Real-World Observational Analysis of Clinical Characteristics and Treatment Patterns of Patients with Chronic Sialorrhea
Abstract
1. Introduction
2. Results
2.1. Primary Objective
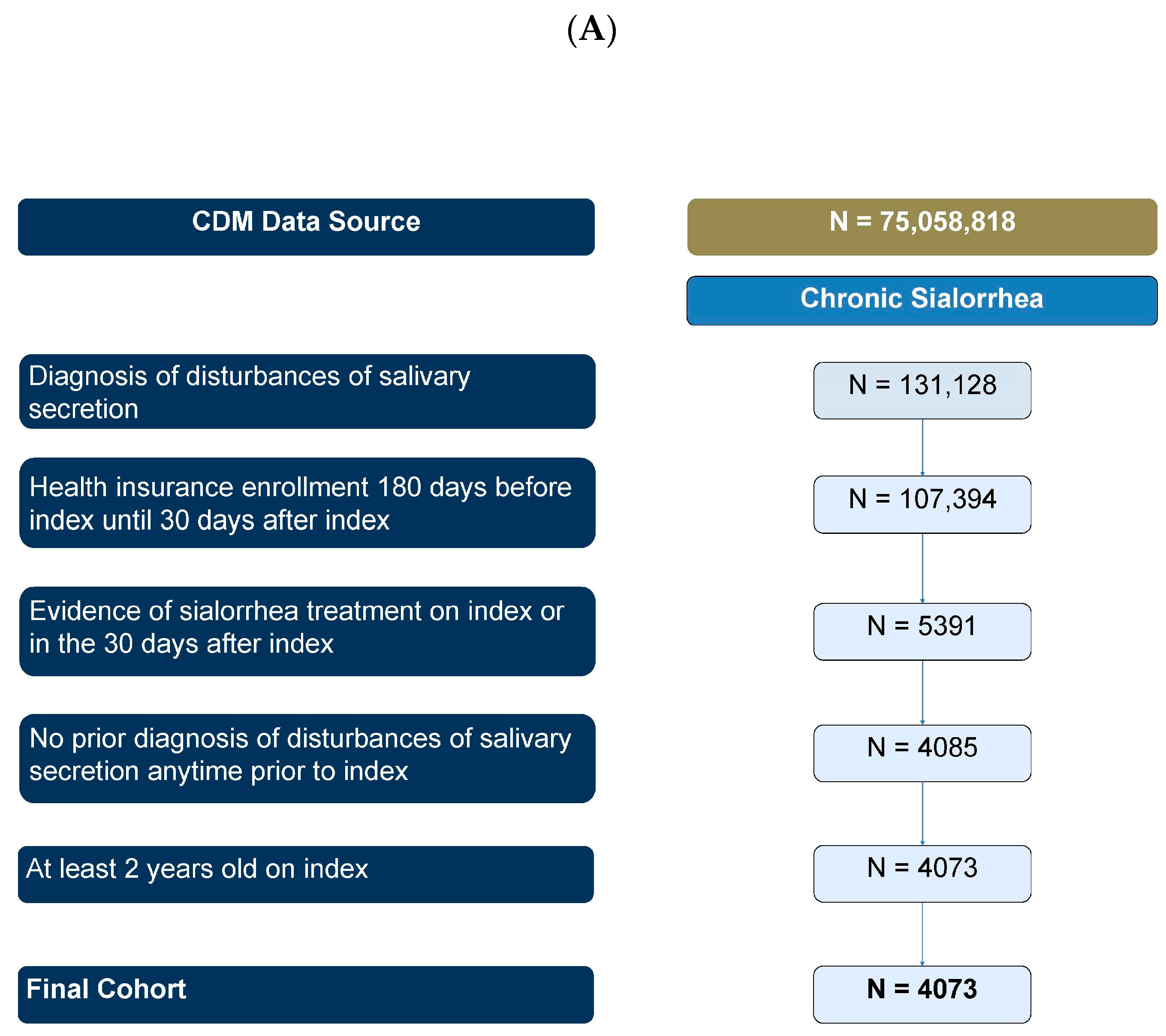
2.1.1. Results Summary
2.1.2. Demographic Characteristics of Sialorrhea Patients
2.1.3. Clinical Characteristics and Treatments before and after Sialorrhea Diagnosis
2.1.4. Treatment Patterns of Sialorrhea Patients in Follow-Up
2.2. Secondary Objective
2.2.1. Results Summary
2.2.2. Demographic Characteristics of IncobotulinumtoxinA Users
2.2.3. Clinical Characteristics and Treatments before and after IncobotulinumtoxinA Injection
2.2.4. IncobotulinumtoxinA Utilization
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Data Source
5.2. Study Design
5.3. Primary Objective Patient Selection
5.4. Secondary Objective Patient Selection
5.5. Patient Demographic and Clinical Characteristics
5.6. Treatments
5.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalf, J.G.; de Swart, B.J.M.; Borm, G.F.; Bloem, B.R.; Munneke, M. Prevalence and definition of drooling in Parkinson’s disease: A systematic review. J. Neurol. 2009, 256, 1391–1396. [Google Scholar] [CrossRef]
- Hockstein, N.G.; Samadi, D.S.; Gendron, K.; Handler, S.D. Sialorrhea: A Management Challenge. Am. Fam. Physician 2004, 69, 2628–2635. [Google Scholar] [PubMed]
- Morgante, F.; Bavikatte, G.; Anwar, F.; Mohamed, B. The burden of sialorrhoea in chronic neurological conditions: Current treatment options and the role of incobotulinumtoxinA (incobotulinumtoxinA®). Ther. Adv. Neurol. Disord. 2019, 12, 1756286419888601. [Google Scholar] [CrossRef] [PubMed]
- Lakraj, A.A.; Moghimi, N.; Jabbari, B. Sialorrhea: Anatomy, Pathophysiology and Treatment with Emphasis on the Role of Botulinum Toxins. Toxins 2013, 5, 1010–1031. [Google Scholar] [CrossRef] [PubMed]
