.H.; Ho, T.C. Residual daily urine volume association with inflammation and nutrition status in maintenance hemodialysis patients. Ren. Fail. 2009, 31, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Moestrup, S.K.; Nielsen, L.B. The role of the kidney in lipid metabolism. Curr. Opin. Lipidol. 2005, 16, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Mitrofanova, A.; Merscher, S.; Fornoni, A. Kidney lipid dysmetabolism and lipid droplet accumulation in chronic kidney disease. Nat. Rev. Nephrol. 2023, 19, 629–645. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D. Dyslipidemia of chronic renal failure: The nature, mechanisms, and potential consequences. Am. J. Physiol. Renal Physiol. 2006, 290, F262–F272. [Google Scholar] [CrossRef] [PubMed]
- Kaysen, G.A. Dyslipidemia in chronic kidney disease: Causes and consequences. Kidney Int. 2006, 70, S55–S58. [Google Scholar] [CrossRef]
- Bulbul, M.C.; Dagel, T.; Afsar, B.; Ulusu, N.N.; Kuwabara, M.; Covic, A.; Kanbay, M. Disorders of Lipid Metabolism in Chronic Kidney Disease. Blood Purif. 2018, 46, 144–152. [Google Scholar] [CrossRef]
- Rroji, M.; Spahia, N.; Seferi, S.; Barbullushi, M.; Spasovski, G. Influence of Residual Renal Function in Carotid Modeling as a Marker of Early Atherosclerosis in Dialysis Patients. Ther. Apher. Dial. 2017, 21, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Chou, C.Y.; Jheng, J.S.; Chen, I.R.; Liang, C.C.; Wang, S.M.; Liu, J.H.; Lin, S.Y.; Kuo, H.L.; Wang, I.K.; et al. Loss of Residual Renal Function is Associated With Vascular Calcification in Hemodialysis Patients. Ther. Apher. Dial. 2016, 20, 27–30. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Davidson, J.A.; Del Prato, S. The role of the kidneys in glucose homeostasis: A new path towards normalizing glycaemia. Diabetes Obes. Metab. 2012, 14, 5–14. [Google Scholar] [CrossRef]
- Burmeister, J.E.; Scapini, A.; da Rosa Miltersteiner, D.; da Costa, M.G.; Campos, B.M. Glucose-added dialysis fluid prevents asymptomatic hypoglycaemia in regular haemodialysis. Nephrol. Dial. Transplant. 2007, 22, 1184–1189. [Google Scholar] [CrossRef]
- Abe, M.; Kaizu, K.; Matsumoto, K. Plasma insulin is removed by hemodialysis: Evaluation of the relation between plasma insulin and glucose by using a dialysate with or without glucose. Ther. Apher. Dial. 2007, 11, 280–287. [Google Scholar] [CrossRef]
- Ferrario, M.; Raimann, J.G.; Thijssen, S.; Signorini, M.G.; Kruse, A.; Diaz-Buxo, J.A.; Cerutti, S.; Levin, N.W.; Kotanko, P. Effects of dialysate glucose concentration on heart rate variability in chronic hemodialysis patients: Results of a prospective randomized trial. Kidney Blood Press. Res. 2011, 34, 334–343. [Google Scholar] [CrossRef]
- Raimann, J.G.; Kruse, A.; Thijssen, S.; Kuntsevich, V.; Diaz-Buxo, J.A.; Levin, N.W.; Kotanko, P. Fatigue in hemodialysis patients with and without diabetes: Results from a randomized controlled trial of two glucose-containing dialysates. Diabetes Care 2010, 33, e121. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Kikuchi, F.; Kaizu, K.; Matsumoto, K. The influence of hemodialysis membranes on the plasma insulin level of diabetic patients on maintenance hemodialysis. Clin. Nephrol 2008, 69, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Okada, K.; Matsumoto, K. Plasma insulin and C-peptide concentrations in diabetic patients undergoing hemodialysis: Comparison with five types of high-flux dialyzer membranes. Diabetes Res. Clin. Pract. 