A Novel Cytotoxic Mechanism for Triple-Negative Breast Cancer Cells Induced by the Type II Heat-Labile Enterotoxin LT-IIc through Ganglioside Ligation
Abstract
1. Background
2. Results
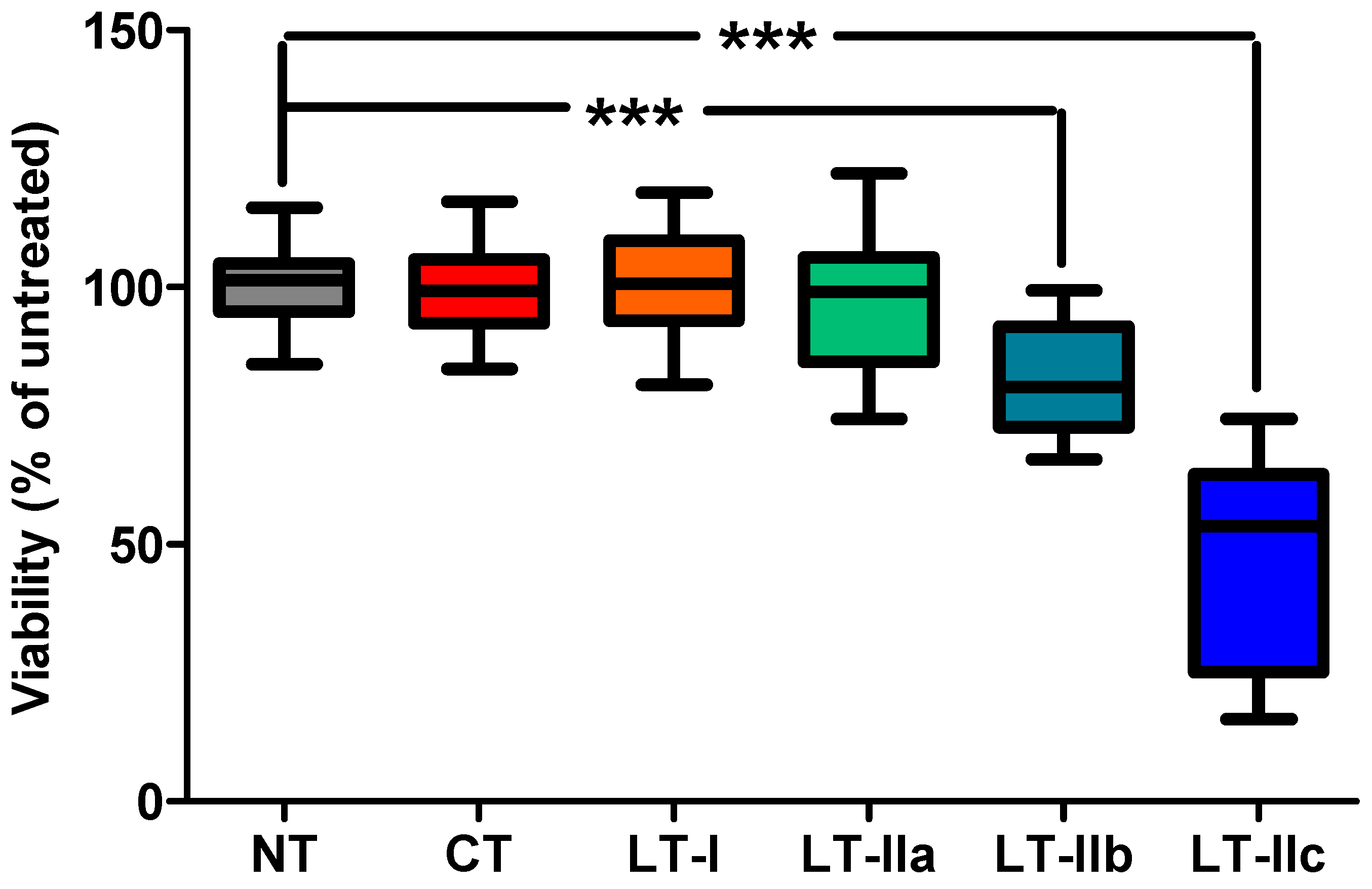
2.1. LT-IIc Elicits a Strong Cytotoxic Response in Primary Triple-Negative Breast Cancer (TNBC) Cells
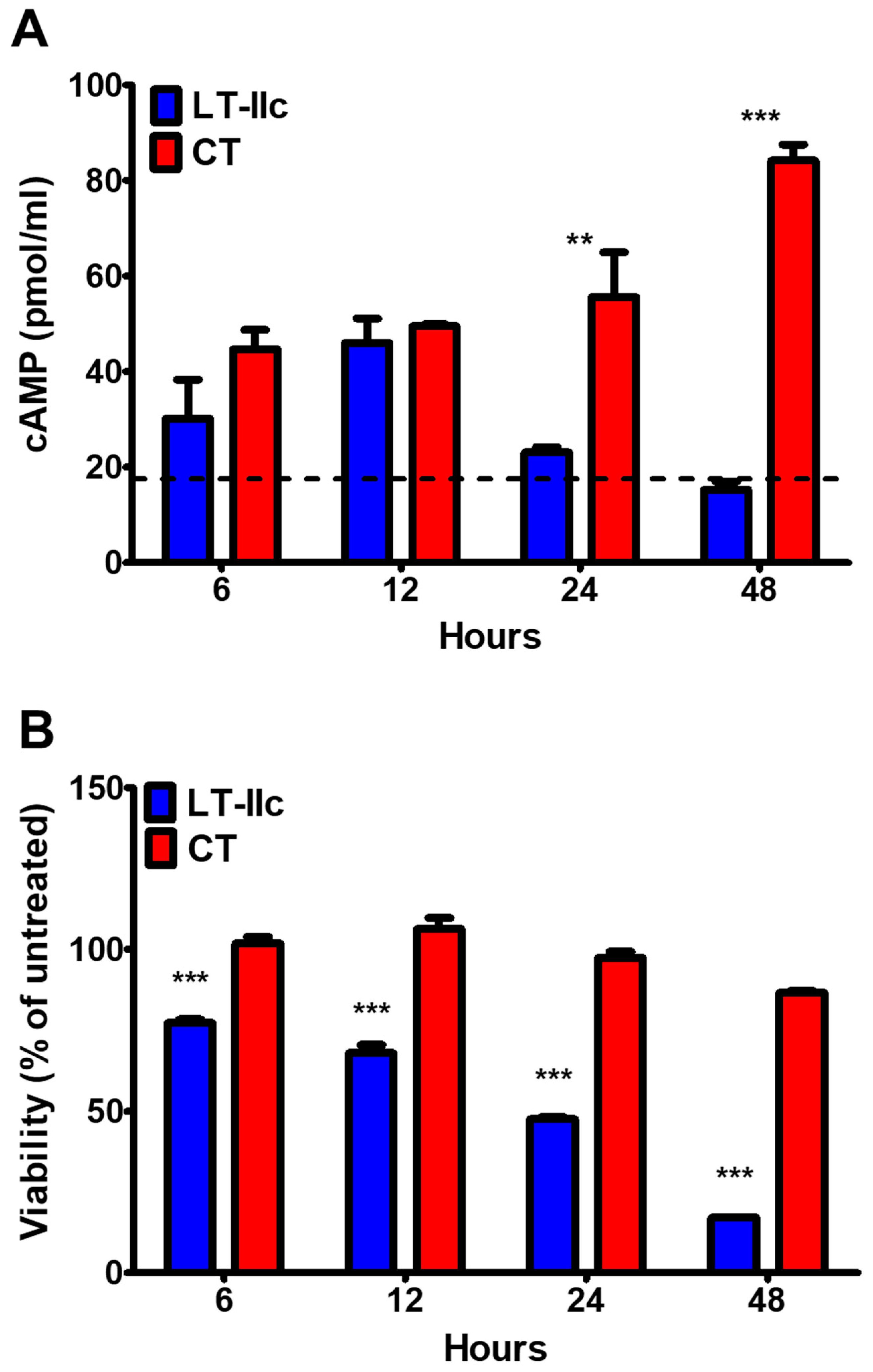
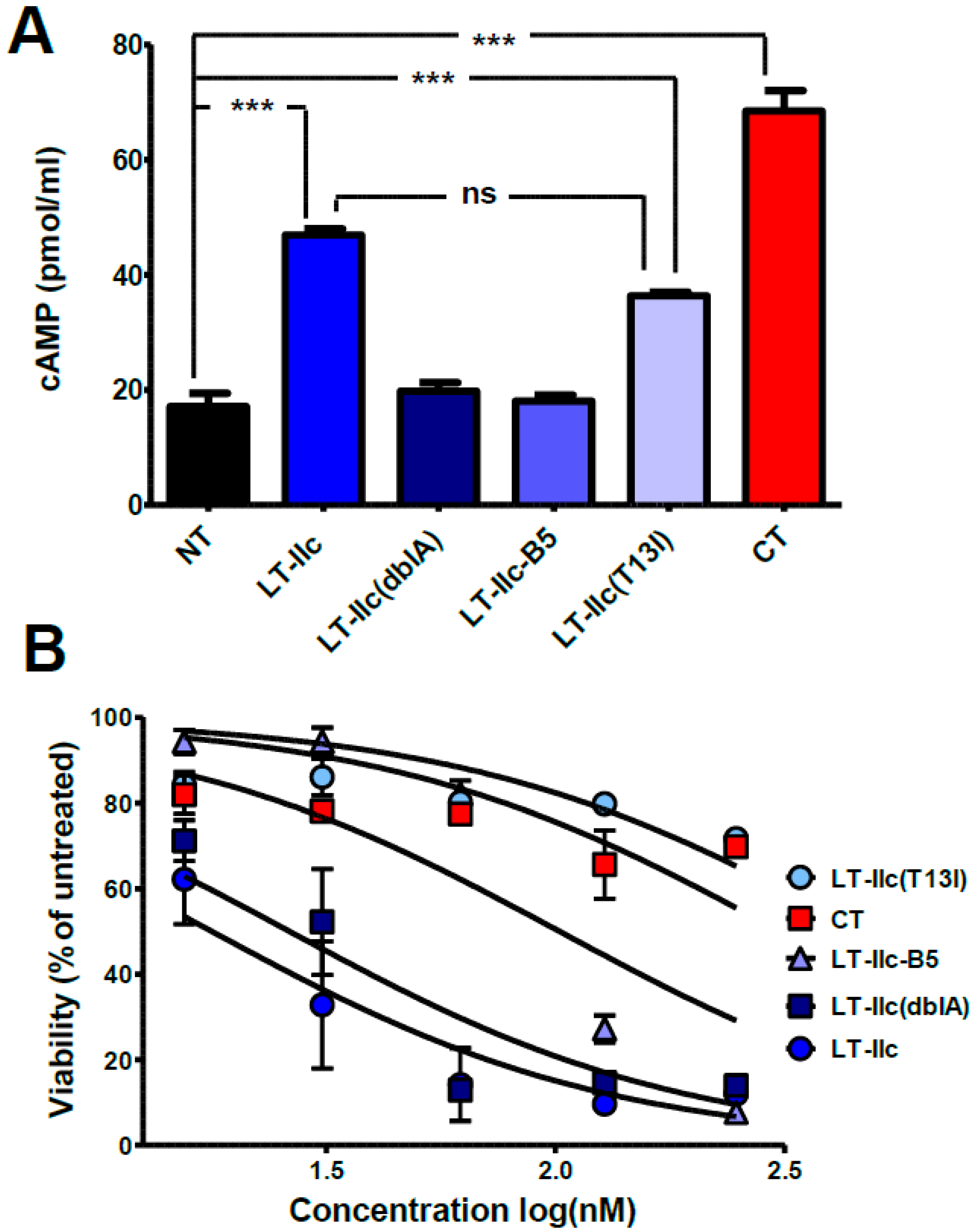
2.2. HLTs Stimulate the cAMP Signaling Pathway in MDA-MB-231
2.3. LT-IIc-Dependent Induction of Cellular cAMP Is Not Required for Cytotoxicity
2.4. HLTs Stimulate PKA Downstream Signaling with Distinctive Kinetics
2.5. ADP-Ribosylation Activity of LT-IIc Is Not Essential for Cytotoxicity
2.6. The A-Subunit of LT-IIc Is Not Necessary for Triggering Cytotoxicity in MDA-MB-231
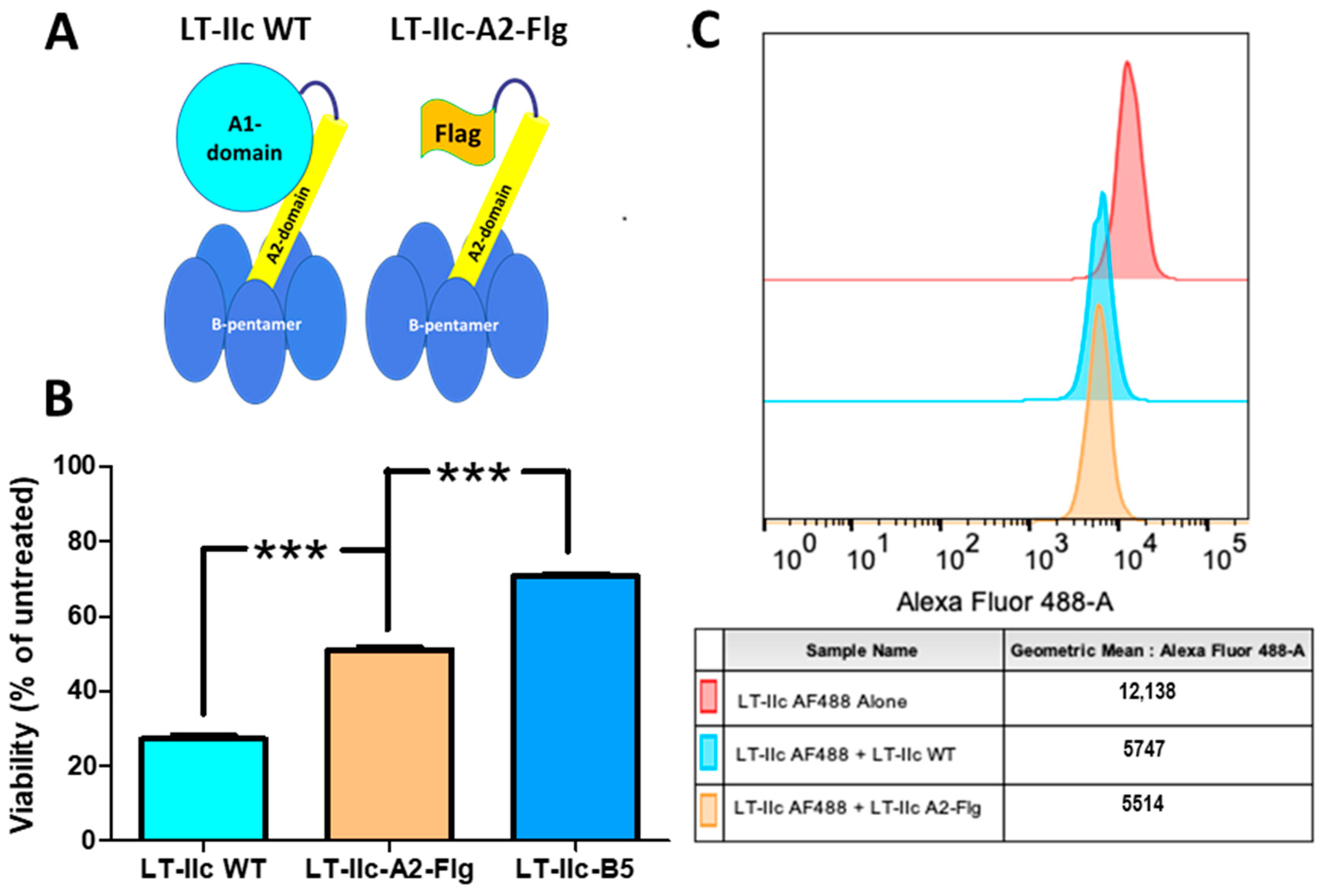
2.7. The A1-Domain of LT-IIc Is Required for Full Cytotoxicity
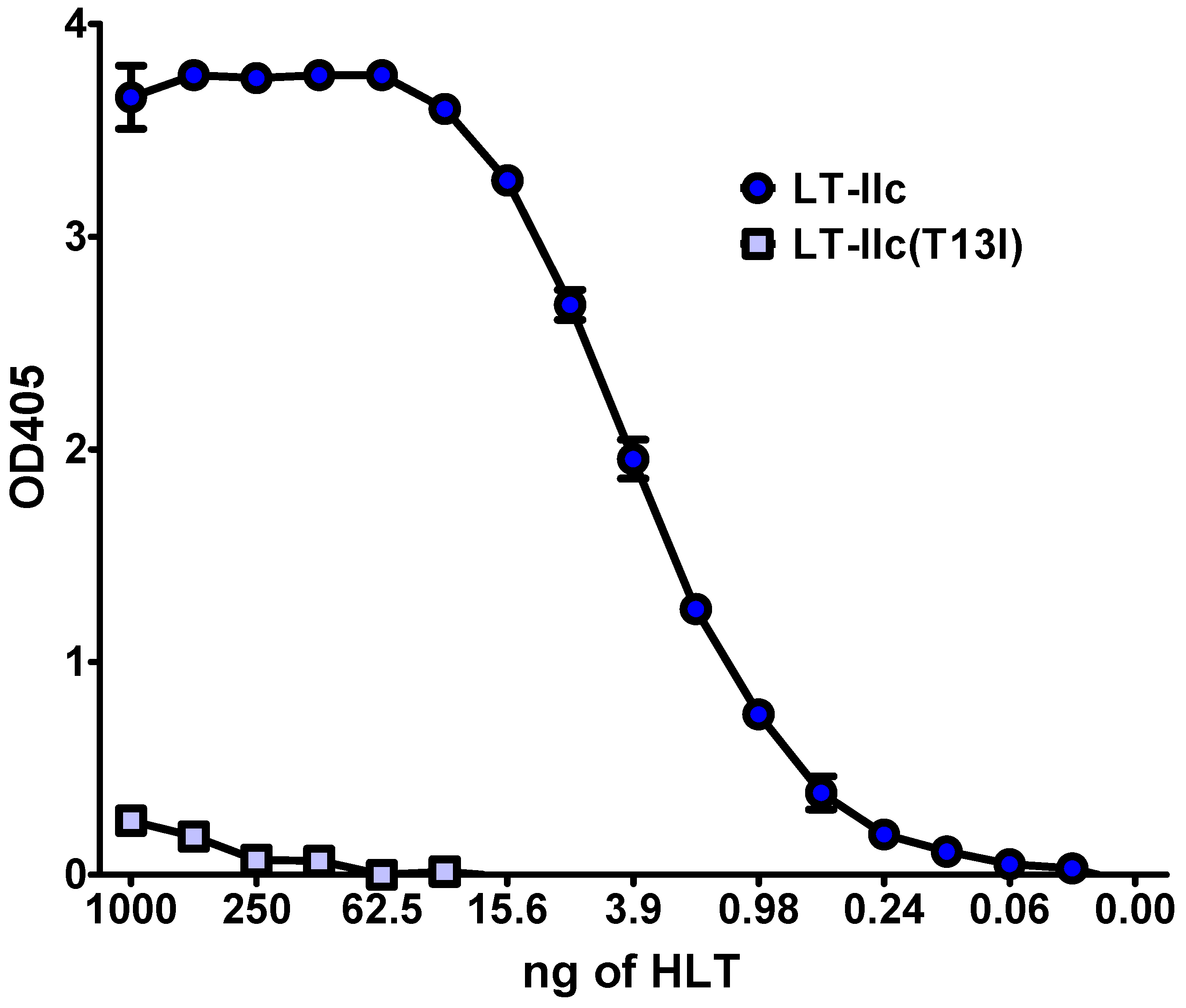
2.8. Cytotoxicity Is Triggered by Ganglioside Ligation
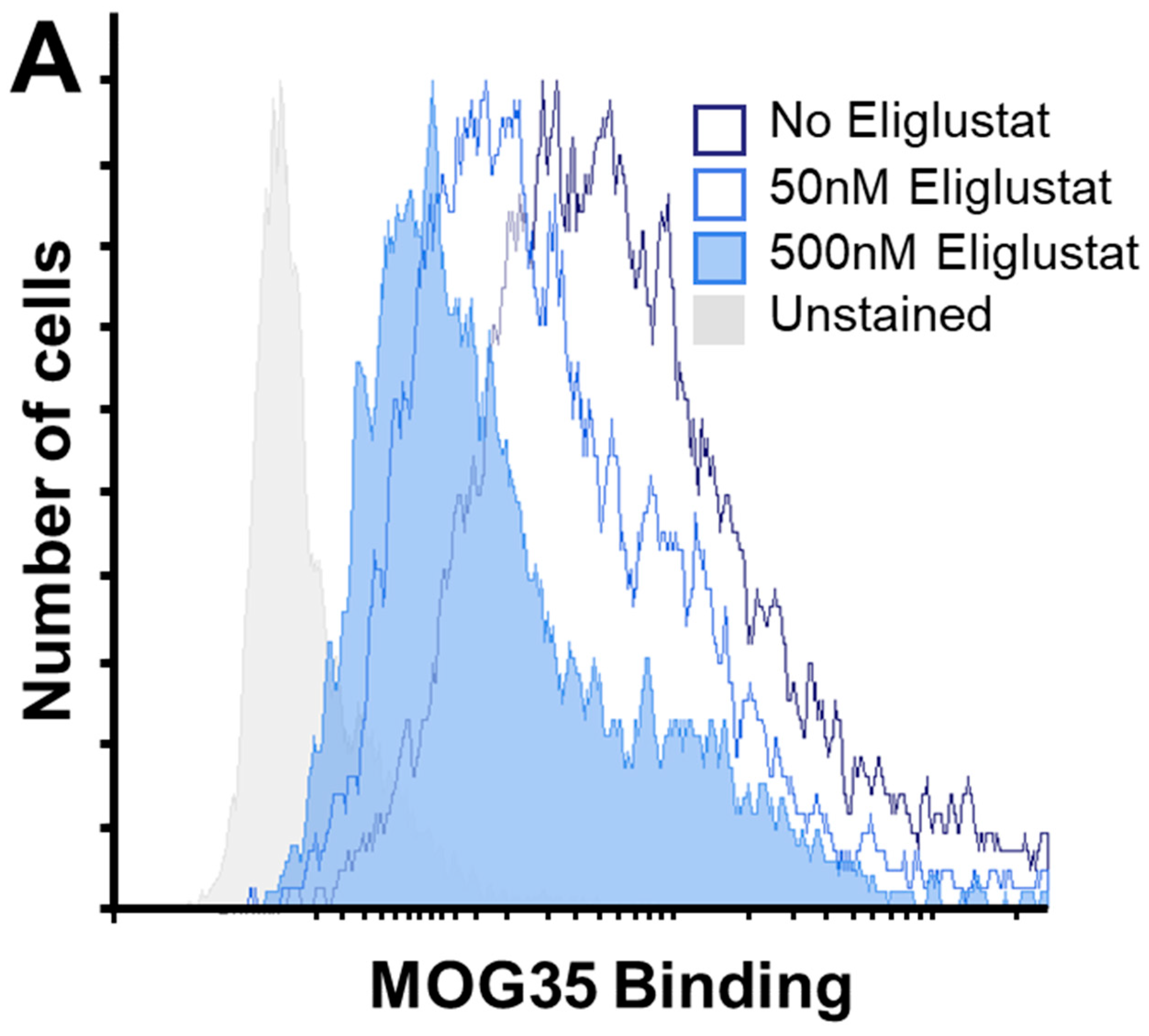
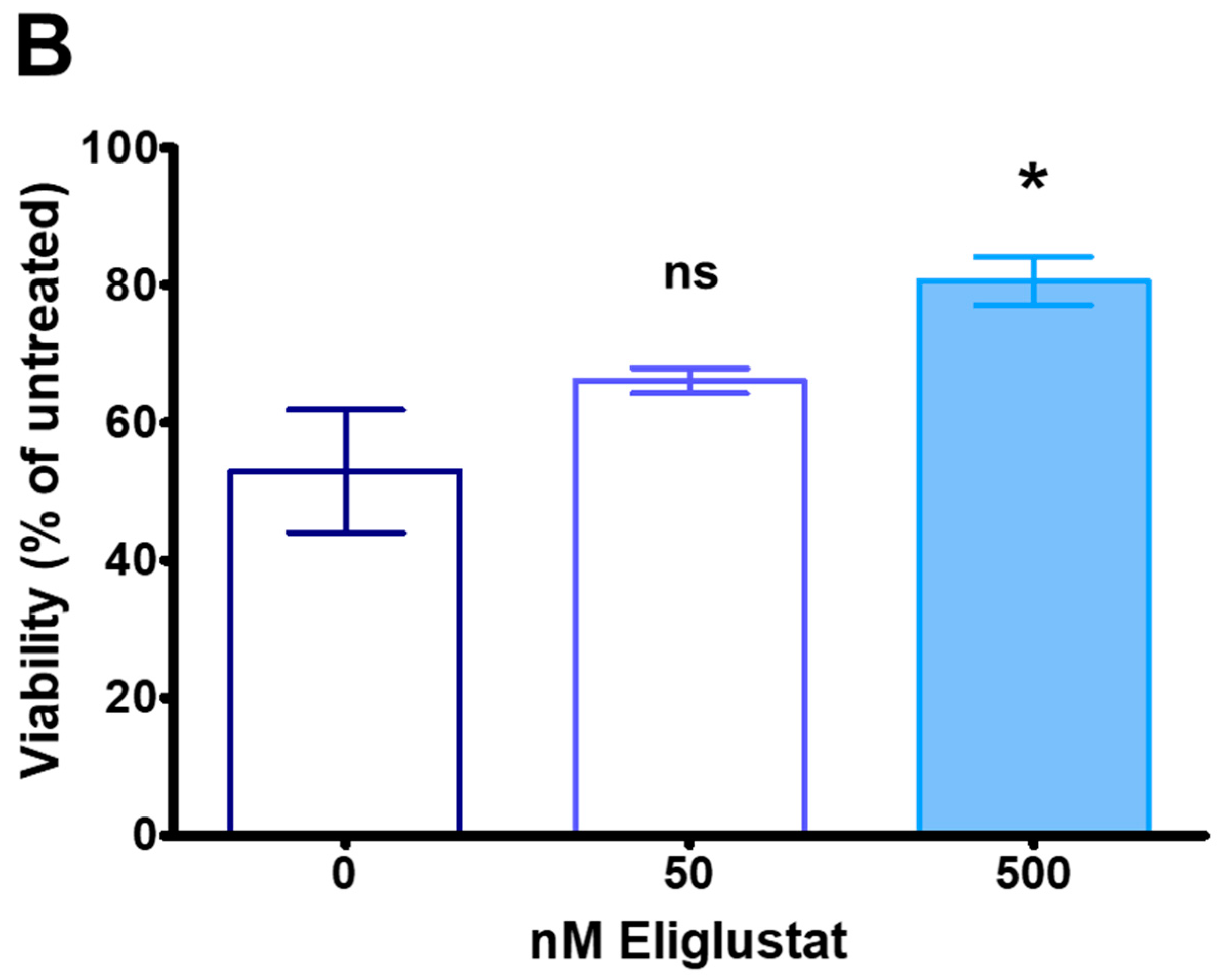
2.9. Inhibiting Ganglioside Synthesis Rescues TNBC Cells from Cytotoxicity
3. Discussion
4. Conclusions
5. Methods
5.1. Cell Lines and Reagents
5.2. Isolating and Culturing Primary Cells from Human, Resected Tumors
5.3. Construction of LT-IIc(T13I), LT-IIc(dblA), LT-IIc-A2-Flg and LT-IIc-B5 Expression Plasmids
5.4. HLT and B-Pentamer Expression and Purification
5.5. cAMP ELISA
5.6. MTT Assay
5.7. Western Immunoblotting
5.8. Competitive Binding Assay
5.9. Whole Cell ELISA
5.10. Ganglioside GD1a Staining
5.11. Data and Material Availability
5.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | adenosine diphosphate |
| BCG | Bacillus Calmette-Guerin |
| cAMP | cyclic adenosine monophosphate |
| CREB | cAMP Response Element-Binding Protein |
| CT | cholera toxin |
| ER | estrogen receptor |
| Gsα | Gs-alpha subunit |
| HER2/Neu/ErbB2 | human epidermal growth factor receptor 2 |
| HLT | heat-labile enterotoxins |
| MFI | mean fluorescent intensity |
| MTT | thiazolyl blue tetrazolium bromide |
| NeuAc | N-acetylneuraminic acid |
| NeuGc | N-glycolylneuraminic acid |
| PKA | protein kinase A |
| PR | progesterone receptor |
| TNBC | triple-negative breast cancer |
References
- Nair, N.; Kasai, T.; Seno, M. Bacteria: Prospective savior in battle against cancer. Anticancer Res. 2014, 34, 6289–6296. [Google Scholar] [PubMed]