- Jost, W.H.; Bäumer, T.; Laskawi, R.; Slawek, J.; Spittau, B.; Steffen, A.; Winterholler, M.; Bavikatte, G. Therapy of Sialorrhea with Botulinum Neurotoxin. Neurol. Ther. 2019, 8, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Garuti, G.; Rao, F.; Ribuffo, V.; Sansone, V.A. Sialorrhea in patients with ALS: Current treatment options. Degener. Neurol. Neuromuscul. Dis. 2019, 9, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Berweck, S.; Bonikowski, M.; Kim, H.; Althaus, M.; Flatau-Baqué, B.; Mueller, D.; Banach, M.D. Placebo-Controlled Clinical Trial of IncobotulinumtoxinA for Sialorrhea in Children. Neurology 2021, 97, e1425–e1436. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, S.H.; Ondo, W.; Jackson, C.E.; Trosch, R.M.; Molho, E.; Pagan, F.; Lew, M.; Dashtipour, K.; Clinch, T.; Espay, A.J.; et al. Safety and Efficacy of RimabotulinumtoxinB for Treatment of Sialorrhea in Adults: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Jost, W.H.; Steffen, A.; Berweck, S. A critical review of incobotulinumtoxinA in the treatment of chronic sialorrhea in pediatric patients. Expert Rev. Neurother. 2021, 21, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Bergmans, B.; Clark, V.; Isaacson, S.H.; Baumer, T. Recommendations for a paradigm shift in approach to increase the recognition and treatment of sialorrhea in Parkinson’s disease. Clin. Park. Relat. Disord. 2023, 9, 100223. [Google Scholar] [CrossRef] [PubMed]
- Dias, B.L.S.; Fernandes, A.R.; de Souza Maia Filho, H. Sialorrhea in children with cerebral palsy. J. Pediatr. 2016, 92, 549–558. [Google Scholar] [CrossRef] [PubMed]
- You, P.; Strychowsky, J.; Gandhi, K.; Chen, B.A. Anticholinergic treatment for sialorrhea in children: A systematic review. Paediatr. Child Health 2021, 27, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, A.; Charu, V. Analysis of sampling bias in large health care claims databases. JAMA Netw. Open 2023, 6, e2249804. [Google Scholar] [CrossRef] [PubMed]
- DeFrank, J.T.; Michael, B.J.; Rimer, B.K.; Gierisch, J.M.; Skinner, C.S. Triangulating Differential Nonresponse by Race in a Telephone Sur13vey. Prev. Chronic Dis. 2007, 4, A60. [Google Scholar] [PubMed]
- Aetion. Real-World Evidence Solutions|RWE Analytics. Available online: https://aetion.com/ (accessed on 24 August 2022).

