Combining Multiple Omics with Molecular Dynamics Reveals SCP2-Mediated Cytotoxicity Effects of Aflatoxin B1 in SW480 Cells
Abstract
1. Introduction
2. Result
2.1. Cell Viability
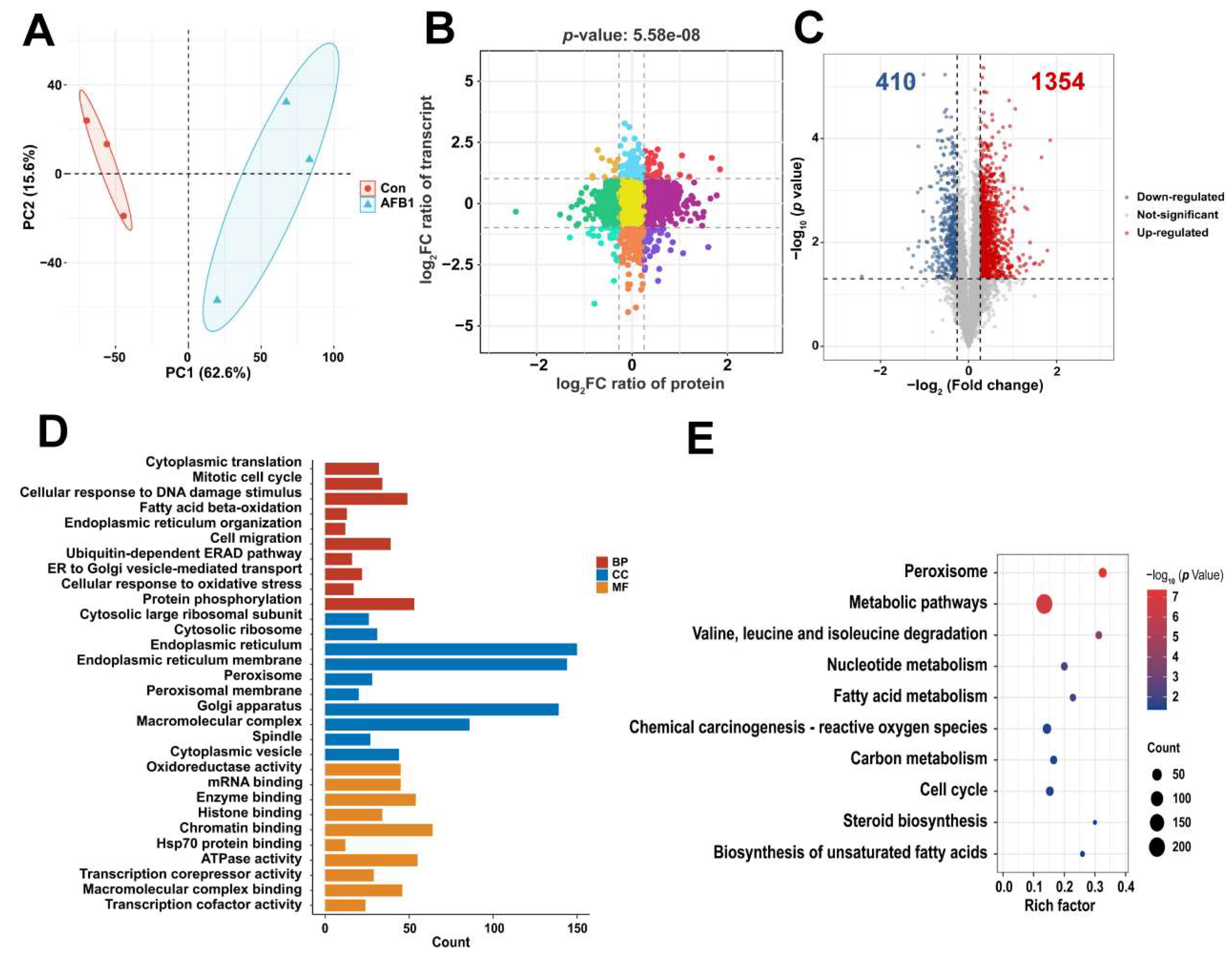
2.2. Transcriptomic Analysis Based on Differentially Expressed Genes (DEGs)
2.3. Proteomics Analysis Based on Differentially Expressed Proteins (DEPs)
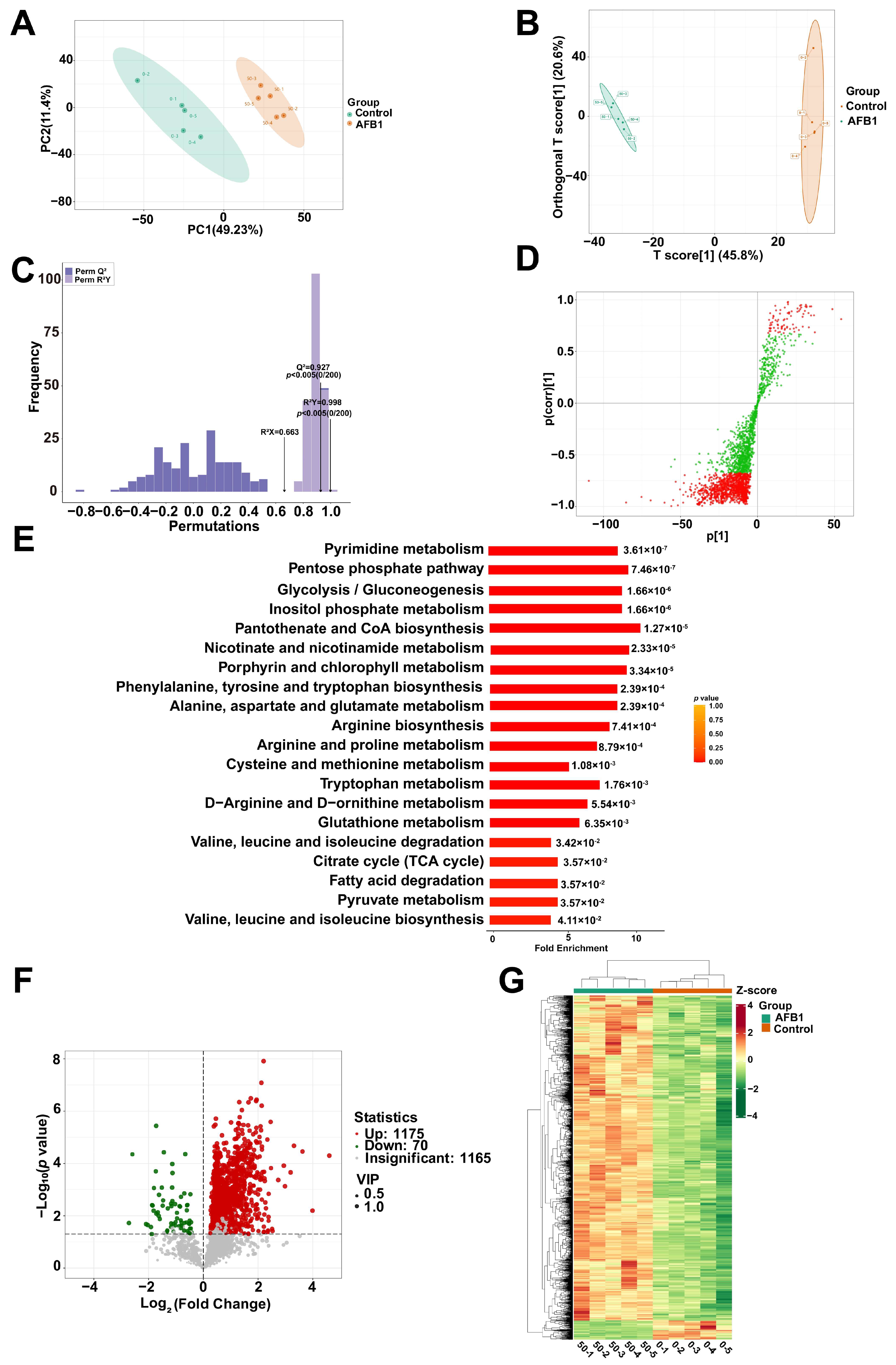
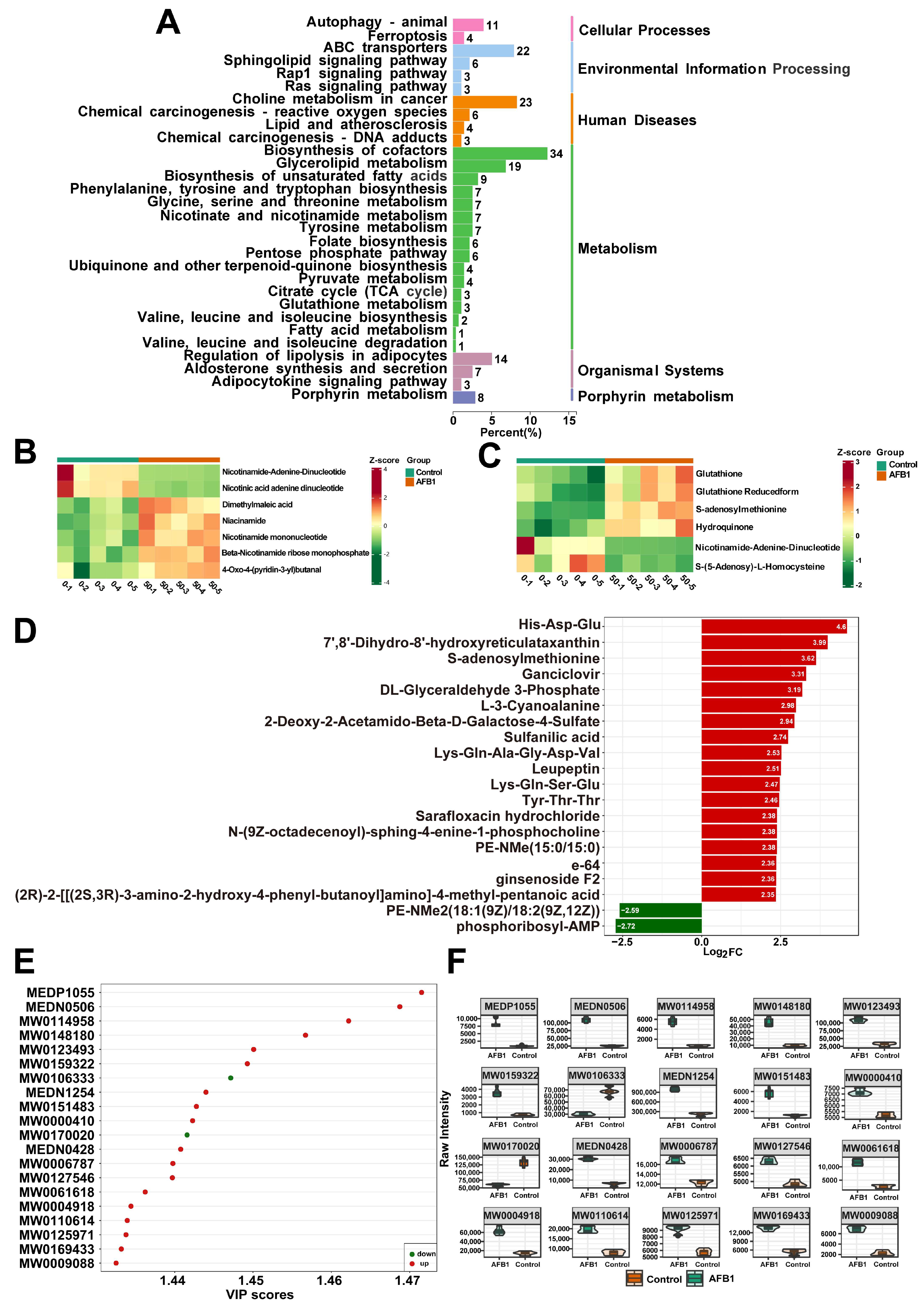
2.4. Analysis of the Metabolomics Data between the Group Treated with AFB1 and the Control Group
2.5. The Potential Target for AFB1 Toxicity
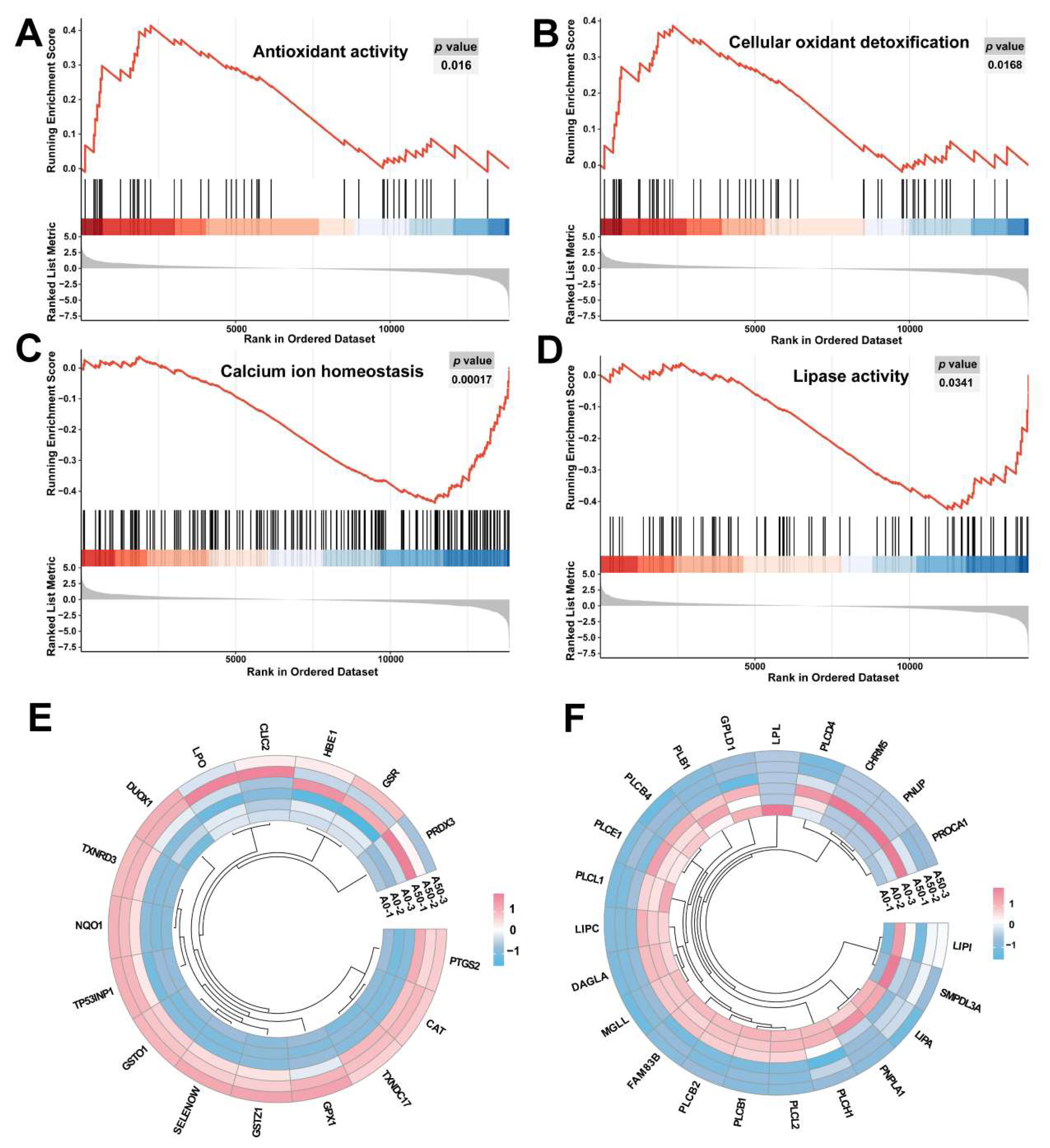
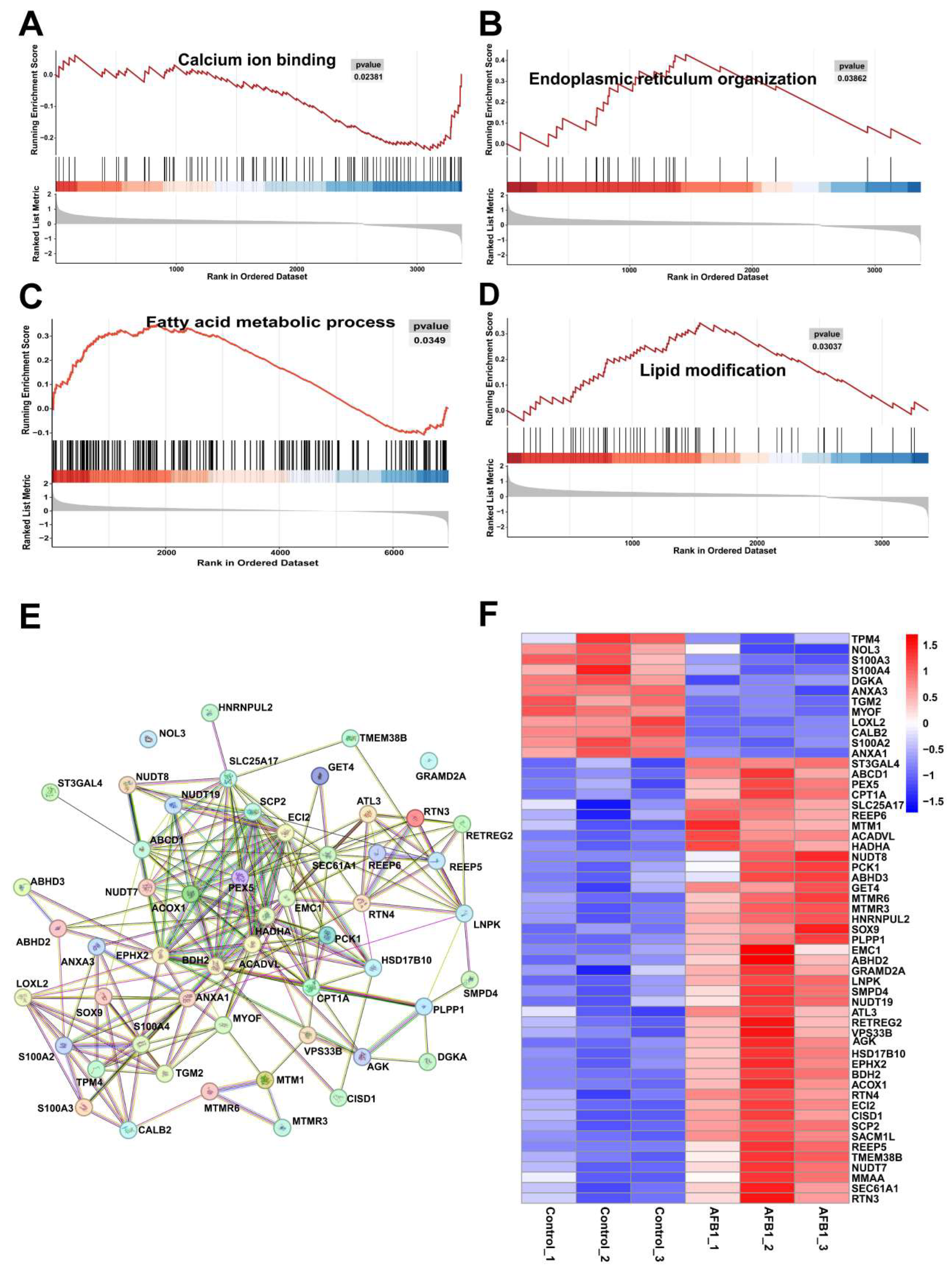
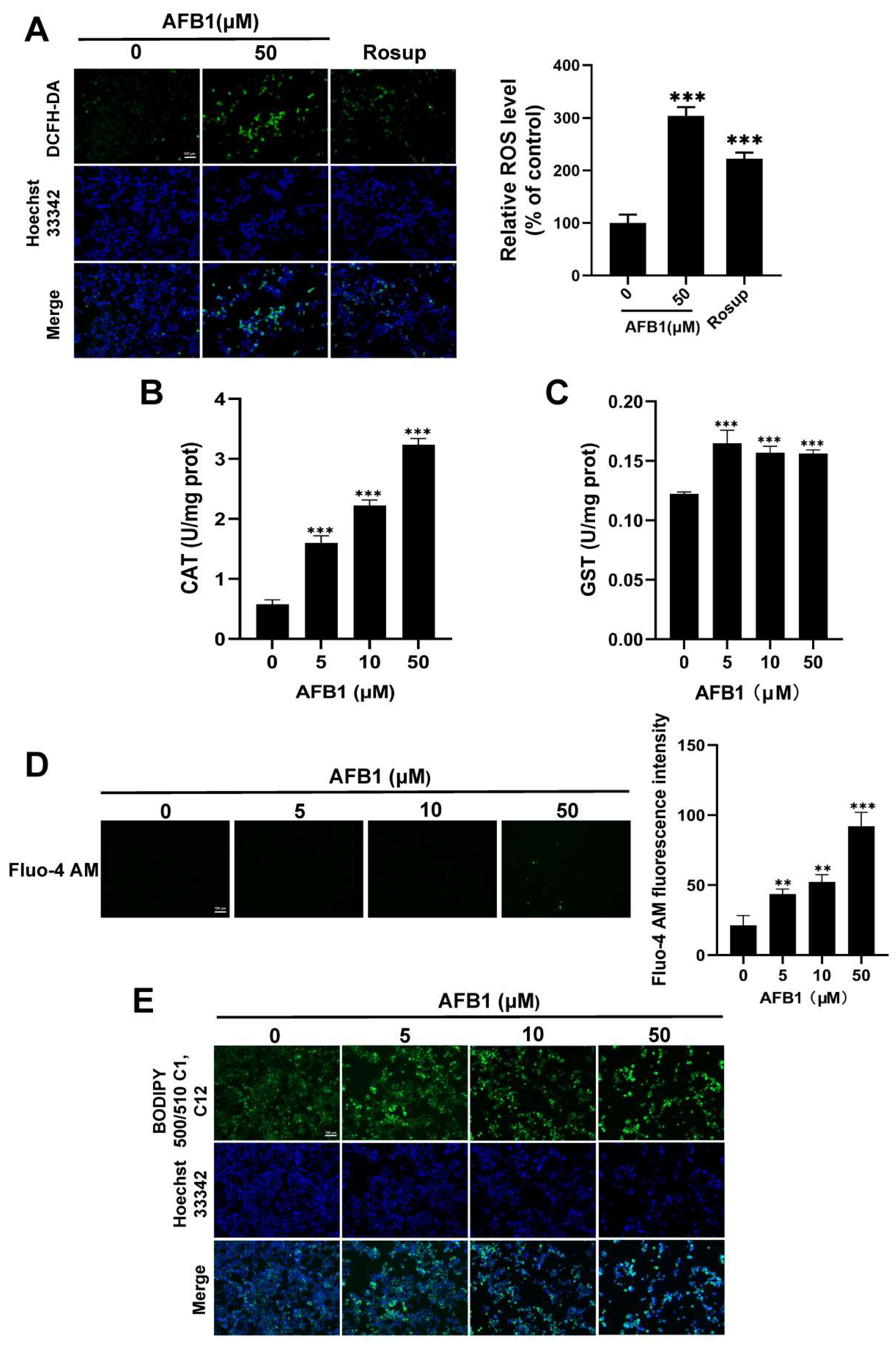
2.6. Oxidative Stress Induced by AFB1
2.7. The Antioxidant Enzyme Activity
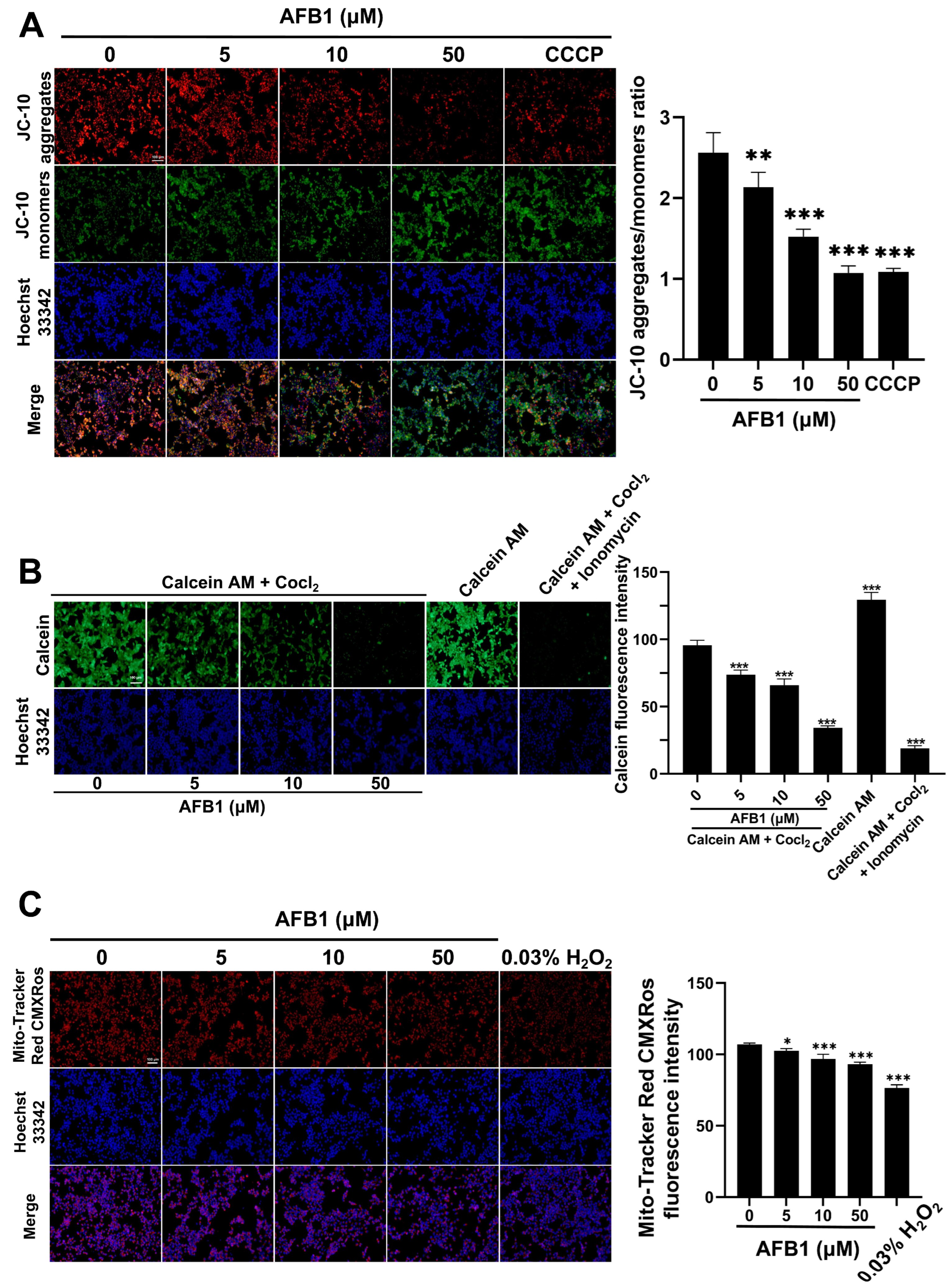
2.8. The Exposure to AFB1 Leads to Excessive Accumulation of Intracellular Ca2+
2.9. Fatty Acid Localization
2.10. AFB1-Induced Mitochondrial Dysfunction
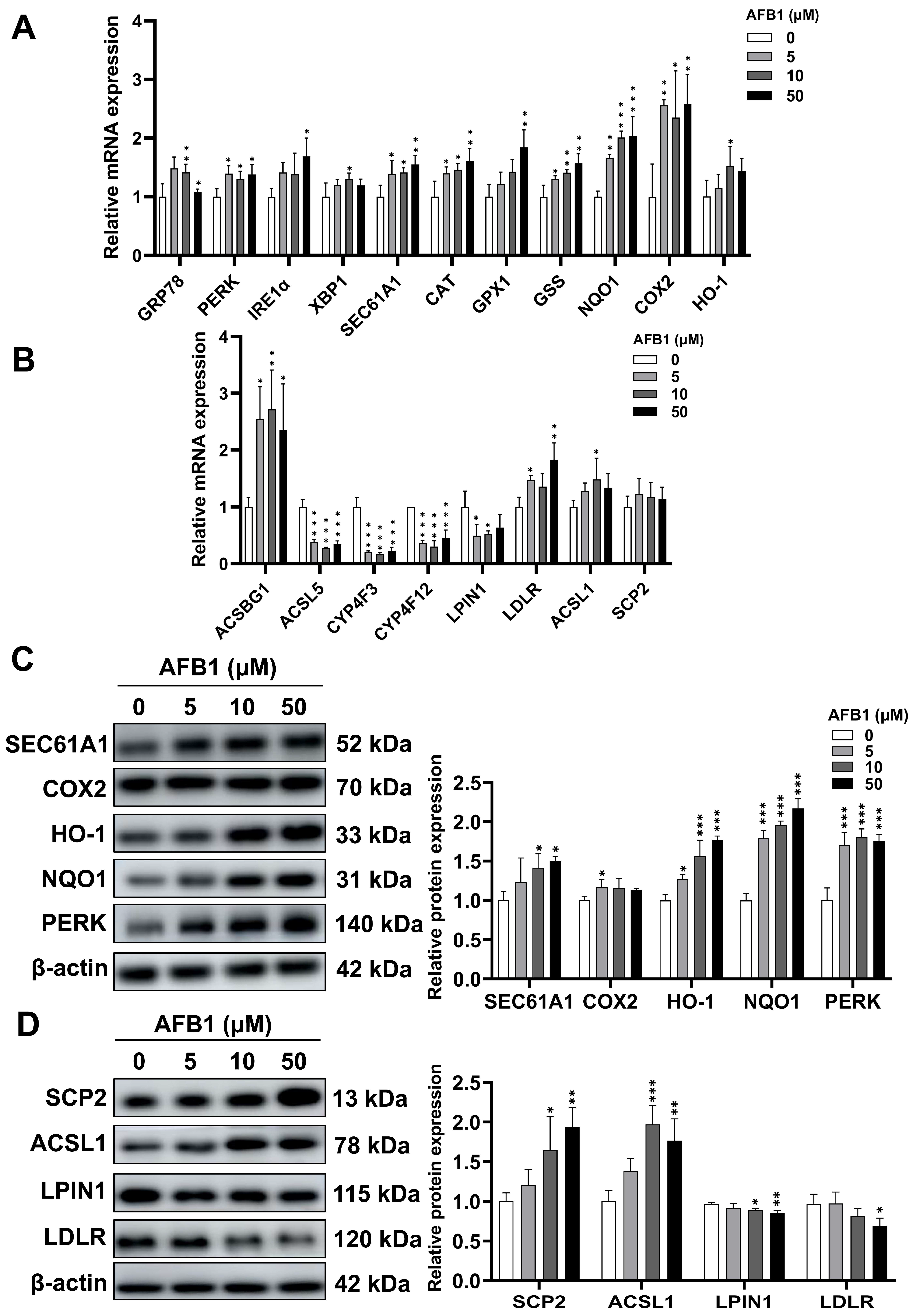
2.11. mRNA and Protein Expression Involved in Oxidative Stress and Lipid Signaling Pathways
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemical Reagent and Cell Culture
5.2. Cell Viability Assay
5.3. RNA Extraction and Transcriptomics
5.4. Tandem Mass Tag Proteomics Analysis
5.5. Sample Preparation and Analysis for Metabolomics
5.6. Molecular Docking
5.7. Molecular Dynamics (MD)
5.8. ROS Measurement by DCFH-DA