- Felgner, S.; Kocijancic, D.; Frahm, M.; Weiss, S. Bacteria in Cancer Therapy: Renaissance of an Old Concept. Int. J. Microbiol. 2016, 2016, 8451728. [Google Scholar] [CrossRef] [PubMed]
- Droller, M.J. Intracavitary bacillus Calmette-Guerin for Superficial Bladder Tumors. J. Urol. 2017, 197, S146–S147. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.T.-Q.; Qin, Y.; You, S.-H.; Min, J.-J. Bacteria-cancer interactions: Bacteria-based cancer therapy. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, K.; Fol, M. Microorganisms in the Treatment of Cancer: Advantages and Limitations. J. Immunol. Res. 2018, 2018, 2397808. [Google Scholar] [CrossRef]
- Jiang, S.-N.; Park, S.-H.; Lee, H.J.; Zheng, J.H.; Kim, H.-S.; Bom, H.-S.; Hong, Y.; Szardenings, M.; Shin, M.G.; Kim, S.-C.; et al. Engineering of bacteria for the visualization of targeted delivery of a cytolytic anticancer agent. Mol. Ther. 2013, 21, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-N.; Phan, T.X.; Nam, T.-K.; Nguyen, V.H.; Kim, H.-S.; Bom, H.-S.; E Choy, H.; Hong, Y.; Min, J.-J. Inhibition of tumor growth and metastasis by a combination of Escherichia coli-mediated cytolytic therapy and radiotherapy. Mol. Ther. 2010, 18, 635–642. [Google Scholar] [CrossRef]
- Jarosz, M.; Jazowiecka-Rakus, J.; Cichoń, T.; Głowala-Kosińska, M.; Smolarczyk, R.; Smagur, A.; Malina, S.; Sochanik, A.; Szala, S. Therapeutic antitumor potential of endoglin-based DNA vaccine combined with immunomodulatory agents. Gene Ther. 2013, 20, 262–273. [Google Scholar] [CrossRef]
- Masuyama, A.; Kondoh, M.; Seguchi, H.; Takahashi, A.; Harada, M.; Fujii, M.; Mizuguchi, H.; Horiguchi, Y.; Watanabe, Y. Role of N-terminal amino acids in the absorption-enhancing effects of the c-terminal fragment of Clostridium perfringens enterotoxin. J. Pharmacol. Exp. Ther. 2005, 314, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Robbins, D.H.; Margulies, I.; Stetler-Stevenson, M.; Kreitman, R.J. Hairy cell leukemia, a B-cell neoplasm that is particularly sensitive to the cytotoxic effect of anti-Tac(Fv)-PE38 (LMB-2). Clin. Cancer Res. 2000, 6, 693–700. [Google Scholar]