2008, 82, e17–e19. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Okada, K.; Ikeda, K.; Matsumoto, S.; Soma, M.; Matsumoto, K. Characterization of insulin adsorption behavior of dialyzer membranes used in hemodialysis. Artif. Organs 2011, 35, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Kaizu, K.; Matsumoto, K. Evaluation of the hemodialysis-induced changes in plasma glucose and insulin concentrations in diabetic patients: Comparison between the hemodialysis and non-hemodialysis days. Ther. Apher. Dial. 2007, 11, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Shimizu, N.; Suzuki, A.; Matoba, K.; Momozono, A.; Masaki, T.; Ogawa, A.; Moriguchi, I.; Takano, K.; Kobayashi, N.; et al. Hemodialysis-Related Glycemic Disarray Proven by Continuous Glucose Monitoring; Glycemic Markers and Hypoglycemia. Diabetes Care 2021, 44, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.M., Jr. Regulation of enzymes of urea and arginine synthesis. Annu. Rev. Nutr. 1992, 12, 81–101. [Google Scholar] [CrossRef]
- Garibotto, G.; Sofia, A.; Saffioti, S.; Bonanni, A.; Mannucci, I.; Verzola, D. Amino acid and protein metabolism in the human kidney and in patients with chronic kidney disease. Clin. Nutr. 2010, 29, 424–433. [Google Scholar] [CrossRef]
- Prado de Negreiros Nogueira Maduro, I.; Elias, N.M.; Nonino Borges, C.B.; Padovan, G.J.; Cardeal da Costa, J.A.; Marchini, J.S. Total nitrogen and free amino acid losses and protein calorie malnutrition of hemodialysis patients: Do they really matter? Nephron Clin. Pract. 2007, 105, c9–c17. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Kremer, D.; Groothof, D.; van der Veen, Y.; de Blaauw, P.; van der Krogt, J.; Kema, I.P.; Westerhuis, R.; Heiner-Fokkema, M.R.; Bakker, S.J.L.; et al. Amino Acid Homeostasis and Fatigue in Chronic Hemodialysis Patients. Nutrients 2022, 14, 2810. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, F.K.; Smeets, J.S.J.; Broers, N.J.H.; van Kranenburg, J.M.X.; van der Sande, F.M.; Kooman, J.P.; van Loon, L.J.C. End-Stage Renal Disease Patients Lose a Substantial Amount of Amino Acids during Hemodialysis. J. Nutr. 2020, 150, 1160–1166. [Google Scholar] [CrossRef]
- Abumrad, N.N.; Williams, P.; Frexes-Steed, M.; Geer, R.; Flakoll, P.; Cersosimo, E.; Brown, L.L.; Melki, I.; Bulus, N.; Hourani, H.; et al. Inter-organ metabolism of amino acids in vivo. Diabetes Metab. Rev. 1989, 5, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Sahay, M.; Kalra, S.; Bandgar, T. Renal endocrinology: The new frontier. Indian J. Endocrinol. Metab. 2012, 16, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Acharya, V.; Olivero, J. The Kidney as an Endocrine Organ. Methodist Debakey Cardiovasc. J. 2018, 14, 305–307. [Google Scholar] [CrossRef]
- Sibbel, S.P.; Koro, C.E.; Brunelli, S.M.; Cobitz, A.R. Characterization of chronic and acute ESA hyporesponse: A retrospective cohort study of hemodialysis patients. BMC Nephrol. 2015, 16, 144. [Google Scholar] [CrossRef]
- Katagiri, D. Benefits and risks of erythrocyte-stimulating agents. World J. Clin. Urol. 2014, 3, 258. [Google Scholar] [CrossRef]
