7-Phenylheptanoic Acid-Hydroxypropyl β-Cyclodextrin Complex Slows the Progression of Renal Failure in Adenine-Induced Chronic Kidney Disease Mice
Abstract
1. Introduction
2. Results
2.1. PB and Structurally Related Compounds Inhibit Tryptophan Indole Lyase In Vitro
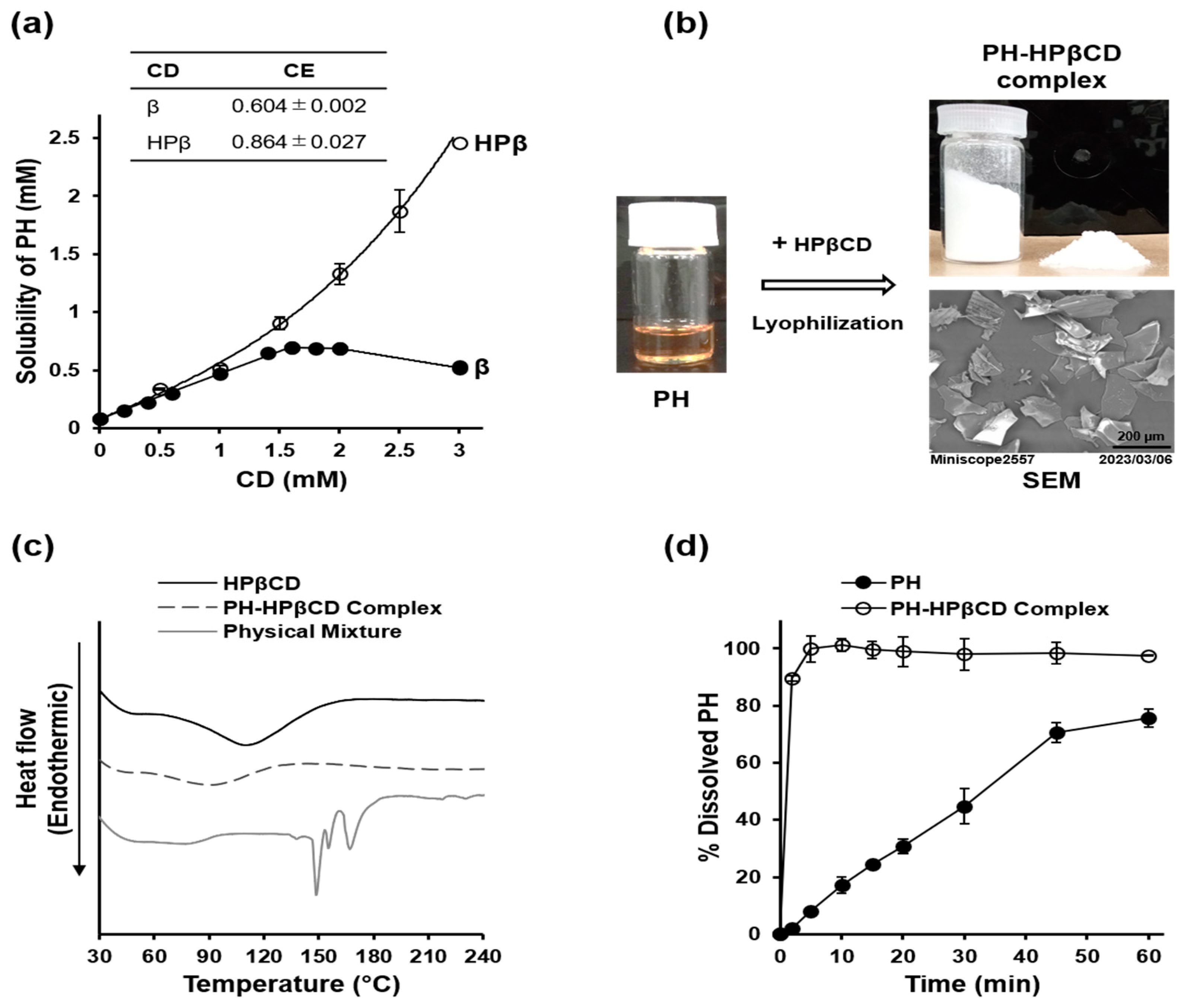
2.2. Preparation and Characterization of PH-HPβCD Solid System
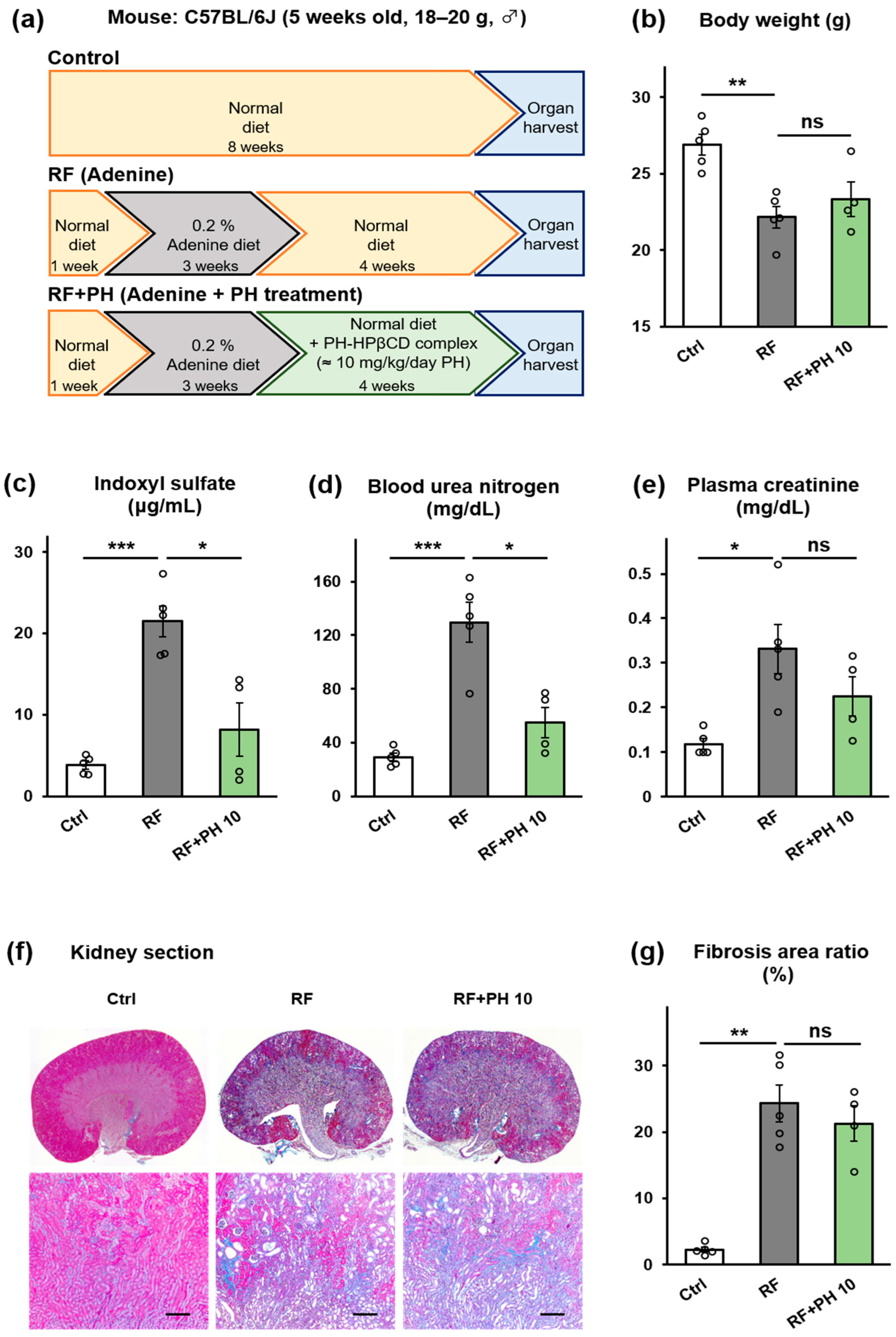
2.3. Prophylactic Supplementation with PH-HPβCD Slowed the Progression of Renal Failure and Fibrosis in Adenine-Induced CKD Mice
2.4. Treatment with PH-HPβCD Improved Plasma IS and BUN but Not Fibrosis in Moderately Advanced Adenine-Induced CKD Mice
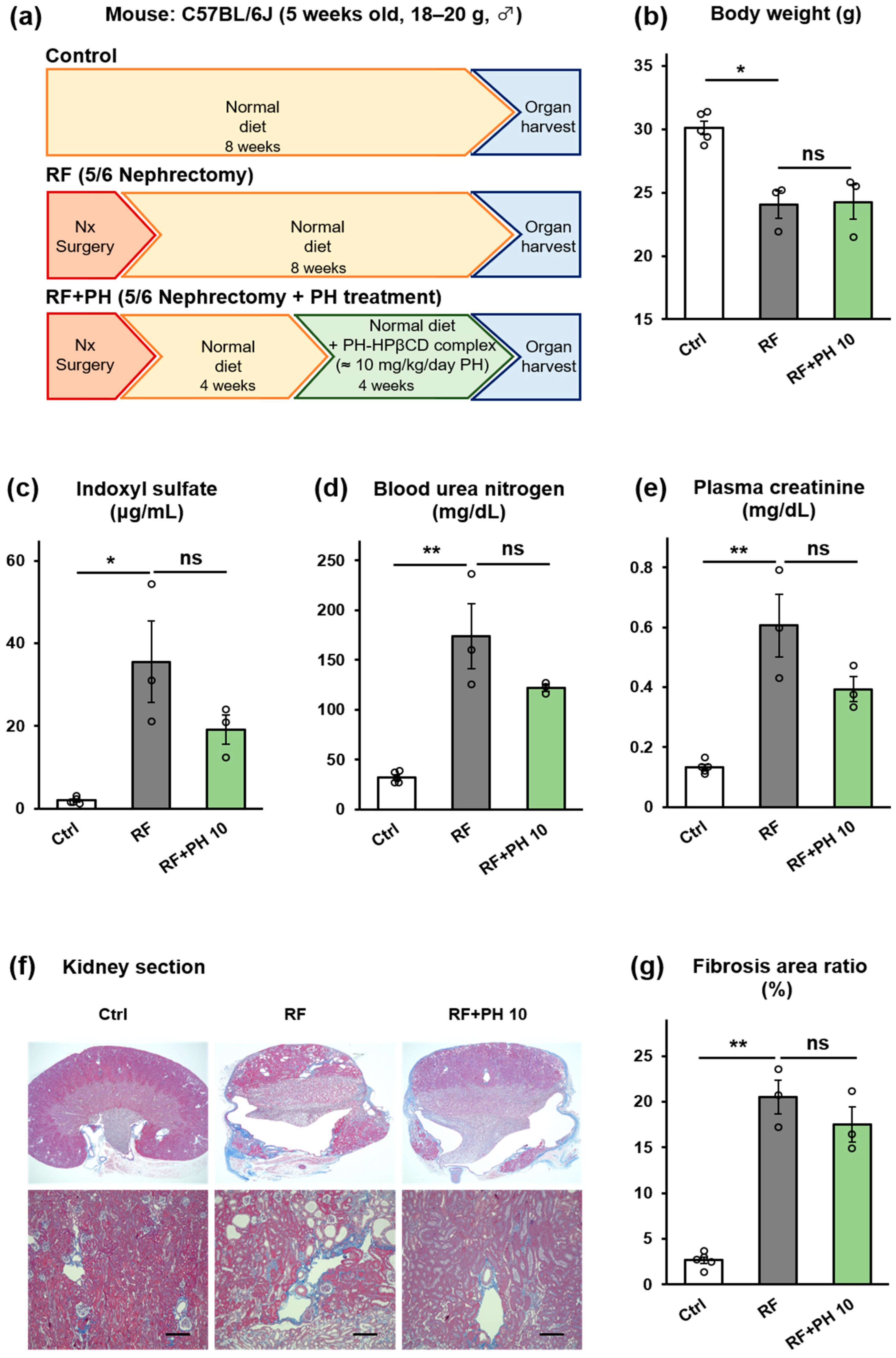
2.5. PH-HPβCD Did Not Improve Renal Function and Fibrosis in 5/6-Nephrectomized Mice
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Inhibition of Tryptophan Indole Lyase Activity In Vitro
5.3. Preparation and Characterization of PH-HPβCD Solid System
5.3.1. Phase Solubility Studies
5.3.2. Preparation of PH-HPβCD Solid System
5.3.3. Characterization of PH-HPβCD Solid System
5.4. Animal Experiments
5.4.1. Animal Care
5.4.2. Prophylactic Supplementation with PH-HPβCD in Adenine-Induced CKD Mice
5.4.3. Treatment with PH-HPβCD in Moderately Advanced Adenine-Induced CKD Mice
5.4.4. Treatment with PH-HPβCD in 5/6-Nephrectomized Mice
5.4.5. Histological Analysis
5.4.6. Measurement of IS, BUN, and CRE in Mouse Plasma
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Boer, I.H.; Caramori, M.L.; Chan, J.C.; Heerspink, H.J.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef]
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A.; European Uremic Toxin Work Group. Normal and Pathologic Concentrations of Uremic Toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Ise, M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J. Lab. Clin. Med. 1994, 124, 96–104. [Google Scholar] [CrossRef]