5.9. Assay of Catalase (CAT) and Glutathione S-Transferase (GST)
5.10. Measurement of Cellular Calcium
5.11. Fatty Acid Green Fluorescence Probe
5.12. Detection of Mitochondrial Membrane Potential (MMP)
5.13. Detection of Mitochondrial Permeability Transition Pore (mPTP)-Opening
5.14. Assessment of Mitochondrial Activity
5.15. RT-qPCR
5.16. Western Blotting
5.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raysyan, A.; Eremin, S.A.; Beloglazova, N.V.; De Saeger, S.; Gravel, I.V. Immunochemical approaches for detection of aflatoxin B1 in herbal medicines. Phytochem. Anal. 2020, 31, 662–669. [Google Scholar] [CrossRef]
- Sun, G.; Wang, S.; Hu, X.; Su, J.; Zhang, Y.; Xie, Y.; Zhang, H.; Tang, L.; Wang, J.S. Co-contamination of aflatoxin B1 and fumonisin B1 in food and human dietary exposure in three areas of China. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Yu, P.; Yang, K.; Cao, D. Aflatoxin B1: Metabolism, toxicology, and its involvement in oxidative stress and cancer development. Toxicol. Mech. Methods 2022, 32, 395–419. [Google Scholar] [CrossRef]
- Damiano, S.; Jarriyawattanachaikul, W.; Girolami, F.; Longobardi, C.; Nebbia, C.; Andretta, E.; Lauritano, C.; Dabbou, S.; Avantaggiato, G.; Schiavone, A.; et al. Curcumin supplementation protects broiler chickens against the renal oxidative stress induced by the dietary exposure to low levels of aflatoxin B1. Front. Vet. Sci. 2022, 8, 822227. [Google Scholar] [CrossRef]