- Penne, E.L.; van der Weerd, N.C.; Grooteman, M.P.; Mazairac, A.H.; van den Dorpel, M.A.; Nube, M.J.; Bots, M.L.; Levesque, R.; ter Wee, P.M.; Blankestijn, P.J. Role of residual renal function in phosphate control and anemia management in chronic hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 281–289. [Google Scholar] [CrossRef]
- Kimura, H.; Sy, J.; Okuda, Y.; Wenziger, C.; Hanna, R.; Obi, Y.; Rhee, C.M.; Kovesdy, C.P.; Kalantar-Zadeh, K.; Streja, E. A faster decline of residual kidney function and erythropoietin stimulating agent hyporesponsiveness in incident hemodialysis patients. Hemodial. Int. 2021, 25, 60–70. [Google Scholar] [CrossRef]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, C.H.; Yoshida, K.; Zhao, P.; Meyer, T.W.; Zhang, L.; Huang, S.M.; Giacomini, K.M. Identification and Quantitative Assessment of Uremic Solutes as Inhibitors of Renal Organic Anion Transporters, OAT1 and OAT3. Mol. Pharm. 2016, 13, 3130–3140. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Nguyen, M.; Chen, Y.; Hoofnagle, A.N.; Becker, J.O.; Zelnick, L.R.; Kundzins, J.; Goodling, A.; Himmelfarb, J.; Kestenbaum, B. Association of Tubular Solute Clearance with Symptom Burden in Incident Peritoneal Dialysis. Clin. J. Am. Soc. Nephrol. 2020, 15, 530–538. [Google Scholar] [CrossRef]
- Chang, A.R.; Zafar, W.; Grams, M.E. Kidney Function in Obesity-Challenges in Indexing and Estimation. Adv. Chronic Kidney Dis. 2018, 25, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Milutinovic, J.; Cutler, R.E.; Hoover, P.; Meijsen, B.; Scribner, B.H. Measurement of residual glomerular filtration rate in the patient receiving repetitive hemodialysis. Kidney Int. 1975, 8, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.M.; Marr, J.; Kapke, A.; Jin, Y.; Pearson, J.; Esposito, D.; Young, E.W. Mortality Risk of Patients Treated in Dialysis Facilities with Payment Reductions under ESRD Quality Incentive Program. Clin. J. Am. Soc. Nephrol. 2023, 18, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Daugirdas, J.T.; Greene, T.; Chertow, G.M.; Depner, T.A. Can rescaling dose of dialysis to body surface area in the HEMO study explain the different responses to dose in women versus men? Clin. J. Am. Soc. Nephrol. 2010, 5, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Daugirdas, J.T. You’re Not Big--You’re Just Tall, That’s All! J. Am. Soc. Nephrol. 2016, 27, 339–341. [Google Scholar] [CrossRef]
- Obi, Y.; Streja, E.; Rhee, C.M.; Ravel, V.; Amin, A.N.; Cupisti, A.; Chen, J.; Mathew, A.T.; Kovesdy, C.P.; Mehrotra, R.; et al. Incremental Hemodialysis, Residual Kidney Function, and Mortality Risk in Incident Dialysis Patients: A Cohort Study. Am. J. Kidney Dis. 2016, 68, 256–265. [Google Scholar] [CrossRef]
- Obi, Y.; Rhee, C.M.; Mathew, A.T.; Shah, G.; Streja, E.; Brunelli, S.M.; Kovesdy, C.P.; Mehrotra, R.; Kalantar-Zadeh, K. Residual Kidney Function Decline and Mortality in Incident Hemodialysis Patients. J. Am. Soc. Nephrol. 2016, 27, 3758–3768. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.I.; Sheth, V.; Kim, J.; Bang, H. Estimating Residual Native Kidney Urea Clearance in Hemodialysis Patients with and without 24-Hour Urine Volume. Kidney Med. 2019, 1, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.; Debowska, M.; Gomez, R.; Waniewski, J.; Lindholm, B. Urine volume as an estimator of residual renal clearance and urinary removal of solutes in patients undergoing peritoneal dialysis. Sci. Rep. 2022, 12, 18755. [Google Scholar] [CrossRef]