- Perou, C.M.; Jeffrey, S.S.; van de Rijn, M.; Rees, C.A.; Eisen, M.B.; Ross, D.T.; Pergamenschikov, A.; Williams, C.F.; Zhu, S.X.; Lee, J.C.F.; et al. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc. Natl. Acad. Sci. USA 1999, 96, 9212–9217. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Kalimutho, M.; Parsons, K.; Mittal, D.; López, J.A.; Srihari, S.; Khanna, K.K. Targeted Therapies for Triple-Negative Breast Cancer: Combating a Stubborn Disease. Trends Pharmacol. Sci. 2015, 36, 822–846. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Fan, S.; Qin, T.; Yang, J.; Sun, Y.; Lu, Y.; Mao, J.; Li, L. Role of autophagy in breast cancer and breast cancer stem cells (Review). Int. J. Oncol. 2018, 52, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Yan, Y.; Yuan, B.; Dasgupta, A.; Sun, J.; Mu, H.; Do, K.A.; Ueno, N.T.; Andreeff, M.; Battula, V.L. ST8SIA1 Regulates Tumor Growth and Metastasis in TNBC by Activating the FAK-AKT-mTOR Signaling Pathway. Mol. Cancer Ther. 2018, 17, 2689–2701. [Google Scholar] [CrossRef]
- De Giorgi, U.; Cohen, E.N.; Gao, H.; Mego, M.; Lee, B.N.; Lodhi, A.; Cristofanilli, M.; Lucci, A.; Reuben, J.M. Mesenchymal stem cells expressing GD2 and CD271 correlate with breast cancer-initiating cells in bone marrow. Cancer Biol. Ther. 2011, 11, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Battula, V.L.; Shi, Y.; Evans, K.W.; Wang, R.-Y.; Spaeth, E.L.; Jacamo, R.O.; Guerra, R.; Sahin, A.A.; Marini, F.C.; Hortobagyi, G.; et al. Ganglioside GD2 identifies breast cancer stem cells and promotes tumorigenesis. J. Clin. Investig. 2012, 122, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Cheung, N.-K.V. Engineering anti-GD2 monoclonal antibodies for cancer immunotherapy. FEBS Lett. 2014, 588, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Connell, T.D.; Holmes, R.K. Mutational analysis of the ganglioside-binding activity of the type II Escherichia coli heat-labile enterotoxin LT-IIb. Mol. Microbiol. 1995, 16, 21–31. [Google Scholar] [CrossRef]
- Holmes, R.K.; Jobling, M.G.; Connell, T.D. Cholera toxins and related enterotoxins of gram-negative bacteria. In Bacterial Toxins and Virulence Factors in Disease; Moss, J., Iglewski, B., Vaughan, M., Tu, A.T., Eds.; Marcel Dekker: New York, NY, USA, 1995; Volume 8, pp. 225–255. [Google Scholar]
- Nawar, H.F.; Greene, C.J.; Lee, C.H.; Mandell, L.M.; Hajishengallis, G.; Connell, T.D. LT-IIc, a new member of the type II heat-labile enterotoxin family, exhibits potent immunomodulatory properties that are different from those induced by LT-IIa or LT-IIb. Vaccine 2011, 29, 721–727. [Google Scholar] [CrossRef]