| Overall Sialorrhea | Pediatric Sialorrhea | Adult Sialorrhea | IncobotulinumtoxinA Users | |
|---|---|---|---|---|
| N = 4073 | N = 398 | N = 3675 | N = 177 | |
| Age (years) | ||||
| Mean (SD) | 59.64 (23.09) | 9.39 (4.64) | 65.09 (16.89) | 68.97 (17.80) |
| Median [IQR] | 67.00 [50.00, 77.00] | 10.00 [5.00, 13.00] | 68.00 [56.00, 78.00] | 73.00 [65.50, 80.50] |
| Age Group, n (%) | ||||
| 2–17 years | 398 (9.8%) | 398 (100.0%) | 6 (3.4%) | |
| 18–44 years | 465 (11.4%) | 465 (12.7%) | 10 (5.6%) | |
| 45–64 years | 1013 (24.9%) | 1013 (27.6%) | 27 (15.3%) | |
| 65+ years | 2197 (53.9%) | 2197 (59.8%) | 134 (75.7%) | |
| Sex, n (%) | ||||
| Male | 2223 (54.6%) | 237 (59.5%) | 1986 (54.0%) | 110 (62.1%) |
| Female | 1850 (45.4%) | 161 (40.5%) | 1689 (46.0%) | 67 (37.9%) |
| Race, n (%) | ||||
| Non-Hispanic White | 2739 (67.2%) | 259 (65.1%) | 2480 (67.5%) | 130 (73.4%) |
| Non-Hispanic Black | 557 (13.7%) | 24 (6.0%) | 533 (14.5%) | 15 (8.5%) |
| Non-Hispanic Asian | 114 (2.8%) | 18 (4.5%) | 96 (2.6%) | 9 (5.1%) |
| Hispanic | 353 (8.7%) | 29 (7.3%) | 324 (8.8%) | 15 (8.5%) |
| Unknown/missing | 310 (7.6%) | 68 (17.1%) | 242 (6.6%) | 8 (4.5%) |
| Region, n (%) | ||||
| Northeast | 512 (12.6%) | 29 (7.3%) | 483 (13.1%) | 27 (15.3%) |
| Midwest | 974 (23.9%) | 157 (39.4%) | 817 (22.2%) | 30 (16.9%) |
| South | 1721 (42.3%) | 149 (37.4%) | 1572 (42.8%) | 64 (36.2%) |
| West | 861 (21.1%) | 60 (15.1%) | 801 (21.8%) | 56 (31.6%) |
| Other | 5 (0.1%) | 3 (0.8%) | 2 (0.1%) | 0 (0.0%) |
| Insurance Type, n (%) | ||||
| Commercial only | 1469 (36.1%) | 398 (100.0%) | 1071 (29.1%) | 39 (22.0%) |
| Medicare only | 2600 (63.8%) | 0 (0.0%) | 2600 (70.7%) | 138 (78.0%) |
| Other | N < 5 | N < 5 | N < 5 | N < 5 |
| Overall Sialorrhea | Pediatric Sialorrhea | Adult Sialorrhea | IncobotulinumtoxinA Users | |||||
|---|---|---|---|---|---|---|---|---|
| N = 4073 | N = 398 | N = 3675 | N = 177 | |||||
| Baseline | Follow-Up | Baseline | Follow-Up | Baseline | Follow-Up | Baseline | Follow-Up | |
| Comorbidities, n (%) | ||||||||
| Alzheimer’s disease/dementia | 358 (8.8%) | 624 (15.3%) | N < 5 | N < 5 | 357 (9.7%) | 623 (17.0%) | 11 (6.2%) | 25 (14.1%) |
| Amyotrophic lateral sclerosis | 245 (6.0%) | 283 (6.9%) | N < 5 | N < 5 | 245 (6.7%) | 282 (7.7%) | 8 (4.5%) | 9 (5.1%) |
| Cerebral Palsy | 328 (8.1%) | 410 (10.1%) | 223 (56.0%) | 269 (67.6%) | 105 (2.9%) | 141 (3.8%) | 7 (4.0%) | 9 (5.1%) |
