The Potential Therapeutic Effects of Botulinum Neurotoxins on Neoplastic Cells: A Comprehensive Review of In Vitro and In Vivo Studies
Abstract
:1. Introduction
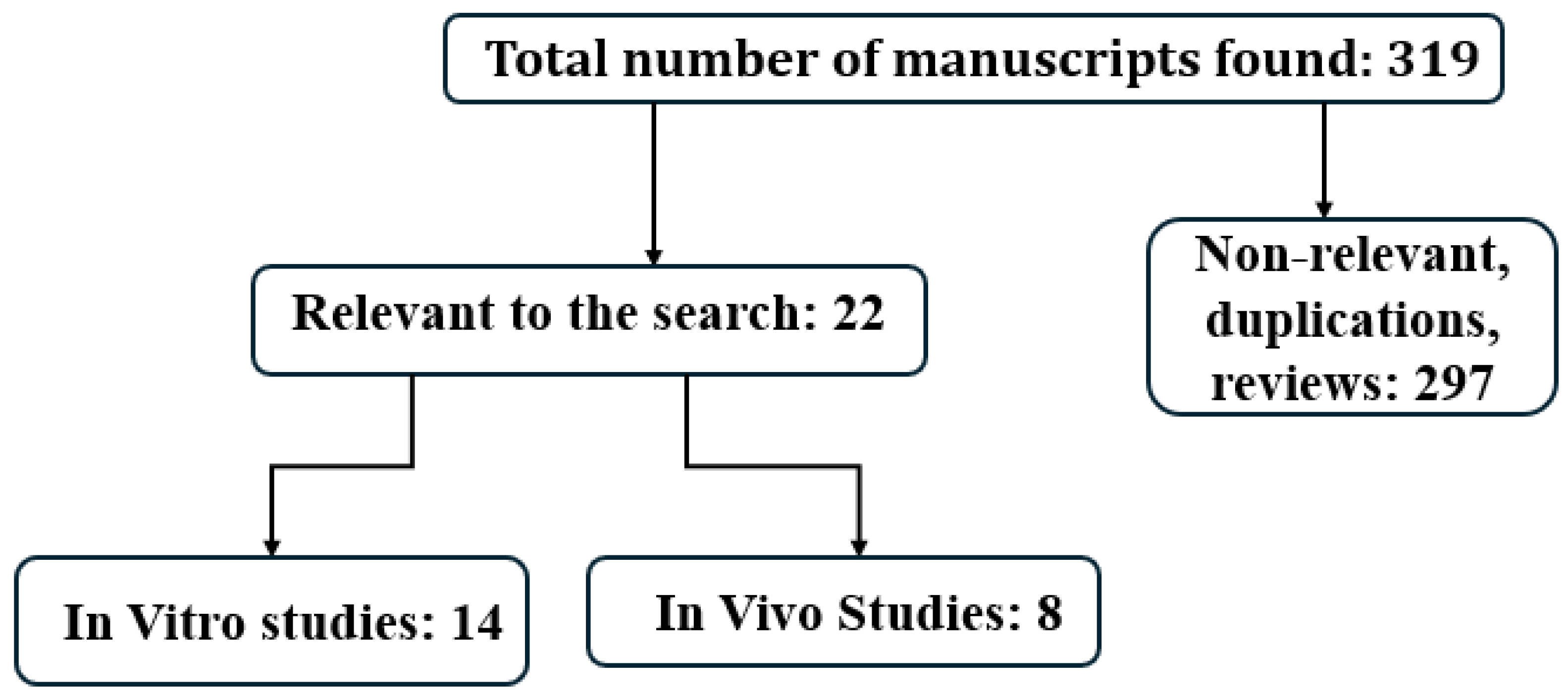
2. Study Design
3. Results
4. In Vitro Studies [Table 1]
5. In Vivo Studies
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jankovic, J. Botulinum toxin: State of the art. Mov. Disord. 2017, 32, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef]
- Matak, I.; Bölcskei, K.; Bach-Rojecky, L.; Helyes, Z. Mechanisms of Botulinum Toxin Type A Action on Pain. Toxins 2019, 11, 459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Babcock, M.S.; Foster, L.; Pasquina, P.; Jabbari, B. Treatment of pain attributed to plantar fasciitis with botulinum toxin a: A short-term, randomized, placebo-controlled, double-blind study. Am. J. Phys. Med. Rehabil. 2005, 84, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Eleopra, R.; Rinaldo, S.; Lettieri, C.; Santamato, A.; Bortolotti, P.; Lentino, C.; Tamborino, C.; Causero, A.; Devigili, G. AbobotulinumtoxinA: A New Therapy for Hip Osteoarthritis. A Prospective Randomized Double-Blind Multicenter Study. Toxins 2018, 10, 448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Placzek, R.; Drescher, W.; Deuretzbacher, G.; Hempfing, A.; Meiss, A.L. Treatment of chronic radial epicondylitis with botulinum toxin A. A double-blind, placebo-controlled, randomized multicenter study. J. Bone Jt. Surg. Am. 2007, 89, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Turkel, C.C.; Aurora, S.; Diener, H.C.; Dodick, D.W.; Lipton, R.B.; Silberstein, S.D.; Brin, M.F. Treatment of chronic migraine with Botox (onabotulinumtoxinA): Development, insights, and impact. Medicine 2023, 102, e32600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Safarpour, D.; Jabbari, B. Botulinum Toxin Treatment for Cancer-Related Disorders: A Systematic Review. Toxins 2023, 15, 689. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mittal, S.; Machado, D.G.; Jabbari, B. OnabotulinumtoxinA for treatment of focal cancer pain after surgery and/or radiation. Pain Med. 2012, 13, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Rostami, R.; Mittal, S.O.; Radmand, R.; Jabbari, B. Incobotulinum Toxin-A Improves Post-Surgical and Post-Radiation Pain in Cancer Patients. Toxins 2016, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Lu, Z.; Linghu, E.; Yang, Y.; Yang, J.; Wang, S.; Yan, B.; Song, J.; Zhou, X.; Wang, X.; et al. Prevention of esophageal strictures after endoscopic submucosal dissection with the injection of botulinum toxin type A. Gastrointest. Endosc. 2016, 84, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, H.; Chen, M.; Ding, C.; Zhang, G.; Si, X. Comparison of endoscopic injection of botulinum toxin and steroids immediately after endoscopic submucosal dissection to prevent esophageal stricture: A prospective cohort study. J. Cancer 2021, 12, 5789–5796. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martin, J.T.; Federico, J.A.; McKelvey, A.A.; Kent, M.S.; Fabian, T. Prevention of delayed gastric emptying after esophagectomy: A single center’s experience with botulinum toxin. Ann. Thorac. Surg. 2009, 87, 1708–1713; discussion 1713–1714. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.S.; Pennathur, A.; Fabian, T.; McKelvey, A.; Schuchert, M.J.; Luketich, J.D.; Landreneau, R.J. A pilot study of botulinum toxin injection for the treatment of delayed gastric emptying following esophagectomy. Surg. Endosc. 