- Crick, F. Central dogma of molecular biology. Nature 1970, 227, 561–563. [Google Scholar] [CrossRef]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [PubMed]
- Waterston, R.H.; Lindblad-Toh, K.; Birney, E.; Rogers, J.; Abril, J.F.; Agarwal, P. Initial sequencing and comparative analysis of the mouse genome. Nature 2002, 420, 520–562. [Google Scholar] [PubMed]
- Gibbs, R.A.; Weinstock, G.M.; Metzker, M.L.; Muzny, D.M.; Sodergren, E.J.; Scherer, S. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature 2004, 428, 493–521. [Google Scholar]
- Chimpanzee Sequencing and Analysis Consortium. Initial sequence of the chimpanzee genome and comparison with the human genome. Nature 2005, 437, 69–87. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, X.; Lin, Y.; Wu, C.; Zhang, J.; Chen, M.; Wen, J.; Wu, Y.; Tian, K.; Bao, W.; et al. Integrating epigenetics, proteomics, and metabolomics to reveal the involvement of Wnt/β-catenin signaling pathway in oridonin-induced reproductive toxicity. Toxics 2024, 12, 339. [Google Scholar] [CrossRef]
- Budayeva, H.G.; Kirkpatrick, D.S. Monitoring protein communities and their responses to therapeutics. Nat. Rev. Drug Discov. 2020, 19, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, Y.; Li, Y.; Li, H.; Gong, Y.; Wu, J.; Ning, Y.; Ding, C.; Xu, Y. Proteomic analysis of copper toxicity in human fungal pathogen Cryptococcus neoformans. Front. Cell. Infect. Microbiol. 2021, 11, 662404. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, S.; Bao, X.; Luo, C.; Yang, H.; Wang, J.; Zhao, S.; Zheng, N. Transcriptional and proteomic analysis revealed a synergistic effect of aflatoxin M1 and ochratoxin A mycotoxins on the intestinal epithelial integrity of differentiated human Caco-2 cells. J. Proteome Res. 2018, 17, 3128–3142. [Google Scholar] [CrossRef] [PubMed]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J. Mass spectrometry-based metabolomics: A guide for annotation, quantification and best reporting practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef]
- Guo, H.H.; Feng, C.L.; Zhang, W.X.; Luo, Z.G.; Zhang, H.J.; Zhang, T.T.; Ma, C.; Zhan, Y.; Li, R.; Wu, S.; et al. Liver-target nanotechnology facilitates berberine to ameliorate cardio-metabolic diseases. Nat. Commun. 2019, 10, 1981. [Google Scholar] [CrossRef] [PubMed]
- Wert, K.J.; Velez, G.; Kanchustambham, V.L.; Shankar, V.; Evans, L.P.; Sengillo, J.D.; Zare, R.N.; Bassuk, A.G.; Tsang, S.H.; Mahajan, V.B. Metabolite therapy guided by liquid biopsy proteomics delays retinal neurodegeneration. EBioMedicine 2020, 52, 102636. [Google Scholar] [CrossRef] [PubMed]
- Michaudel, C.; Danne, C.; Agus, A.; Magniez, A.; Aucouturier, A.; Spatz, M.; Lefevre, A.; Kirchgesner, J.; Rolhion, N.; Wang, Y.; et al. Rewiring the altered tryptophan metabolism as a novel therapeutic strategy in inflammatory bowel diseases. Gut 2023, 72, 1296–1307. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, L.; Wang, J.S. Assessment of the adverse impacts of aflatoxin B1 on gut-microbiota dependent metabolism in F344 rats. Chemosphere 2019, 217, 618–628. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Gao, Y.; Wang, J.; Zheng, N. Integrated metabolomics and lipidomics analysis reveals lipid metabolic disorder in NCM460 cells caused by aflatoxin B1 and aflatoxin M1 alone and in combination. Toxins 2023, 15, 255. [Google Scholar] [CrossRef]
- Harrison, J.C.; Carvajal, M.; Garner, R.C. Does aflatoxin exposure in the United Kingdom constitute a cancer risk? Environ. Health Perspect. 1993, 99, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Yuan, Q.; Yan, H.; Tian, G.; Chen, D.; He, J.; Zheng, P.; Yu, J.; Mao, X.; Huang, Z.; et al. Effects of chronic exposure to low levels of dietary aflatoxin B1 on growth performance, apparent total tract digestibility and intestinal health in pigs. Animals 2021, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Galano, M.; Venugopal, S.; Papadopoulos, V. Role of STAR and SCP2/SCPx in the transport of cholesterol and other lipids. Int. J. Mol. Sci. 2022, 23, 12115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, Q.; Wang, J.; Sun, J.; Xiang, Y.; Jin, X. Aflatoxin B1 disrupts the intestinal barrier integrity by reducing junction protein and promoting apoptosis in pigs and mice. Ecotoxicol. Environ. Saf. 2022, 247, 114250. [Google Scholar] [CrossRef]