| Multiple sclerosis | 62 (1.5%) | 84 (2.1%) | N < 5 | N < 5 | 61 (1.7%) | 82 (2.2%) | N < 5 | N < 5 |
| Parkinson’s disease | 710 (17.4%) | 957 (23.5%) | N < 5 | N < 5 | 710 (19.3%) | 955 (26.0%) | 110 (62.1%) | 115 (65.0%) |
| Stroke | 430 (10.6%) | 655 (16.1%) | 12 (3.0%) | 21 (5.3%) | 418 (11.4%) | 634 (17.3%) | 15 (8.5%) | 29 (16.4%) |
| Traumatic brain injury | 87 (2.1%) | 176 (4.3%) | 9 (2.3%) | 18 (4.5%) | 78 (2.1%) | 158 (4.3%) | N < 5 | 9 (5.1%) |
| Clinical Symptoms, n (%) | ||||||||
| Anxiety | 848 (20.8%) | 1402 (34.4%) | 22 (5.5%) | 52 (13.1%) | 826 (22.5%) | 1350 (36.7%) | 27 (15.3%) | 39 (22.0%) |
| Depression | 815 (20.0%) | 1338 (32.9%) | 6 (1.5%) | 10 (2.5%) | 809 (22.0%) | 1328 (36.1%) | 40 (22.6%) | 54 (30.5%) |
| Dehydration | 385 (9.5%) | 916 (22.5%) | 27 (6.8%) | 77 (19.3%) | 358 (9.7%) | 839 (22.8%) | N < 5 | 37 (20.9%) |
| Dysphagia | 1482 (36.4%) | 2198 (54.0%) | 141 (35.4%) | 206 (51.8%) | 1341 (36.5%) | 1992 (54.2%) | 66 (37.3%) | 94 (53.1%) |
| Speech impairment | 222 (5.5%) | 393 (9.6%) | 26 (6.5%) | 51 (12.8%) | 196 (5.3%) | 342 (9.3%) | 7 (4.0%) | 11 (6.2%) |
| incobotulinumtoxinA Indications, n (%) | ||||||||
| Blepharospasm | 53 (1.3%) | 95 (2.3%) | N < 5 | N < 5 | 52 (1.4%) | 93 (2.5%) | 11 (6.2%) | 21 (11.9%) |
| Cervical Dystonia | 91 (2.2%) | 180 (4.4%) | 5 (1.3%) | 12 (3.0%) | 86 (2.3%) | 168 (4.6%) | 15 (8.5%) | 23 (13.0%) |
| Upper Limb Spasticity | 153 (3.8%) | 288 (7.1%) | 78 (19.6%) | 148 (37.2%) | 75 (2.0%) | 140 (3.8%) | 8 (4.5%) | 13 (7.3%) |
| Anticholinergic Therapy, n (%) | ||||||||
| Any anticholinergic | 1525 (37.4%) | 2827 (69.4%) | 195 (49.0%) | 340 (85.4%) | 1330 (36.2%) | 2487 (67.7%) | 27 (15.3%) | 25 (14.1%) |
| Glycopyrrolate | 580 (14.2%) | 1332 (32.7%) | 149 (37.4%) | 292 (73.4%) | 431 (11.7%) | 1040 (28.3%) | 16 (9.0%) | 14 (7.9%) |
| Benztropine | 407 (10.0%) | 477 (11.7%) | 6 (1.5%) | 12 (3.0%) | 401 (10.9%) | 465 (12.7%) | N < 5 | N < 5 |
| Scopolamine | 617 (15.1%) | 1308 (32.1%) | 49 (12.3%) | 89 (22.4%) | 568 (15.5%) | 1219 (33.2%) | 11 (6.2%) | 12 (6.8%) |
| Tropicamide | N < 5 | N < 5 | N < 5 | N < 5 | N < 5 | N < 5 | N < 5 | N < 5 |
| Biperiden | N < 5 | N < 5 | N < 5 | N < 5 | N < 5 | N < 5 | N < 5 | N < 5 |
| Botulinum Toxin Therapy, n (%) | ||||||||
| Any BoNT | 306 (7.5%) | 1389 (34.1%) | 50 (12.6%) | 152 (38.2%) | 256 (7.0%) | 1237 (33.7%) | 46 (26.0%) | 177 (100.0%) |
| incobotulinumtoxinA | 9 (0.2%) | 92 (2.3%) | N < 5 | 6 (1.5%) | 7 (0.2%) | 86 (2.3%) | N < 5 | 177 (100.0%) |