2007, 21, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Laskawi, R.; Winterhoff, J.; Köhler, S.; Kottwitz, L.; Matthias, C. Botulinum toxin treatment of salivary fistulas following parotidectomy: Follow-up results. Oral Maxillofac. Surg. 2013, 17, 281–285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marchese, M.R.; Bussu, F.; Settimi, S.; Scarano, E.; Almadori, G.; Galli, J. Not only gustatory sweating and flushing: Signs and symptoms associated to the Frey syndrome and the role of botulinum toxin A therapy. Head Neck 2021, 43, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, B. Botulinum Toxin Treatment of Pain Disorders, 2nd ed.; Springer Nature: Berlin/Heidelberg, Germany, 2022; pp. 291–293. [Google Scholar]
- Grenda, T.; Grenda, A.; Krawczyk, P.; Kwiatek, K. Botulinum toxin in cancer therapy-current perspectives and limitations. Appl. Microbiol. Biotechnol. 2022, 106, 485–495. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lomneth, R.; Martin, T.F.; DasGupta, B.R. Botulinum neurotoxin light chain inhibits norepinephrine secretion in PC12 cells at an intracellular membranous or cytoskeletal site. J. Neurochem. 1991, 57, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.S.; Duggan, M.J.; Shone, C.C.; Foster, K.A. The effect of botulinum neurotoxins on the release of insulin from the insulinoma cell lines HIT-15 and RINm5F. J. Biol. Chem. 1995, 270, 18216–18218. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wheeler, M.B.; Kang, Y.H.; Sheu, L.; Lukacs, G.L.; Trimble, W.S.; Gaisano, H.Y. Truncated SNAP-25 (1-197), like botulinum neurotoxin A, can inhibit insulin secretion from HIT-T15 insulinoma cells. Mol. Endocrinol. 1998, 12, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Purkiss, J.R.; Friis, L.M.; Doward, S.; Quinn, C.P. Clostridium botulinum neurotoxins act with a wide range of potencies on SH-SY5Y human neuroblastoma cells. Neurotoxicology 2001, 22, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G.; Rocha, J.; Chevalier, S.; Scarlata, E.; Andrieu, C.; Zouanat, F.Z.; Rocchi, P.; Giusiano, S.; Elzayat, E.A.; Corcos, J. Botulinum toxin type A inhibits the growth of LNCaP human prostate cancer cells in vitro and in vivo. Prostate 2009, 69, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Nardicchi, V.; Porena, M.; Giannantoni, A. Attività della tossina botulinica A in linee cellulari di cancro prostatico [Botulinum toxin type-A toxin activity on prostate cancer cell lines]. Urologia 2012, 79, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Bandala, C.; Perez-Santos, J.L.; Lara-Padilla, E.; Delgado Lopez, G.; Anaya-Ruiz, M. Effect of botulinum toxin A on proliferation and apoptosis in the T47D breast cancer cell line. Asian Pac. J. Cancer Prev. 2013, 14, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Bandala, C.; Cortés-Algara, A.L.; Mejía-Barradas, C.M.; Ilizaliturri-Flores, I.; Dominguez-Rubio, R.; Bazán-Méndez, C.I.; Floriano-Sánchez, E.; Luna-Arias, J.P.; Anaya-Ruiz, M.; Lara-Padilla, E. Botulinum neurotoxin type A inhibits synaptic vesicle 2 expression in breast cancer cell lines. Int. J. Clin. Exp. Pathol. 2015, 8, 8411–8418. [Google Scholar] [PubMed] [PubMed Central]