- Liabeuf, S.; Barreto, D.V.; Barreto, F.C.; Meert, N.; Glorieux, G.; Schepers, E.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; et al. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol. Dial. Transpl. 2010, 25, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Owada, A.; Nakao, M.; Koike, J.; Ujiie, K.; Tomita, K.; Shiigai, T. Effects of oral adsorbent AST-120 on the progression of chronic renal failure: A randomized controlled study. Kidney Int. Suppl. 1997, 63, S188–S190. [Google Scholar]
- Su, P.Y.; Lee, Y.H.; Kuo, L.N.; Chen, Y.-C.; Chen, C.; Kang, Y.-N.; Chang, E.H. Efficacy of AST-120 for Patients with Chronic Kidney Disease: A Network Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2021, 12, 676345. [Google Scholar] [CrossRef]
- Evenepoel, P.; Poesen, R.; Meijers, B. The gut–kidney axis. Pediatr. Nephrol. 2017, 32, 2005–2014. [Google Scholar] [CrossRef]
- Bartochowski, P.; Gayrard, N.; Bornes, S.; Druart, C.; Argilés, A.; Cordaillat-Simmons, M.; Duranton, F. Gut–Kidney Axis Investigations in Animal Models of Chronic Kidney Disease. Toxins 2022, 14, 626. [Google Scholar] [CrossRef]
- Huang, Y.; Xin, W.; Xiong, J.; Yao, M.; Zhang, B.; Zhao, J. The Intestinal Microbiota and Metabolites in the Gut-Kidney-Heart Axis of Chronic Kidney Disease. Front. Pharmacol. 2022, 13, 837500. [Google Scholar] [CrossRef]
- Do, Q.T.; Nguyen, G.T.; Celis, V.; Phillips, R.S. Inhibition of Escherichia coli tryptophan indole-lyase by tryptophan homologues. Arch. Biochem. Biophys. 2014, 560, 20–26. [Google Scholar] [CrossRef]
- Mishima, E.; Fukuda, S.; Mukawa, C.; Yuri, A.; Kanemitsu, Y.; Matsumoto, Y.; Akiyama, Y.; Fukuda, N.N.; Tsukamoto, H.; Asaji, K.; et al. Evaluation of the impact of gut microbiota on uremic solute accumulation by a CE-TOFMS–based metabolomics approach. Kidney Int. 2017, 92, 634–645. [Google Scholar] [CrossRef]