- Bragg-Gresham, J.L.; Fissell, R.B.; Mason, N.A.; Bailie, G.R.; Gillespie, B.W.; Wizemann, V.; Cruz, J.M.; Akiba, T.; Kurokawa, K.; Ramirez, S.; et al. Diuretic use, residual renal function, and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Pattern Study (DOPPS). Am. J. Kidney Dis. 2007, 49, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Park, J.T.; Park, K.S.; Kwon, Y.E.; Oh, H.J.; Yoo, T.H.; Kim, Y.L.; Kim, Y.S.; Yang, C.W.; Kim, N.H.; et al. Prognostic Value of Residual Urine Volume, GFR by 24-hour Urine Collection, and eGFR in Patients Receiving Dialysis. Clin. J. Am. Soc. Nephrol. 2017, 12, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Hoek, F.J.; Korevaar, J.C.; Dekker, F.W.; Boeschoten, E.W.; Krediet, R.T. Estimation of residual glomerular filtration rate in dialysis patients from the plasma cystatin C level. Nephrol. Dial. Transplant. 2007, 22, 1633–1638. [Google Scholar] [CrossRef]
- Vilar, E.; Boltiador, C.; Wong, J.; Viljoen, A.; Machado, A.; Uthayakumar, A.; Farrington, K. Plasma Levels of Middle Molecules to Estimate Residual Kidney Function in Haemodialysis without Urine Collection. PLoS ONE 2015, 10, e0143813. [Google Scholar] [CrossRef]
- Wong, J.; Sridharan, S.; Berdeprado, J.; Vilar, E.; Viljoen, A.; Wellsted, D.; Farrington, K. Predicting residual kidney function in hemodialysis patients using serum beta-trace protein and beta2-microglobulin. Kidney Int. 2016, 89, 1090–1098. [Google Scholar] [CrossRef]
- Shafi, T.; Michels, W.M.; Levey, A.S.; Inker, L.A.; Dekker, F.W.; Krediet, R.T.; Hoekstra, T.; Schwartz, G.J.; Eckfeldt, J.H.; Coresh, J. Estimating residual kidney function in dialysis patients without urine collection. Kidney Int. 2016, 89, 1099–1110. [Google Scholar] [CrossRef]
- Steubl, D.; Fan, L.; Michels, W.M.; Inker, L.A.; Tighiouart, H.; Dekker, F.W.; Krediet, R.T.; Simon, A.L.; Foster, M.C.; Karger, A.B.; et al. Development and Validation of Residual Kidney Function Estimating Equations in Dialysis Patients. Kidney Med. 2019, 1, 104–114. [Google Scholar] [CrossRef]
- van Olden, R.W.; van Acker, B.A.; Koomen, G.C.; Krediet, R.T.; Arisz, L. Time course of inulin and creatinine clearance in the interval between two haemodialysis treatments. Nephrol. Dial. Transplant. 1995, 10, 2274–2280. [Google Scholar] [CrossRef]
- Daugirdas, J.T. Equations to Estimate the Normalized Creatinine Generation Rate (CGRn) in 3/Week Dialysis Patients With or Without Residual Kidney Function. J. Ren. Nutr. 2021, 31, 90–95. [Google Scholar] [CrossRef]
- Colton, C.K.; Smith, K.A.; Merrill, E.R.; Friedman, S. Diffusion of urea in flowing blood. AIChE J. 1971, 17, 800–808. [Google Scholar] [CrossRef]
- Obi, Y.; Kalantar-Zadeh, K.; Streja, E.; Daugirdas, J.T. Prediction equation for calculating residual kidney urea clearance using urine collections for different hemodialysis treatment frequencies and interdialytic intervals. Nephrol. Dial. Transplant. 2018, 33, 530–539. [Google Scholar] [CrossRef]