- Cheng, Y.T.; Chung, Y.H.; Kang, H.Y.; Tai, M.H.; Chancellor, M.B.; Chuang, Y.C. OnobotulinumtoxinA Has No Effects on Growth of LNCaP and PC3 Human Prostate Cancer Cells. Low Urin. Tract Symptoms 2013, 5, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Hajighasemlou, S.; Alebouyeh, M.; Rastegar, H.; Manzari, M.T.; Mirmoghtadaei, M.; Moayedi, B.; Ahmadzadeh, M.; Parvizpour, F.; Johari, B.; Naeini, M.M.; et al. Preparation of Immunotoxin Herceptin-Botulinum and Killing Effects on Two Breast Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2015, 16, 5977–5981. [Google Scholar] [CrossRef] [PubMed]
- Rust, A.; Leese, C.; Binz, T.; Davletov, B. Botulinum neurotoxin type C protease induces apoptosis in differentiated human neuroblastoma cells. Oncotarget 2016, 7, 33220–33228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shebl, R.I. Anti-cancer Potential of Captopril and Botulinum Toxin Type-A and Associated p53 Gene Apoptotic Stimulating Activity. Iran J. Pharm. Res. 2019, 18, 1967–1977. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akpınar, O.; Özşimşek, A.; Güzel, M.; Nazıroğlu, M. Clostridium botulinum neurotoxin A induces apoptosis and mitochondrial oxidative stress via activation of TRPM2 channel signaling pathway in neuroblastoma and glioblastoma tumor cells. J. Recept. Signal Transduct. Res. 2020, 40, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Duman, İ.; Nazıroğlu, M. Synergic actions of botulinum neurotoxin A and oxaliplatin on colorectal tumour cell death through the upregulation of TRPM2 channel-mediated oxidative stress. Clin. Exp. Pharmacol. Physiol. 2024, 51, e13844. [Google Scholar] [CrossRef] [PubMed]
- Ansiaux, R.; Baudelet, C.; Cron, G.O.; Segers, J.; Dessy, C.; Martinive, P.; De Wever, J.; Verrax, J.; Wauthier, V.; Beghein, N.; et al. Botulinum toxin potentiates cancer radiotherapy and chemotherapy. Clin. Cancer Res. 2006, 12, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Vezdrevanis, K. Prostatic carcinoma shrunk after intraprostatic injection of botulinum toxin. Urol. J. 2011, 8, 239–241. [Google Scholar] [PubMed]
- Zhao, C.M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ulloa, F.; Gonzàlez-Juncà, A.; Meffre, D.; Barrecheguren, P.J.; Martínez-Mármol, R.; Pazos, I.; Olivé, N.; Cotrufo, T.; Seoane, J.; Soriano, E. Blockade of the SNARE protein syntaxin 1 inhibits glioblastoma tumor growth. PLoS ONE 2015, 10, e0119707. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, D.; Manzoni, A.; Florentin, D.; Fisher, W.; Ding, Y.; Lee, M.; Ayala, G. Biologic effect of neurogenesis in pancreatic cancer. Hum. Pathol. 2016, 52, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Coarfa, C.; Florentin, D.; Putluri, N.; Ding, Y.; Au, J.; He, D.; Ragheb, A.; Frolov, A.; Michailidis, G.; Lee, M.; et al. Influence of the neural microenvironment on prostate cancer. Prostate 2018, 78, 128–139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kwak, S.; Lee, J.Y.; Kim, M.J.; Lee, H.J.; Lee, D.K.; Kang, J.; Kang, W.H.; Son, W.C.; Cruz, D.J.M. Combination of PD-1 Checkpoint Blockade and Botulinum Toxin Type A1 Improves Antitumor Responses in Mouse Tumor Models of Melanoma and Colon Carcinoma. Immunol. Investig. 2023, 52, 749–766. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rossetto, O.; Pirazzini, M.; Montecucco, C. Botulinum neurotoxins: Genetic, structural and mechanistic insights. Nat. Rev. Microbiol. 