- Nawar, H.F.; King-Lyons, N.D.; Hu, J.C.; Pasek, R.C.; Connell, T.D. LT-IIc, a New Member of the Type II Heat-Labile Enterotoxin Family Encoded by an Escherichia coli Strain Obtained from a Nonmammalian Host. Infect. Immun. 2010, 78, 4705–4713. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.M.; Clements, J.D.; Robertson, D.C.; A Finkelstein, R. Subunit number and arrangement in Escherichia coli heat-labile enterotoxin. Infect. Immun. 1981, 33, 677–682. [Google Scholar] [CrossRef]
- Mekalanos, J.J.; Collier, R.J.; Romig, W.R. Enzymic activity of cholera toxin. II. Relationships to proteolytic processing, disulfide bond reduction, and subunit composition. J. Biol. Chem. 1979, 254, 5855–5861. [Google Scholar] [CrossRef] [PubMed]
- Cieplak, W., Jr.; Messer, R.J.; Konkel, M.E.; Grant, C.C.R. Role of a potential endoplasmic reticulum retention sequence (RDEL) and the Golgi complex in the cytotonic activity of Escherichia coli heat-labile enterotoxin. Mol. Microbiol. 1995, 16, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Cassel, D.; Selinger, Z. Mechanism of adenylate cyclase activation by cholera toxin: Inhibition of GTP hydrolysis at the regulatory site. Proc. Natl. Acad. Sci. USA 1977, 74, 3307–3311. [Google Scholar] [CrossRef] [PubMed]
- Daniel, P.B.; Walker, W.H.; Habener, J.F. Cyclic AMP signaling and gene regulation. Annu. Rev. Nutr. 1998, 18, 353–383. [Google Scholar] [CrossRef] [PubMed]
- Mayr, B.; Montminy, M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2001, 2, 599–609. [Google Scholar] [CrossRef]
- Masso-Welch, P.; Berlingeri, S.G.; King-Lyons, N.D.; Mandell, L.; Hu, J.; Greene, C.J.; Federowicz, M.; Cao, P.; Connell, T.D.; Heakal, Y. LT-IIc, A Bacterial Type II Heat-Labile Enterotoxin, Induces Specific Lethality in Triple Negative Breast Cancer Cells by Modulation of Autophagy and Induction of Apoptosis and Necroptosis. Int. J. Mol. Sci. 2018, 20, 85. [Google Scholar] [CrossRef]
- Cho-Chung, Y.S.; Clair, T.; Bodwin, J.S.; Berghoffer, B. Growth Arrest and Morphological Change of Human Breast Cancer Cells by Dibutyryl Cyclic AMP and L-Arginine. Science 1981, 214, 77–79. [Google Scholar] [CrossRef]
- Kim, S.N.; Ahn, Y.H.; Kim, S.G.; Park, S.D.; Cho-Chung, Y.S.; Hong, S.H. 8-Cl-cAMP induces cell cycle-specific apoptosis in human cancer cells. Int. J. Cancer 2001, 93, 33–41. [Google Scholar] [CrossRef]
- Chen, J.; Bander, J.A.; Santore, T.A.; Chen, Y.; Ram, P.T.; Smit, M.J.; Iyengar, R. Expression of Q227L-galphas in MCF-7 human breast cancer cells inhibits tumorigenesis. Proc. Natl. Acad. Sci. USA 1998, 95, 2648–2652. [Google Scholar] [CrossRef] [PubMed]