| rimabotulinumtoxinB | 18 (0.4%) | 529 (13.0%) | 4 (1.0%) | 11 (2.8%) | 14 (0.4%) | 518 (14.1%) | 26 (14.7%) | 27 (15.3%) |
| onabotulinumtoxinA | 270 (6.6%) | 894 (21.9%) | 42 (10.6%) | 133 (33.4%) | 228 (6.2%) | 761 (20.7%) | 20 (11.3%) | 15 (8.5%) |
| abobotulinumtoxinA | 10 (0.2%) | 36 (0.9%) | N < 5 | 13 (3.3%) | 8 (0.2%) | 23 (0.6%) | N < 5 | N < 5 |
| Overall Sialorrhea | Pediatric Sialorrhea | Adult Sialorrhea | |
|---|---|---|---|
| N = 4073 | N = 398 | N = 3675 | |
| First Treatment, n (%) | |||
| Patients with a treatment claim in follow-up | 3241 | 366 | 2875 |
| Anticholinergics | 2013 (62.1%) | 275 (75.1%) | 1738 (75.1%) |
| BoNT monotherapy | 1155 (35.6%) | 77 (21.0%) | 1078 (37.5%) |
| incobotulinumtoxinA | 53 (1.6%) | 51 (0.5%) | |
| rimabotulinumtoxinB | 428 (13.2%) | 422 (1.6%) | |
| onabotulinumtoxinA | 653 (20.1%) | 588 (17.8%) | |
| abobotulinumtoxinA | 21 (0.6%) | 17 (1.1%) | |
| Other combinations | 73 (2.3%) | 14 (3.8%) | 59 (3.8%) |
| Second Treatment, n (%) | |||
| Patients with a second treatment claim in follow-up | 1270 | 201 | 1069 |
| Anticholinergics | 689 (54.3%) | 116 (57.7%) | 573 (53.6%) |
| BoNT monotherapy | 468 (36.9%) | 53 (26.4%) | 415 (38.8%) |
| incobotulinumtoxinA | 21 (1.7%) | 19 (1.8%) | |
| rimabotulinumtoxinB | 179 (14.1%) | 173 (16.2%) | |
| onabotulinumtoxinA | 255 (20.1%) | 214 (20.0%) | |
| abobotulinumtoxinA | 13 (1.0%) | 9 (0.8%) | |
| Other combinations | 113 (8.9%) | 32 (15.9%) | 81 (7.6%) |
| Third Treatment, n (%) | |||
| Patients with a third treatment claim in follow-up | 661 | 134 | 527 |
| Anticholinergics | 362 (54.8%) | 82 (61.2%) | 280 (53.1%) |
| BoNT monotherapy | 240 (36.3%) | 34 (25.4%) | 206 (39.1%) |
| incobotulinumtoxinA | 11 (1.7%) | ||
| rimabotulinumtoxinB | 94 (14.2%) | ||
| onabotulinumtoxinA | 129 (19.5%) | ||
| abobotulinumtoxinA | 6 (0.9%) | ||
| Other combinations | 59 (8.9%) | 18 (13.4%) | 41 (7.8%) |
| Time until First Treatment in Follow-Up, days | |||
| Patients with any treatment claim | 3874 | 391 | 3483 |
| mean (SD) until any treatment | 17.23 (88.00) | 13.25 (28.58) | 17.68 (92.31) |
| median [IQR] until any treatment | 3.00 [0.50, 16.00] | 4.00 [0.50, 18.00] | 2.00 [0.50, 16.00] |
| Patients with an anticholinergic claim | 2827 | 340 | 2487 |
| mean (SD) until anticholinergic claim | 37.42 (172.07) | 52.25 (259.49) | 35.39 (156.34) |
| median [IQR] until anticholinergic claim | 6.00 [0.50, 20.00] | 6.00 [0.50, 20.75] | 6.00 [0.50, 20.00] |