- National Kidney Fundation. NKF-K/DOQI Clinical Practice Guidelines for Peritoneal Dialysis Adequacy: Update 2000. Am. J. Kidney Dis. 2001, 37, S65–S136. [Google Scholar] [CrossRef]
- National Kidney Foundation. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 Update. Am. J. Kidney Dis. 2015, 66, 884–930. [Google Scholar] [CrossRef] [PubMed]
- Daugirdas, J.T. Estimating Weekly Urine Flow Rate And Residual Kidney Urea Clearance: A Method To Deal With Interdialytic Variability. Semin. Dial. 2016, 29, 510–514. [Google Scholar] [CrossRef]
- Kestenbaum, B.; Ix, J.H.; Gansevoort, R.; Granda, M.L.; Bakker, S.J.L.; Groothof, D.; Kieneker, L.M.; Hoofnagle, A.N.; Chen, Y.; Wang, K.; et al. Population-Based Limits of Urine Creatinine Excretion. Kidney Int. Rep. 2022, 7, 2474–2483. [Google Scholar] [CrossRef]
- Agarwal, R.; Delanaye, P. Glomerular filtration rate: When to measure and in which patients? Nephrol. Dial. Transplant. 2019, 34, 2001–2007. [Google Scholar] [CrossRef]
- Delanaye, P.; Ebert, N.; Melsom, T.; Gaspari, F.; Mariat, C.; Cavalier, E.; Bjork, J.; Christensson, A.; Nyman, U.; Porrini, E.; et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: A review. Part 1: How to measure glomerular filtration rate with iohexol? Clin. Kidney J. 2016, 9, 682–699. [Google Scholar] [CrossRef]
- Shafi, T.; Levey, A.S.; Inker, L.A.; Schwartz, G.J.; Knight, C.; Abraham, A.G.; Eckfeldt, J.H.; Coresh, J. Plasma Iohexol Clearance for Assessing Residual Kidney Function in Dialysis Patients. Am. J. Kidney Dis. 2015, 66, 728–730. [Google Scholar] [CrossRef]
- Agarwal, R.; Bills, J.E.; Yigazu, P.M.; Abraham, T.; Gizaw, A.B.; Light, R.P.; Bekele, D.M.; Tegegne, G.G. Assessment of iothalamate plasma clearance: Duration of study affects quality of GFR. Clin. J. Am. Soc. Nephrol. 2009, 4, 77–85. [Google Scholar] [CrossRef]
- White, C.A.; Akbari, A.; Allen, C.; Day, A.G.; Norman, P.A.; Holland, D.; Adams, M.A.; Knoll, G.A. Simultaneous glomerular filtration rate determination using inulin, iohexol, and (99m)Tc-DTPA demonstrates the need for customized measurement protocols. Kidney Int. 2021, 99, 957–966. [Google Scholar] [CrossRef]
- Shafi, T.; Levey, A.S. Measurement and Estimation of Residual Kidney Function in Patients on Dialysis. Adv. Chronic Kidney Dis. 2018, 25, 93–104. [Google Scholar] [CrossRef]
- Sambataro, M.; Thomaseth, K.; Pacini, G.; Robaudo, C.; Carraro, A.; Bruseghin, M.; Brocco, E.; Abaterusso, C.; DeFerrari, G.; Fioretto, P.; et al. Plasma clearance rate of 51Cr-EDTA provides a precise and convenient technique for measurement of glomerular filtration rate in diabetic humans. J. Am. Soc. Nephrol. 1996, 7, 118–127. [Google Scholar] [CrossRef]
- Dowling, T.C.; Frye, R.F.; Fraley, D.S.; Matzke, G.R. Comparison of iothalamate clearance methods for measuring GFR. Pharmacotherapy 1999, 19, 943–950. [Google Scholar] [CrossRef]
- Odlind, B.; Hallgren, R.; Sohtell, M.; Lindstrom, B. Is 125I iothalamate an ideal marker for glomerular filtration? Kidney Int. 1985, 27, 9–16. [Google Scholar] [CrossRef]
- Seegmiller, J.C.; Eckfeldt, J.H.; Lieske, J.C. Challenges in Measuring Glomerular Filtration Rate: A Clinical Laboratory Perspective. Adv. Chronic Kidney Dis. 2018, 25, 84–92. [Google Scholar] [CrossRef]