- Bullock, B.P.; Habener, J.F. Phosphorylation of the cAMP Response Element Binding Protein CREB by cAMP-Dependent Protein Kinase A and Glycogen Synthase Kinase-3 Alters DNA-Binding Affinity, Conformation, and Increases Net Charge. Biochemistry 1998, 37, 3795–3809. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.J.; Hu, J.C.; Vance, D.J.; Rong, Y.; Mandell, L.; King-Lyons, N.; Masso-Welch, P.; Mantis, N.J.; Connell, T.D. Enhancement of humoral immunity by the type II heat-labile enterotoxin LT-IIb is dependent upon IL-6 and neutrophils. J. Leukoc. Biol. 2016, 100, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Beddoe, T.; Paton, A.W.; Le Nours, J.; Rossjohn, J.; Paton, J.C. Structure, biological functions and applications of the AB5 toxins. Trends Biochem. Sci. 2010, 35, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Connell, T.D. Cholera toxin, LT-I, LT-IIa and LT-IIb: The critical role of ganglioside binding in immunomodulation by type I and type II heat-labile enterotoxins. Expert. Rev. Vaccines 2007, 6, 821–834. [Google Scholar] [CrossRef]
- Berenson, C.S.; Nawar, H.F.; Yohe, H.C.; A Castle, S.; Ashline, D.J.; Reinhold, V.N.; Hajishengallis, G.; Connell, T.D. Mammalian cell ganglioside-binding specificities of E. coli enterotoxins LT-IIb and variant LT-IIb(T13I). Glycobiology 2010, 20, 41–54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cazet, A.; Bobowski, M.; Rombouts, Y.; Lefebvre, J.; Steenackers, A.; Popa, I.; Guérardel, Y.; Le Bourhis, X.; Tulasne, D.; Delannoy, P. The ganglioside G(D2) induces the constitutive activation of c-Met in MDA-MB-231 breast cancer cells expressing the G(D3) synthase. Glycobiology 2012, 22, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Berenson, C.S.; Nawar, H.F.; Kruzel, R.L.; Mandell, L.M.; Connell, T.D. Ganglioside-binding specificities of E. coli enterotoxin LT-IIc: Importance of long-chain fatty acyl ceramide. Glycobiology 2013, 23, 23–31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shayman, J.A. ELIGLUSTAT TARTRATE: Glucosylceramide Synthase Inhibitor Treatment of Type 1 Gaucher Disease. Drugs Future 2010, 35, 613–620. [Google Scholar] [CrossRef]
- Goodfellow, J.A.; Bowes, T.; Sheikh, K.; Odaka, M.; Halstead, S.K.; Humphreys, P.D.; Wagner, E.R.; Yuki, N.; Furukawa, K.; Furukawa, K.; et al. Overexpression of GD1a Ganglioside Sensitizes Motor Nerve Terminals to Anti-GD1a Antibody-Mediated Injury in a Model of Acute Motor Axonal Neuropathy. J. Neurosci. 2005, 25, 1620–1628. [Google Scholar] [CrossRef]