2014, 12, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Rothman, J.E. The principle of membrane fusion in the cell (Nobel lecture). Angew. Chem. Int. Ed. Engl. 2014, 53, 12676–12694. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Baker, M.R.; Chatterjee, S.; Kumar, H. Botulinum Toxin: An Update on Pharmacology and Newer Products in Development. Toxins 2021, 13, 58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nam, H.J.; Kang, J.K.; Chang, J.S.; Lee, M.S.; Nam, S.T.; Jung, H.W.; Kim, S.K.; Ha, E.M.; Seok, H.; Son, S.W.; et al. Cells transformed by PLC-gamma 1 overexpression are highly sensitive to clostridium difficile toxin A-induced apoptosis and mitotic inhibition. J. Microbiol. Biotechnol. 2012, 22, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Prepens, U.; Just, I.; von Eichel-Streiber, C.; Aktories, K. Inhibition of Fc epsilon-RI-mediated activation of rat basophilic leukemia cells by Clostridium difficile toxin B (monoglucosyltransferase). J. Biol. Chem. 1996, 271, 7324–7329. [Google Scholar] [CrossRef] [PubMed]
- Verschueren, H.; van der Taelen, I.; Dewit, J.; De Braekeleer, J.; De Baetselier, P.; Aktories, K.; Just, I. Effects of Clostridium botulinum C2 toxin and cytochalasin D on in vitro invasiveness, motility and F-actin content of a murine T-lymphoma cell line. Eur. J. Cell Biol. 1995, 66, 335–341. [Google Scholar] [PubMed]
- Kusama, T.; Mukai, M.; Iwasaki, T.; Tatsuta, M.; Matsumoto, Y.; Akedo, H.; Nakamura, H. Inhibition of epidermal growth factor-induced RhoA translocation and invasion of human pancreatic cancer cells by 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors. Cancer Res. 2001, 61, 4885–4891. [Google Scholar] [PubMed]
- Diaz, L.A., Jr.; Cheong, I.; Foss, C.A.; Zhang, X.; Peters, B.A.; Agrawal, N.; Bettegowda, C.; Karim, B.; Liu, G.; Khan, K.; et al. Pharmacologic and toxicologic evaluation of C. novyi-NT spores. Toxicol. Sci. 2005, 88, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Abedi Jafari, F.; Abdoli, A.; Pilehchian, R.; Soleimani, N.; Hosseini, S.M. The oncolytic activity of Clostridium novyi nontoxic spores in breast cancer. Bioimpacts 2022, 12, 405–414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roberts, N.J.; Zhang, L.; Janku, F.; Collins, A.; Bai, R.Y.; Staedtke, V.; Rusk, A.W.; Tung, D.; Miller, M.; Roix, J.; et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci. Transl. Med. 2014, 6, 249ra111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dailey, K.M.; Small, J.M.; Pullan, J.E.; Winfree, S.; Vance, K.E.; Orr, M.; Mallik, S.; Bayles, K.W.; Hollingsworth, M.A.; Brooks, A.E. An intravenous pancreatic cancer therapeutic: Characterization of CRISPR/Cas9n-modified Clostridium novyi-Non Toxic. PLoS ONE 2023, 18, e0289183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Angulo, M.C.; Kozlov, A.S.; Charpak, S.; Audinat, E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J. Neurosci. 2004, 24, 6920–6927. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yi, H.; Talmon, G.; Wang, J. Glutamate in cancers: From metabolism to signaling. J. Biomed. Res. 2019, 34, 260–270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- da Silva, L.B.; Poulsen, J.N.; Arendt-Nielsen, L.; Gazerani, P. Botulinum neurotoxin type A modulates vesicular release of glutamate from satellite glial cells. J. Cell Mol. Med. 2015, 19, 1900–1909. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fonfria, E.; Donald, S.; Cadd, V.A. Botulinum neurotoxin A and an engineered derivate targeted secretion inhibitor (TSI) A enter cells via different vesicular compartments. J. Recept. Signal Transduct. Res. 2016, 36, 79–88. [Google Scholar] [PubMed]