- Yang, Q.; Li, R.; Zhong, Z.; Mao, H.; Fan, J.; Lin, J.; Yang, X.; Wang, X.; Li, Z.; Yu, X. Is cystatin C a better marker than creatinine for evaluating residual renal function in patients on continuous ambulatory peritoneal dialysis? Nephrol. Dial. Transplant. 2011, 26, 3358–3365. [Google Scholar] [CrossRef]
- Mathew, A.T.; Fishbane, S.; Obi, Y.; Kalantar-Zadeh, K. Preservation of residual kidney function in hemodialysis patients: Reviving an old concept. Kidney Int. 2016, 90, 262–271. [Google Scholar] [CrossRef]
- D’Onofrio, G.; Simeoni, M.; Rizza, P.; Caroleo, M.; Capria, M.; Mazzitello, G.; Sacco, T.; Mazzuca, E.; Panzino, M.T.; Cerantonio, A.; et al. Quality of life, clinical outcome, personality and coping in chronic hemodialysis patients. Ren. Fail. 2017, 39, 45–53. [Google Scholar] [CrossRef]
- Okazaki, M.; Obi, Y.; Shafi, T.; Rhee, C.M.; Kovesdy, C.P.; Kalantar-Zadeh, K. Residual Kidney Function and Cause-Specific Mortality Among Incident Hemodialysis Patients. Kidney Int. Rep. 2023, 8, 1989–2000. [Google Scholar] [CrossRef]
- Hemodialysis Adequacy 2006 Work Group. Clinical practice guidelines for hemodialysis adequacy, update 2006. Am. J. Kidney Dis. 2006, 48 (Suppl. S1), S2–S90. [Google Scholar]
- Rhee, C.M.; Obi, Y.; Mathew, A.T.; Kalantar-Zadeh, K. Precision Medicine in the Transition to Dialysis and Personalized Renal Replacement Therapy. Semin. Nephrol. 2018, 38, 325–335. [Google Scholar] [CrossRef]
- Li, T.; Wilcox, C.S.; Lipkowitz, M.S.; Gordon-Cappitelli, J.; Dragoi, S. Rationale and Strategies for Preserving Residual Kidney Function in Dialysis Patients. Am. J. Nephrol. 2019, 50, 411–421. [Google Scholar] [CrossRef]
- Patel, S.M.; Kang, Y.M.; Im, K.; Neuen, B.L.; Anker, S.D.; Bhatt, D.L.; Butler, J.; Cherney, D.Z.I.; Claggett, B.L.; Fletcher, R.A.; et al. Sodium Glucose Co-transporter 2 Inhibitors and Major Adverse Cardiovascular Outcomes: A SMART-C Collaborative Meta-Analysis. Circulation 2024, 149, 1789–1801. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Berger, S.; Gansevoort, R.T.; Renal Life Cycle Trial Investigators. Will SGLT2 Inhibitors Be Effective and Safe in Patients with Severe CKD, Dialysis, or Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2023, 18, 1500–1502. [Google Scholar] [CrossRef]
- Daugirdas, J.T.; Greene, T.; Rocco, M.V.; Kaysen, G.A.; Depner, T.A.; Levin, N.W.; Chertow, G.M.; Ornt, D.B.; Raimann, J.G.; Larive, B.; et al. Effect of frequent hemodialysis on residual kidney function. Kidney Int. 2013, 83, 949–958. [Google Scholar] [CrossRef]
- Rocco, M.V.; Daugirdas, J.T.; Greene, T.; Lockridge, R.S.; Chan, C.; Pierratos, A.; Lindsay, R.; Larive, B.; Chertow, G.M.; Beck, G.J.; et al. Long-term Effects of Frequent Nocturnal Hemodialysis on Mortality: The Frequent Hemodialysis Network (FHN) Nocturnal Trial. Am. J. Kidney Dis. 2015, 66, 459–468. [Google Scholar] [CrossRef]
- Liu, S.; Diao, Z.; Zhang, D.; Ding, J.; Cui, W.; Liu, W. Preservation of residual renal function by not removing water in new hemodialysis patients: A randomized, controlled study. Int. Urol. Nephrol. 2014, 46, 83–90. [Google Scholar] [CrossRef]

 ). Compared with low-flux dialyzer clearance, high-flux dialyzers and hemodiafiltration are more effective in the removal of medium-molecular-weight uremic toxins (MUTs, denoted in yellow rectangles