- Shayman, J.A. The design and clinical development of inhibitors of glycosphingolipid synthesis: Will invention be the mother of necessity? Trans. Am. Clin. Climatol. Assoc. 2013, 124, 46–60. [Google Scholar]
- Mathias-Santos, C.; Rodrigues, J.F.; Sbrogio-Almeida, M.E.; Connell, T.D.; Ferreira, L.C.S. Distinctive Immunomodulatory and Inflammatory Properties of the Escherichia coli Type II Heat-Labile Enterotoxin LT-IIa and Its B Pentamer following Intradermal Administration. Clin. Vaccine Immunol. 2011, 18, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.; Zhu, J.Y.; Fang, D.; Ding, H.-F.; Dong, Z.; Jing, Q.; Su, S.-B.; Huang, S. Triple negative breast cancer development can be selectively suppressed by sustaining an elevated level of cellular cyclic AMP through simultaneously blocking its efflux and decomposition. Oncotarget 2016, 7, 87232–87245. [Google Scholar] [CrossRef]
- Hu, J.C.; Mathias-Santos, C.; Greene, C.J.; King-Lyons, N.D.; Rodrigues, J.F.; Hajishengallis, G.; Ferreira, L.C.S.; Connell, T.D. Intradermal Administration of the Type II Heat-Labile Enterotoxins LT-IIb and LT-IIc of Enterotoxigenic Escherichia coli Enhances Humoral and CD8+ T Cell Immunity to a Co-Administered Antigen. PLoS ONE 2014, 9, e113978. [Google Scholar] [CrossRef]
- Hu, J.C.; Greene, C.J.; King-Lyons, N.D.; Connell, T.D. The Divergent CD8+ T Cell Adjuvant Properties of LT-IIb and LT-IIc, Two Type II Heat-Labile Enterotoxins, Are Conferred by Their Ganglioside-Binding B Subunits. PLoS ONE 2015, 10, e0142942. [Google Scholar] [CrossRef]
- Nawar, H.F.; Berenson, C.S.; Hajishengallis, G.; Takematsu, H.; Mandell, L.; Clare, R.L.; Connell, T.D. Binding to Gangliosides Containing N-Acetylneuraminic Acid Is Sufficient to Mediate the Immunomodulatory Properties of the Nontoxic Mucosal Adjuvant LT-IIb(T13I). Clin. Vaccine Immunol. 2010, 17, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Nawar, H.F.; Arce, S.; Russell, M.W.; Connell, T.D. Mucosal Adjuvant Properties of Mutant LT-IIa and LT-IIb Enterotoxins That Exhibit Altered Ganglioside-Binding Activities. Infect. Immun. 2005, 73, 1330–1342. [Google Scholar] [CrossRef] [PubMed]
- Schnaar, R.L. Gangliosides of the Vertebrate Nervous System. J. Mol. Biol. 2016, 428, 3325–3336. [Google Scholar] [CrossRef]
- Lipina, C.; Hundal, H.S. Ganglioside GM3 as a gatekeeper of obesity-associated insulin resistance: Evidence and mechanisms. FEBS Lett. 2015, 589, 3221–3227. [Google Scholar] [CrossRef]