- Romero, A.; Ares, I.; Ramos, E.; Castellano, V.; Martínez, M.; Martínez-Larrañaga, M.R.; Anadón, A.; Martínez, M.A. Mycotoxins modify the barrier function of Caco-2 cells through differential gene expression of specific claudin isoforms: Protective effect of illite mineral clay. Toxicology 2016, 353–354, 21–33. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, D.; Zhang, J.; Tang, H.; Li, F.; Peng, Y.; Duan, X.; Meng, E.; Zhang, C.; Zeng, T.; et al. Role of macrophage AHR/TLR4/STAT3 signaling axis in the colitis induced by non-canonical AHR ligand aflatoxin B1. J. Hazard. Mater. 2023, 452, 131262. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Bao, W.; Ren, J.; Li, C.; Chen, M.; Zhang, D.; Zhu, A. Integrated profiles of transcriptome and mRNA m6A modification reveal the intestinal cytotoxicity of aflatoxin B1 on HCT116 cells. Genes 2022, 14, 79. [Google Scholar] [CrossRef]
- Feng, C.; Bai, H.; Chang, X.; Wu, Z.; Dong, W.; Ma, Q.; Yang, J. Aflatoxin B1-induced early developmental hepatotoxicity in larvae zebrafish. Chemosphere 2023, 340, 139940. [Google Scholar] [CrossRef]
- Gao, Y.N.; Yang, X.; Wang, J.Q.; Liu, H.M.; Zheng, N. Multi-omics reveal additive cytotoxicity effects of aflatoxin B1 and aflatoxin M1 toward intestinal NCM460 cells. Toxins 2022, 14, 368. [Google Scholar] [CrossRef]
- Dai, T.; Xue, X.; Huang, J.; Yang, Z.; Xu, P.; Wang, M.; Xu, W.; Feng, Z.; Zhu, W.; Xu, Y.; et al. SCP2 mediates the transport of lipid hydroperoxides to mitochondria in chondrocyte ferroptosis. Cell Death Discov. 2023, 9, 234. [Google Scholar] [CrossRef]