| Patients with a BoNT claim | 1389 | 152 | 1237 |
| mean (SD) until BoNT claim | 58.75 (224.86) | 187.29 (359.36) | 42.95 (196.78) |
| median [IQR] until BoNT claim | 0.50 [0.50, 20.00] | 31.00 [0.50, 154.00] | 0.50 [0.50, 16.00] |
| Patients with surgical excision | 13 | N < 5 | 12 |
| mean (SD) until surgical excision | 5.88 (9.17) | 3.96 (6.25) | |
| median [IQR] until surgical excision | 0.50 [0.50, 12.50] | 0.50 [0.50, 7.75] | |
| Combination Therapy | |||
| Patients with evidence of concurrent anticholinergic and BoNT use, n (%) | 241 (5.9%) | 73 (18.3%) | 168 (4.6%) |
| mean (SD) number of days of overlap | 184.56 (230.50) | 226.79 (261.39) | 166.21 (213.96) |
| median [IQR] number days of overlap | 113.00 [59.00, 215.50] | 133.00 [73.50, 264.50] | 101.00 [55.25, 196.75] |
| Switching | |||
| Patients with an anticholinergic treatment claim in follow-up | 2827 | 340 | 2487 |
| Proportion of patients who switch to BoNT therapy, n (%) | 309 (10.9%) | 88 (25.9%) | 221 (8.9%) |
| Patients with a BoNT claim in follow-up | 1389 | 152 | 1237 |
| Proportion of patients who switch to anticholinergic; n (%) | 282 (20.3%) | 92 (60.5%) | 190 (15.4%) |
| Number of Different Toxins, n (%) | |||
| Zero | 2684 (65.9%) | 246 (61.8%) | 2438 (66.3%) |
| One | 1233 (30.3%) | 141 (35.4%) | 1092 (29.7%) |
| Two or more | 156 (3.8%) | 11 (2.8%) | 145 (3.9%) |
| IncobotulinumtoxinA Users | Sensitivity Cohort (Excluding Patients with Claims <4 or >32 Weeks Apart) | |
|---|---|---|
| N = 177 | N = 153 | |
| Follow-Up, Years | ||
| Mean (SD) | 1.65 (1.75) | 0.54 (0.51) |
| Median [IQR] | 1.01 [0.48, 2.37] | 0.31 [0.31, 0.63] |
| Number of Injections | ||
| Mean number of injections per person (SD) | 2.46 (2.63) | 1.99 (1.80) |
| Median number of injections per person [IQR] | 1.00 [1.00, 3.00] | 1.00 [1.00, 2.00] |
| Mean number of injections per person-year (SD) | 1.47 (1.08) | 1.73 (1.00) |
| Median number of injections per person-year [IQR] | 1.00 [0.80, 2.00] | 1.00 [1.00, 2.00] |
| Weeks between Injections | ||
| Number of patients with more than 1 injection | 87 (49.2%) | 65 (42.5%) |
| Mean number of weeks between injections per person (SD) | 19.86 (15.09) | 15.66 (4.10) |
| Median number of weeks between injections per person [IQR] | 15.00 [13.00, 20.00] | 14.14 [13.00, 17.14] |
| Time until Treatment Switch, days | ||
| number of patients with a switch to non-incobotulinumtoxinA toxin | 40 | 19 |