- Sekino-Suzuki, N.; Yuyama, K.; Miki, T.; Kaneda, M.; Suzuki, H.; Yamamoto, N.; Yamamoto, T.; Oneyama, C.; Okada, M.; Kasahara, K. Involvement of gangliosides in the process of Cbp/PAG phosphorylation by Lyn in developing cerebellar growth cones. J. Neurochem. 2013, 124, 514–522. [Google Scholar] [CrossRef]
- Prendergast, J.; Umanah, G.K.; Yoo, S.-W.; Lagerlöf, O.; Motari, M.G.; Cole, R.N.; Huganir, R.L.; Dawson, T.M.; Dawson, V.L.; Schnaar, R.L. Ganglioside Regulation of AMPA Receptor Trafficking. J. Neurosci. 2014, 34, 13246–13258. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Ohkawa, Y.; Yamauchi, Y.; Hamamura, K.; Ohmi, Y. Fine tuning of cell signals by glycosylation. J. Biochem. 2012, 151, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Hamamura, K.; Furukawa, K.; Hayashi, T.; Hattori, T.; Nakano, J.; Nakashima, H.; Okuda, T.; Mizutani, H.; Hattori, H.; Ueda, M.; et al. Ganglioside GD3 promotes cell growth and invasion through p130Cas and paxillin in malignant melanoma cells. Proc. Natl. Acad. Sci. USA 2005, 102, 11041–11046. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, Y.; Miyazaki, S.; Hamamura, K.; Kambe, M.; Miyata, M.; Tajima, O.; Ohmi, Y.; Yamauchi, Y.; Furukawa, K.; Furukawa, K. Ganglioside GD3 enhances adhesion signals and augments malignant properties of melanoma cells by recruiting integrins to glycolipid-enriched microdomains. J. Biol. Chem. 2010, 285, 27213–27223. [Google Scholar] [CrossRef]
- Yoon, S.-J.; Nakayama, K.-I.; Hikita, T.; Handa, K.; Hakomori, S.-I. Epidermal growth factor receptor tyrosine kinase is modulated by GM3 interaction with N-linked GlcNAc termini of the receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 18987–18991. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ikeda, K.; Hamamura, K.; Zhang, Q.; Kondo, Y.; Matsumoto, Y.; Ohmi, Y.; Yamauchi, Y.; Furukawa, K.; Taguchi, R.; et al. GM1/GD1b/GA1 synthase expression results in the reduced cancer phenotypes with modulation of composition and raft-localization of gangliosides in a melanoma cell line. Cancer Sci. 2010, 101, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Tsurifune, T.; Ito, T.; Li, X.J.; Yamashiro, S.; Okada, M.; Kanematsu, T.; Shiku, H.; Furukawa, K. Alteration of tumor phenotypes of B16 melanoma after genetic remodeling of the ganglioside profile. Int. J. Oncol. 2000, 17, 159–224. [Google Scholar] [CrossRef] [PubMed]