- Morino, Y.; Snell, E.E. [54] Tryptophanase (Escherichia coli B). Meth. Enzymol. 1970, 17, 439–446. [Google Scholar] [CrossRef]
- Atoh, K.; Itoh, H.; Haneda, M. Serum indoxyl sulfate levels in patients with diabetic nephropathy: Relation to renal function. Diabetes Res. Clin. Pract. 2009, 83, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Motojima, M.; Hosokawa, A.; Yamato, H.; Muraki, T.; Yoshioka, T. Uremic toxins of organic anions up-regulate PAI-1 expression by induction of NF-κB and free radical in proximal tubular cells. Kidney Int. 2003, 63, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Ise, M.; Seo, H.; Niwa, T. Indoxyl sulfate increases the gene expressions of TGF-beta 1, TIMP-1 and pro-alpha 1(I) collagen in uremic rat kidneys. Kidney Int. Suppl. 1997, 62, S15–S22. [Google Scholar]
- Kikuchi, K.; Saigusa, D.; Kanemitsu, Y.; Matsumoto, Y.; Thanai, P.; Suzuki, N.; Mise, K.; Yamaguchi, H.; Nakamura, T.; Asaji, K.; et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat. Commun. 2019, 10, 1835. [Google Scholar] [CrossRef]
- Mikami, D.; Kobayashi, M.; Uwada, J.; Yazawa, T.; Kamiyama, K.; Nishimori, K.; Nishikawa, Y.; Nishikawa, S.; Yokoi, S.; Kimura, H.; et al. Short-chain fatty acid mitigates adenine-induced chronic kidney disease via FFA2 and FFA3 pathways. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158666. [Google Scholar] [CrossRef]
- Pluznick, J.L. Gut microbiota in renal physiology: Focus on short-chain fatty acids and their receptors. Kidney Int. 2016, 90, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wei, W.; Zhang, Y.; Xiang, Z.; Peng, D.; Kasimumali, A.; Rong, S. Gut microbial metabolites SCFAs and chronic kidney disease. J. Transl. Med. 2024, 22, 172. [Google Scholar] [CrossRef]