| mean (SD) | 219.35 (255.09) | 86.05 (78.51) |
| median [IQR] | 122.00 [85.50, 250.25] | 84.00 [0.50, 116.00] |
| Switching (n, %) | ||
| Patients with an administration of a non-incobotulinumtoxinA treatment during follow-up | 40 (22.6%) | 19 (12.4%) |
| onabotulinumtoxinA use | 15 (8.5%) | N < 5 |
| rimabotulinumtoxinB use | 27 (15.3%) | 15 (9.8%) |
| abobotulinumtoxinA use | N < 5 | N < 5 |
| incobotulinumtoxinA Treatment Time (until switch or discontinuation), days | ||
| Mean number of weeks (SD) | 187.22 (212.80) | 175.35 (170.94) |
| Median number of weeks [IQR] | 113.00 [99.00, 226.00] | 113.00 [91.00, 219.00] |
| Provider Types Administering incobotulinumtoxinA | ||
| Total number of injection events | 435 | 305 |
| Number of injection events administered by general/family medicine, n (%) | 49 (11.3%) | 48 (15.7%) |
| Number of injection events administered by neurology, n (%) | 249 (57.2%) | 186 (61.0%) |
| Number of injection events administered by physical medicine and rehabilitation specialist, n (%) | 7 (1.6%) | 7 (2.3%) |
| Number of injection events administered by ear, nose, and throat, n (%) | 12 (2.8%) | 7 (2.3%) |
| Number of injection events administered by psychiatry, n (%) | 22 (5.1%) | 22 (7.2%) |
| Number of injection events administered by other provider type; n (%) | 96 (22.1%) | 35 (11.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hast, M.A.; Kong, A.M.; Abdelhadi, J.; Shah, R.; Szendrey, A.; Holmes, J. Real-World Observational Analysis of Clinical Characteristics and Treatment Patterns of Patients with Chronic Sialorrhea. Toxins 2024, 16, 366. https://doi.org/10.3390/toxins16080366
Hast MA, Kong AM, Abdelhadi J, Shah R, Szendrey A, Holmes J. Real-World Observational Analysis of Clinical Characteristics and Treatment Patterns of Patients with Chronic Sialorrhea. Toxins. 2024; 16(8):366. https://doi.org/10.3390/toxins16080366
Chicago/Turabian StyleHast, Michael A., Amanda M. Kong, Jenna Abdelhadi, Rohan Shah, Andrew Szendrey, and Jordan Holmes. 2024. "Real-World Observational Analysis of Clinical Characteristics and Treatment Patterns of Patients with Chronic Sialorrhea" Toxins 16, no. 8: 366. https://doi.org/10.3390/toxins16080366
APA StyleHast, M. A., Kong, A. M., Abdelhadi, J., Shah, R., Szendrey, A., & Holmes, J. (2024). Real-World Observational Analysis of Clinical Characteristics and Treatment Patterns of Patients with Chronic Sialorrhea. Toxins, 16(8), 366. https://doi.org/10.3390/toxins16080366