- Dewald, J.H.; Cavdarli, S.; Steenackers, A.; Delannoy, C.P.; Mortuaire, M.; Spriet, C.; Noël, M.; Groux-Degroote, S.; Delannoy, P. TNF differentially regulates ganglioside biosynthesis and expression in breast cancer cell lines. PLoS ONE 2018, 13, e0196369. [Google Scholar] [CrossRef] [PubMed]
- Fujinaga, Y.; Wolf, A.A.; Rodighiero, C.; Wheeler, H.; Tsai, B.; Allen, L.; Jobling, M.G.; Rapoport, T.; Holmes, R.K.; Lencer, W.I. Gangliosides That Associate with Lipid Rafts Mediate Transport of Cholera and Related Toxins from the Plasma Membrane to Endoplasmic Reticulm. Mol. Biol. Cell 2003, 14, 4783–4793. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Barrientos, R.; Shiota, T.; Madigan, V.; Misumi, I.; McKnight, K.L.; Sun, L.; Li, Z.; Meganck, R.M.; Li, Y.; et al. Gangliosides are essential endosomal receptors for quasi-enveloped and naked hepatitis A virus. Nat. Microbiol. 2020, 5, 1069–1078. [Google Scholar] [CrossRef]
- Tl, R.; Moravec, R.A.; Al, N.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell viability assays. In Assay Guidance Manual; Markossian, S., Ed.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
King-Lyons, N.D.; Bhati, A.S.; Hu, J.C.; Mandell, L.M.; Shenoy, G.N.; Willison, H.J.; Connell, T.D. A Novel Cytotoxic Mechanism for Triple-Negative Breast Cancer Cells Induced by the Type II Heat-Labile Enterotoxin LT-IIc through Ganglioside Ligation. Toxins 2024, 16, 311. https://doi.org/10.3390/toxins16070311
King-Lyons ND, Bhati AS, Hu JC, Mandell LM, Shenoy GN, Willison HJ, Connell TD. A Novel Cytotoxic Mechanism for Triple-Negative Breast Cancer Cells Induced by the Type II Heat-Labile Enterotoxin LT-IIc through Ganglioside Ligation. Toxins. 2024; 16(7):311. https://doi.org/10.3390/toxins16070311
Chicago/Turabian StyleKing-Lyons, Natalie D., Aryana S. Bhati, John C. Hu, Lorrie M. Mandell, Gautam N. Shenoy, Hugh J. Willison, and Terry D. Connell. 2024. "A Novel Cytotoxic Mechanism for Triple-Negative Breast Cancer Cells Induced by the Type II Heat-Labile Enterotoxin LT-IIc through Ganglioside Ligation" Toxins 16, no. 7: 311. https://doi.org/10.3390/toxins16070311
APA StyleKing-Lyons, N. D., Bhati, A. S., Hu, J. C., Mandell, L. M., Shenoy, G. N., Willison, H. J., & Connell, T. D. (2024). A Novel Cytotoxic Mechanism for Triple-Negative Breast Cancer Cells Induced by the Type II Heat-Labile Enterotoxin LT-IIc through Ganglioside Ligation. Toxins, 16(7), 311. https://doi.org/10.3390/toxins16070311