- Carlisle, R.E.; Brimble, E.; Werner, K.E.; Cruz, G.L.; Ask, K.; Ingram, A.J.; Dickhout, J.G. 4-Phenylbutyrate Inhibits Tunicamycin-Induced Acute Kidney Injury via CHOP/GADD153 Repression. PLoS ONE 2014, 9, e84663. [Google Scholar] [CrossRef] [PubMed]
- Upagupta, C.; Carlisle, R.E.; Dickhout, J.G. Analysis of the potency of various low molecular weight chemical chaperones to prevent protein aggregation. Biochem. Biophys. Res. Commun. 2017, 486, 163–170. [Google Scholar] [CrossRef]
- Kim, K.; Anderson, E.M.; Thome, T.; Lu, G.; Salyers, Z.R.; Cort, T.A.; O’Malley, K.A.; Scali, S.T.; Ryan, T.E. Skeletal myopathy in CKD: A comparison of adenine-induced nephropathy and 5/6 nephrectomy models in mice. Am. J. Physiol. Renal Physiol. 2021, 321, F106–F119. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Al-Salam, S.; Al Za’abi, M.; Waly, M.I.; Ramkumar, A.; Beegam, S.; Al-Lawati, I.; Adham, S.A.; Nemmar, A. New model for adenine-induced chronic renal failure in mice, and the effect of gum acacia treatment thereon: Comparison with rats. J. Pharmacol. Toxicol. Methods 2013, 68, 384–393. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef]
- Commey, K.L.; Nakatake, A.; Enaka, A.; Nakamura, R.; Nishi, K.; Tsukigawa, K.; Ikeda, H.; Yamaguchi, K.; Iohara, D.; Hirayama, F.; et al. Study of the Structural Chemistry of the Inclusion Complexation of 4-Phenylbutyrate and Related Compounds with Cyclodextrins in Solution: Differences in Inclusion Mode with Cavity Size Dependency. Int. J. Mol. Sci. 2023, 24, 15091. [Google Scholar] [CrossRef]
- Wang, Z.; Roberts, A.B.; Buffa, J.A.; Levison, B.S.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.K.; et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015, 163, 1585–1595. [Google Scholar] [CrossRef]
- Lee, H.H.; Molla, M.N.; Cantor, C.R.; Collins, J.J. Bacterial charity work leads to population-wide resistance. Nature 2010, 467, 82–85. [Google Scholar] [CrossRef]