). Compared with low-flux dialyzer clearance, high-flux dialyzers and hemodiafiltration are more effective in the removal of medium-molecular-weight uremic toxins (MUTs, denoted in yellow rectangles  ) and protein-bound uremic toxins (PBUTs, denoted in purple rectangles
) and protein-bound uremic toxins (PBUTs, denoted in purple rectangles  ), but all are inferior when clearing these molecules compared with residual kidney function.
), but all are inferior when clearing these molecules compared with residual kidney function.
 ). Compared with low-flux dialyzer clearance, high-flux dialyzers and hemodiafiltration are more effective in the removal of medium-molecular-weight uremic toxins (MUTs, denoted in yellow rectangles
). Compared with low-flux dialyzer clearance, high-flux dialyzers and hemodiafiltration are more effective in the removal of medium-molecular-weight uremic toxins (MUTs, denoted in yellow rectangles  ) and protein-bound uremic toxins (PBUTs, denoted in purple rectangles
) and protein-bound uremic toxins (PBUTs, denoted in purple rectangles  ), but all are inferior when clearing these molecules compared with residual kidney function.
), but all are inferior when clearing these molecules compared with residual kidney function.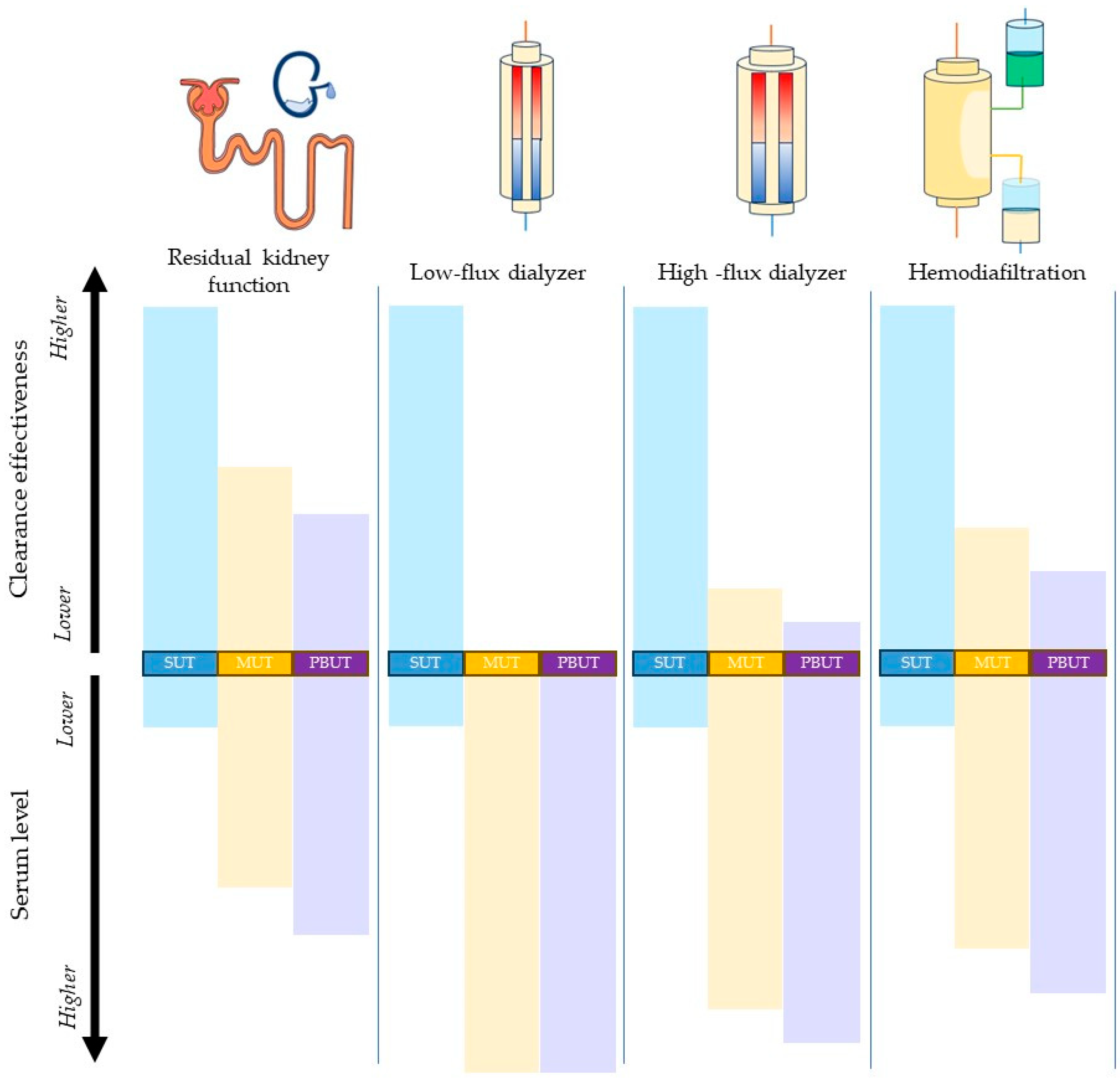
| Function | Natural Kidney | Artificial Kidney | Advantages Conferred by Residual Kidney Function |
|---|---|---|---|
| Solute and acid–base homeostasis |
|
|
|
| Fluid balance | Adjusts the volume and the concentration of the urine to maintain the balance of water. | Controlled removal of water through ultrafiltration. | Lower interdialytic weight gain. |
| Immune function |
| Aids immune function through clearance of uremic toxins. | Lower inflammation. |
| Lipid regulation | Clearance of lipoproteins and cholesterol. | None. | Better lean body mass. Lower risk of atherosclerosis.Lower vascular calcification burden. |
| Glucose homeostasis |
|
| Better nutritional status. |
| Protein metabolism |
|
| Better nutritional status. |
| Endocrine function | Erythropoietin. 1,25-dihydroxy vitamin D. | Necessitates the addition of pharmacologic products: erythropoietin-stimulating agents (ESAs), active vitamin D analogs. | Lower ESA requirements. Better anemia control. |
| Hoek, F.J. et al. [108] | Internal validation only |
| Equation | GFR (mL/min per 1.73 m2) = −0.77 + {21/Cystatin C (mg/L)} * |
| - Performance indices | Systemic bias 0.24 (SD, 1.24), 95% limits of agreement (−2.2, 2.68), r = 0.48 |
| Vilar, E. et al. [109] | Internal validation only |
| Equation (1) | GFR (mL/min) = (160.3/B2MG) − 4.2 |
| - Performance indices | Systemic bias 1.4 (SD, 1.92), 95% limits of agreement (−2.35, 5.16), r ^ 2 = 0.55 |
| Equation (2) | GFR (mL/min per 1.73 m2) = {142.2/B2MD (mg/L)} + {899.8/Creatinine (μmol/L)} + 0.0.13 × Pre-HD Weight (kg) − 5.63 |
| - Performance indices | Not available |
| Wong, J. et al. [110] | Internal validation only |
| Equation | GFR (mL/min) = {13.471/BTP (mg/L)} + {52.379/B2MG (mg/L)} + {782.909/Creatinine (μmol/L)} − 3.939 + 0.519 (if male) |
| - Performance indices | Systemic bias −0.64 (95%CI, −0.89 to −0.39), 95% limits of agreement (−2.84, 1.57), r = 0.783 |
| Shafi, T. et al. [111] | External validation |