- Mu, X.; Qi, S.; Wang, H.; Yuan, L.; Wang, C.; Li, Y.; Qiu, J. Bisphenol analogues induced metabolic effects through eliciting intestinal cell heterogeneous response. Environ. Int. 2022, 165, 107287. [Google Scholar] [CrossRef] [PubMed]
- Warth, B.; Raffeiner, P.; Granados, A.; Huan, T.; Fang, M.; Forsberg, E.M.; Benton, H.P.; Goetz, L.; Johnson, C.H.; Siuzdak, G. Metabolomics reveals that dietary xenoestrogens alter cellular metabolism induced by palbociclib/letrozole combination cancer therapy. Cell Chem. Biol. 2018, 25, 291–300. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, J.; Xu, Y.; Liao, G.; Jia, Q.; Pan, Y.; Wang, T.; Qian, Y. Integrated non-targeted lipidomics and metabolomics analyses for fluctuations of neonicotinoids imidacloprid and acetamiprid on Neuro-2a cells. Environ. Pollut. 2021, 284, 117327. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, C.; De Spiegeleer, M.; Van Meulebroek, L.; De Vos, W.H.; Vanhaecke, L. Validated comprehensive metabolomics and lipidomics analysis of colon tissue and cell lines. Anal. Chim. Acta 2019, 1066, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Surendran, P.; Stewart, I.D.; Au Yeung, V.P.W.; Pietzner, M.; Raffler, J.; Wörheide, M.A.; Li, C.; Smith, R.F.; Wittemans, L.B.L.; Bomba, L.; et al. Rare and common genetic determinants of metabolic individuality and their effects on human health. Nat. Med. 2022, 28, 2321–2332. [Google Scholar] [CrossRef]