- Di Martino, P.; Fursy, R.; Bret, L.; Sundararaju, B.; Phillips, R.S. Indole can act as an extracellular signal to regulate biofilm formation of Escherichia coli and other indole-producing bacteria. Can. J. Microbiol. 2003, 49, 443–449. [Google Scholar] [CrossRef]
- Anyanful, A.; Dolan-Livengood, J.M.; Lewis, T.; Sheth, S.; DeZalia, M.N.; Sherman, M.A.; Kalman, L.V.; Benian, G.M.; Kalman, D. Paralysis and killing of Caenorhabditis elegans by enteropathogenic Escherichia coli requires the bacterial tryptophanase gene. Mol. Microbiol. 2005, 57, 988–1007. [Google Scholar] [CrossRef]
- Banoglu, E.; King, R.S. Sulfation of indoxyl by human and rat aryl (phenol) sulfotransferases to form indoxyl sulfate. Eur. J. Drug Metab. Pharmacokinet. 2002, 27, 135–140. [Google Scholar] [CrossRef]
- Stella, V.J.; Rajewski, R.A. Cyclodextrins: Their future in drug formulation and delivery. Pharm. Res. 1997, 14, 556–567. [Google Scholar] [CrossRef]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef]
- Irie, T.; Uekama, K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef]
- European Medicines Agency. Background Review for Cyclodextrins Used as Excipients. Available online: https://www.ema.europa.eu/en/documents/report/background-review-cyclodextrins-used-excipients-context-revision-guideline-excipients-label-and-package-leaflet-medicinal-products-human-use-draft-report_en.pdf (accessed on 13 May 2024).
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. The complexation efficiency. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 545–552. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef]
- Figueiras, A.; Carvalho, R.A.; Ribeiro, L.; Torres-Labandeira, J.J.; Veiga, F.J.B. Solid-state characterization and dissolution profiles of the inclusion complexes of omeprazole with native and chemically modified β-cyclodextrin. Eur. J. Pharm. Biopharm. 2007, 67, 531–539. [Google Scholar] [CrossRef]