- Whitt, J.; Hong, W.S.; Telange, R.R.; Lin, C.P.; Bibb, J.; Beebe, D.J.; Chen, H.; Jaskula-Sztul, R. Non-toxic fragment of botulinum neurotoxin type A and monomethyl auristatin E conju-gate for targeted therapy for neuroendocrine tumors. Cancer Gene Ther. 2020, 27, 898–909. [Google Scholar] [CrossRef]
- Ward, N.L.; Kavlick, K.D.; Diaconu, D.; Dawes, S.M.; Michaels, K.A.; Gilbert, E. Botulinum neurotoxin A decreases infiltrating cutaneous lymphocytes and improves acanthosis in the KC-Tie2 mouse model. J. Investig. Dermatol. 2012, 132, 1927–1930. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Popescu, M.N.; Beiu, C.; Iliescu, M.G.; Mihai, M.M.; Popa, L.G.; Stănescu, A.M.A.; Berteanu, M. Botulinum Toxin Use for Modulating Neuroimmune Cutaneous Activity in Psoriasis. Medicina 2022, 58, 813. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corasaniti, M.T.; Bagetta, G.; Nicotera, P.; Tarsitano, A.; Tonin, P.; Sandrini, G.; Lawrence, G.W.; Scuteri, D. Safety of Onabotulinumtoxin A in Chronic Migraine: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Toxins 2023, 15, 332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wissel, J.; Bensmail, D.; Ferreira, J.J.; Molteni, F.; Satkunam, L.; Moraleda, S.; Rekand, T.; McGuire, J.; Scheschonka, A.; Flatau-Baqué, B.; et al. Safety and efficacy of incobotulinumtoxinA doses up to 800 U in limb spasticity: The TOWER study. Neurology 2017, 88, 1321–1328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brin, M.F.; Kirby, R.S.; Slavotinek, A.; Adams, A.M.; Parker, L.; Ukah, A.; Radulian, L.; Elmore, M.R.P.; Yedigarova, L.; Yushmanova, I. Pregnancy Outcomes in Patients Exposed to OnabotulinumtoxinA Treatment: A Cumulative 29-Year Safety Update. Neurology 2023, 101, e103–e113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Author and Year | Origin/Type of Tumor | Cell Lines | Type of Toxin | Result |
|---|---|---|---|---|
| Lomnette et al., 1991 [19] | Pheochromocytoma | PC-12 | OnaA | Reduced the secretion of catecholamines |
| Boyd et al., 1995 [20] | Pancreas | Beta cell line: HIT-15 and RIN-m5F | Ona-A Rima-B | OnaA: >90% inhibition of insulin secretion RimaB: 60% inhibition of insulin secretion |
| Huang et al., 1998 [21] | Endocrine | Insulin secreting HIT-T15 | Ona-A | Regulated insulin secretion |
| Purkiss et al., 2001 [22] | Neuroblastoma | Shsy-5y | OnaA | Very sensitive |
| Karsenty et al., 2009 [23] | Prostate | PC3 and LNCaP | Ona-A | Reduced proliferation and increased apoptosis of LNCaP cells |
| Proietti et al., 2012 [24] | Prostate | PC3 and LNCaP | Inco-A | Reduction in cell growth PC3 25%, LNCaP 20% |
| Bandala et al., 2013 [25] | Breast | T47D | Ona-A | Greater cytotoxicity to T47D cells than normal cells |
| Bandala et al., 2015 [26] | Breast | T47D, MDA-MB-231, MDA-MB-453 | Ona-A | onaA diminished Sv2 expression in all three cell lines |
| Cheng et al., 2013 [27] | Prostate | PC3and LNCaP | Ona-A | No effect during day 1 to 6 exposure |
| Hajighasemlou et al., 2015 [28] | Breast | SK-BR3 and BT-474 | Ona-A | Herceptin–onaA conjugate improved Herceptin efficacy against breast cancer cells |