| Equation (1) | GFR (mL/min per 1.73 m2) = 2852 × B2MG (mg/L) ^ (2.417) × 1.592 if male ** |
| - Performance indices | Systemic bias 1.0 (95%CI, 0.9 to 1.1), interquartile range of bias 1.9 (95%Ci, 1.7 to 2.1) |
| Equation (2) | GFR (mL/min per 1.73 m2) = 673 × BTP (mg/L) ^ (−1.406) × B2MG (mg/L) ^ (−1.096) × 1.670 if male ** |
| - Performance indices | Systemic bias 0.7 (95%CI, 0.6 to 0.8), IQR of bias 1.8 (95%Ci, 1.6 to 1.9) |
| Steubl, D. et al. [112] | External validation |
| Equation (1) | GFR (mL/min per 1.73 m2) = 39 × {B2MG (mg/L)/23} ^ (0.144) × Creatinine (mg/dL) ^ (−1.152) [For B2M ≤ 23 mg/L] |
| GFR (mL/min per 1.73 m2) = 39 × {B2MG (mg/L)/23} ^ (−2.129) × Creatinine (mg/dL) ^ (−1.152) [For B2M > 23 mg/L] | |
| - Performance indices | Systemic bias 0.4 (95%CI, 0.4 to 0.5), IQR of bias 1.8 (95%CI, 1.6 to 2.0) |
| Equation (2) | GFR (mL/min per 1.73 m2) = 32 × BTP (mg/L) ^ (−1.126) × {B2MG (mg/L)/23} ^ (0.271) [For B2M ≤ 23 mg/L] |
| GFR (mL/min per 1.73 m2) = 32 × BTP (mg/L) ^ (−1.126) × {B2MG (mg/L)/23} ^ (−2.133) [For B2M > 23 mg/L] | |
| - Performance indices | Systemic bias 0.1 (95%CI, 0.0 to 0.3), IQR of bias 1.8 (95%CI, 1.6 to 2.0) |
| Wong, J. et al. [110] | Internal validation only |
| Equation | CLurea (mL/min) = {90.97/BTP (mg/L)} + {37.568/B2MG (mg/L)} − 2.049 + 0.402 (if Caucasian) |
| - Perfomance indices | Systemic bias −0.50 (95%CI, −0.25 to −0.75), 95% limits of agreement (−2.03, 1.04), r = 0.762 |
| Shafi, T. et al. [111] | External validation |
| Equation (1) | CLurea (mL/min) = 2852 × B2MG (mg/L) ^ (2.417) × 1.592 if male * |
| - Perfomance indices | Systemic bias 0.7 (95%CI, 0.6 to 0.8), IQR of bias 1.6 (95%CI, 1.5 to 1.7) |
| Equation (2) | CLurea (mL/min) = 673 × BTP (mg/L) ^ (−1.406) × B2MG (mg/L) ^ (−1.096) × 1.670 if male * |
| - Perfomance indices | Systemic bias 0.5 (95%CI, 0.4 to 0.6), IQR of bias 1.5 (95%CI, 1.4 to 1.7) |
| Steubl, D. et al. [112] | External validation |
| Equation (1) | CLurea (mL/min) = 2 × {B2MG (mg/L)/24} ^ (−0.678) [For B2M < 24 mg/L] |
| CLurea (mL/min) = 2 × {B2MG (mg/L)/24} ^ (−2.880) [For B2M > 24 mg/L] | |
| - Perfomance indices | Systemic bias 0.6 (95%CI, 0.6 to 0.7), IQR of bias 1.5 (95%CI, 1.4 to 1.7) |
| Equation (2) | CLurea (mL/min) = 16 × BTP (mg/L) ^ (−1.02) × {B2MG (mg/L)/24} ^ (0.159) [For B2M < 24 mg/L] |
| CLurea (mL/min) = 16 × BTP (mg/L) ^ (−1.02) × {B2MG (mg/L)/24} ^ (−2.187) [For B2M > 24 mg/L] | |
| - Perfomance indices | Systemic bias 0.4 (95%CI, 0.3 to 0.5), IQR of bias 1.5 (95%CI, 1.3 to 1.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obi, Y.; Raimann, J.G.; Kalantar-Zadeh, K.; Murea, M. Residual Kidney Function in Hemodialysis: Its Importance and Contribution to Improved Patient Outcomes. Toxins 2024, 16, 298. https://doi.org/10.3390/toxins16070298
Obi Y, Raimann JG, Kalantar-Zadeh K, Murea M. Residual Kidney Function in Hemodialysis: Its Importance and Contribution to Improved Patient Outcomes. Toxins. 2024; 16(7):298. https://doi.org/10.3390/toxins16070298
Chicago/Turabian StyleObi, Yoshitsugu, Jochen G. Raimann, Kamyar Kalantar-Zadeh, and Mariana Murea. 2024. "Residual Kidney Function in Hemodialysis: Its Importance and Contribution to Improved Patient Outcomes" Toxins 16, no. 7: 298. https://doi.org/10.3390/toxins16070298