- Commey, K.L.; Enaka, A.; Nakamura, R.; Yamamoto, A.; Tsukigawa, K.; Nishi, K.; Iohara, D.; Hirayama, F.; Otagiri, M.; Yamasaki, K. Development of α-Cyclodextrin-Based Orally Disintegrating Tablets for 4-Phenylbutyrate. Pharmaceutics 2024, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Klinkhammer, B.M.; Djudjaj, S.; Kunter, U.; Palsson, R.; Edvardsson, V.O.; Wiech, T.; Thorsteinsdottir, M.; Hardarson, S.; Foresto-Neto, O.; Mulay, S.R.; et al. Cellular and Molecular Mechanisms of Kidney Injury in 2,8-Dihydroxyadenine Nephropathy. J. Am. Soc. Nephrol. 2020, 31, 799–816. [Google Scholar] [CrossRef] [PubMed]
- Kumakura, S.; Sato, E.; Sekimoto, A.; Hashizume, Y.; Yamakage, S.; Miyazaki, M.; Ito, S.; Harigae, H.; Takahashi, N. Nicotinamide Attenuates the Progression of Renal Failure in a Mouse Model of Adenine-Induced Chronic Kidney Disease. Toxins 2021, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Diwan, V.; Brown, L.; Gobe, G.C. Adenine-induced chronic kidney disease in rats. Nephrology 2018, 23, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Iohara, D.; Wada, K.; Taguchi, K.; Maruyama, T.; Otagiri, M.; Uekama, K.; Hirayama, F. Antioxidant and renoprotective activity of 2-hydroxypropyl-β-cyclodextrin in nephrectomized rats. J. Pharm. Pharmacol. 2016, 68, 608–614. [Google Scholar] [CrossRef]
- Sueyoshi, M.; Fukunaga, M.; Mei, M.; Nakajima, A.; Tanaka, G.; Murase, T.; Narita, Y.; Hirata, S.; Kadowaki, D. Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats. Clin. Exp. Nephrol. 2019, 23, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–118. [Google Scholar]