| Rust et al., 2016 [29] | Neuroblastoma | SiMa and SH-SY-5Y | BTX-C | Caused apoptotic changes only in neuroblastoma cells |
| Shebl et al., 2019 [30] | Colon and Prostate | HC-T116 and DU145 | Ona-A | Inhibitory effect on cell proliferation and ability of cancer cells to migrate |
| Akpinar et al., 2020 [31] | Neuroblastoma and glioblastoma | DBTRG and SH-SY5Y cells | Ona-A | Apoptosis and death of cancer cells via increase in ROS production |
| Demir et al., 2024 [32] | Colon | H229 | OnaA | Caused oxidative distress and apoptosis of tumor cells |
| Author and Year | Location of Cancer | Tested Subject | Type of Toxin and Dose | Results |
|---|---|---|---|---|
| Ansiauox et al., 2006 [33] | Fiborsarcoma, hepatocarcinoma | Injected into Mouse tumor | onaA, 29 units/Kg | Increased tumor oxygenation and response to chemotherapy |
| Vezdrevanis et al., 2011 [34] | Prostate | Injected into Patient’s tumor | abo-A, 1000 units | 30% reduction in tumor size in 4 weeks. |
| Cheng et al., 2013 [27] | Prostate | Injected LNCaP and PC3 cancer cells into the prostate of the mice | onaA, 2 units (two weeks later) | Pre-treatment with onaA had no effect on cancer |
| Zhao et al., 2014 [35] | Stomach | Injected into the greater curvature of the stomach of INS-GNS mice with spontaneous gastric cancer | onaA, 0.1 unit/mouse every month for 6 months. | Slowed development and progression of the cancer |
| Ulloa et al., 2015 [36] | Brain glioblastoma multiforme (GBM) | Mice, U373 cells with BoNT-C1 injected into striatum | BoNT—C1, 375 pg | Impaired GBM cell proliferation by blocking the function of syntaxin-1 |
| He et al., 2016 [37] | Pancreas cancer | Injected into tumor with MIA-PaCa2 cells treated with OnaA | onaA, 20 units/Kg | Increased apoptosis, decreased malignant tumor size |
| Coarfa et al., 2018 [38] | Mice Prostate Prostate (Human Clinical trial) | injected into prostate of mouse before implanting VCaP cancer cells Into prostate malignant prostate tumor | onaA, 10 units onaA, 100 units | Reduction in tumor incidence and malignant tumor size Increased apoptosis of cancer cells |
| Kwak et al., 2023 [39] | Melanoma, colon, lung, and breast carcinoma | Single injection into implanted tumor cells in B16-F10 and MC38 tumor-bearing mice | onaA, 15 units/Kg | Improved anti-cancer activity of PD1 check point blockade |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safarpour, D.; Tavassoli, F.A.; Jabbari, B. The Potential Therapeutic Effects of Botulinum Neurotoxins on Neoplastic Cells: A Comprehensive Review of In Vitro and In Vivo Studies. Toxins 2024, 16, 355. https://doi.org/10.3390/toxins16080355
Safarpour D, Tavassoli FA, Jabbari B. The Potential Therapeutic Effects of Botulinum Neurotoxins on Neoplastic Cells: A Comprehensive Review of In Vitro and In Vivo Studies. Toxins. 2024; 16(8):355. https://doi.org/10.3390/toxins16080355
Chicago/Turabian StyleSafarpour, Delaram, Fattaneh A. Tavassoli, and Bahman Jabbari. 2024. "The Potential Therapeutic Effects of Botulinum Neurotoxins on Neoplastic Cells: A Comprehensive Review of In Vitro and In Vivo Studies" Toxins 16, no. 8: 355. https://doi.org/10.3390/toxins16080355
APA StyleSafarpour, D., Tavassoli, F. A., & Jabbari, B. (2024). The Potential Therapeutic Effects of Botulinum Neurotoxins on Neoplastic Cells: A Comprehensive Review of In Vitro and In Vivo Studies. Toxins, 16(8), 355. https://doi.org/10.3390/toxins16080355