- Commey, K.; Nakatake, A.; Enaka, A.; Nishi, K.; Tsukigawa, K.; Yamaguchi, K.; Ikeda, H.; Iohara, D.; Hirayama, F.; Otagiri, M.; et al. Study of the inclusion complexes formed between 4-phenylbutyrate and α-, β- and γ-cyclodextrin in solution and evaluation on their taste-masking properties. J. Pharm. Pharmacol. 2023, 75, 236–244. [Google Scholar] [CrossRef]
- Khan, K.A. The concept of dissolution efficiency. J. Pharm. Pharmacol. 2011, 27, 48–49. [Google Scholar] [CrossRef]
- Al Za’abi, M.; Ali, B.; Al Toubi, M. HPLC-Fluorescence Method for Measurement of the Uremic Toxin Indoxyl Sulfate in Plasma. J. Chromatogr. Sci. 2013, 51, 40–43. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Commey, K.L.; Enaka, A.; Nakamura, R.; Yamamoto, A.; Tsukigawa, K.; Nishi, K.; Otagiri, M.; Yamasaki, K. 7-Phenylheptanoic Acid-Hydroxypropyl β-Cyclodextrin Complex Slows the Progression of Renal Failure in Adenine-Induced Chronic Kidney Disease Mice. Toxins 2024, 16, 316. https://doi.org/10.3390/toxins16070316
Commey KL, Enaka A, Nakamura R, Yamamoto A, Tsukigawa K, Nishi K, Otagiri M, Yamasaki K. 7-Phenylheptanoic Acid-Hydroxypropyl β-Cyclodextrin Complex Slows the Progression of Renal Failure in Adenine-Induced Chronic Kidney Disease Mice. Toxins. 2024; 16(7):316. https://doi.org/10.3390/toxins16070316
Chicago/Turabian StyleCommey, Kindness Lomotey, Airi Enaka, Ryota Nakamura, Asami Yamamoto, Kenji Tsukigawa, Koji Nishi, Masaki Otagiri, and Keishi Yamasaki. 2024. "7-Phenylheptanoic Acid-Hydroxypropyl β-Cyclodextrin Complex Slows the Progression of Renal Failure in Adenine-Induced Chronic Kidney Disease Mice" Toxins 16, no. 7: 316. https://doi.org/10.3390/toxins16070316
APA StyleCommey, K. L., Enaka, A., Nakamura, R., Yamamoto, A., Tsukigawa, K., Nishi, K., Otagiri, M., & Yamasaki, K. (2024). 7-Phenylheptanoic Acid-Hydroxypropyl β-Cyclodextrin Complex Slows the Progression of Renal Failure in Adenine-Induced Chronic Kidney Disease Mice. Toxins, 16(7), 316. https://doi.org/10.3390/toxins16070316