- Wang, L.; Ahn, Y.J.; Asmis, R. Sexual dimorphism in glutathione metabolism and glutathione-dependent responses. Redox. Biol. 2020, 31, 101410. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef]
- Currie, E.; Schulze, A.; Zechner, R.; Walther, T.C.; Farese, R.V., Jr. Cellular fatty acid metabolism and cancer. Cell Metab. 2013, 18, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, H.; Lin, X.; Chen, M.; Bao, W.; Wu, Y.; Li, C.; Gao, Y.; Hou, S.; Yang, Q.; et al. Euphorbia factor L1 inhibited transport channel and energy metabolism in human colon adenocarcinoma cell line Caco-2. Biomed. Pharmacother. 2023, 169, 115919. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, X.; Bao, W.; Hong, X.; Li, C.; Lu, J.; Zhang, D.; Zhu, A. Effect of humantenine on mRNA m6A modification and expression in human colon cancer cell line HCT116. Genes 2022, 13, 781. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, J.; Wu, Y.; Chen, M.; Hu, H.; Gao, X.; Li, C.; Li, M.; Zhang, Y.; Lin, X.; et al. Gelsenicine disrupted the intestinal barrier of Caenorhabditis elegans. Chem. Biol. Interact. 2024, 395, 111036. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Bao, W.; Gao, Y.; Chen, M.; Wu, Y.; Wang, S.; Li, C.; Zhang, J.; Zhang, D.; Wang, Q.; et al. Integrated analysis of transcriptome and microRNA profile reveals the toxicity of euphorbia factors toward human colon adenocarcinoma cell line Caco-2. Molecules 2022, 27, 6931. [Google Scholar] [CrossRef] [PubMed]


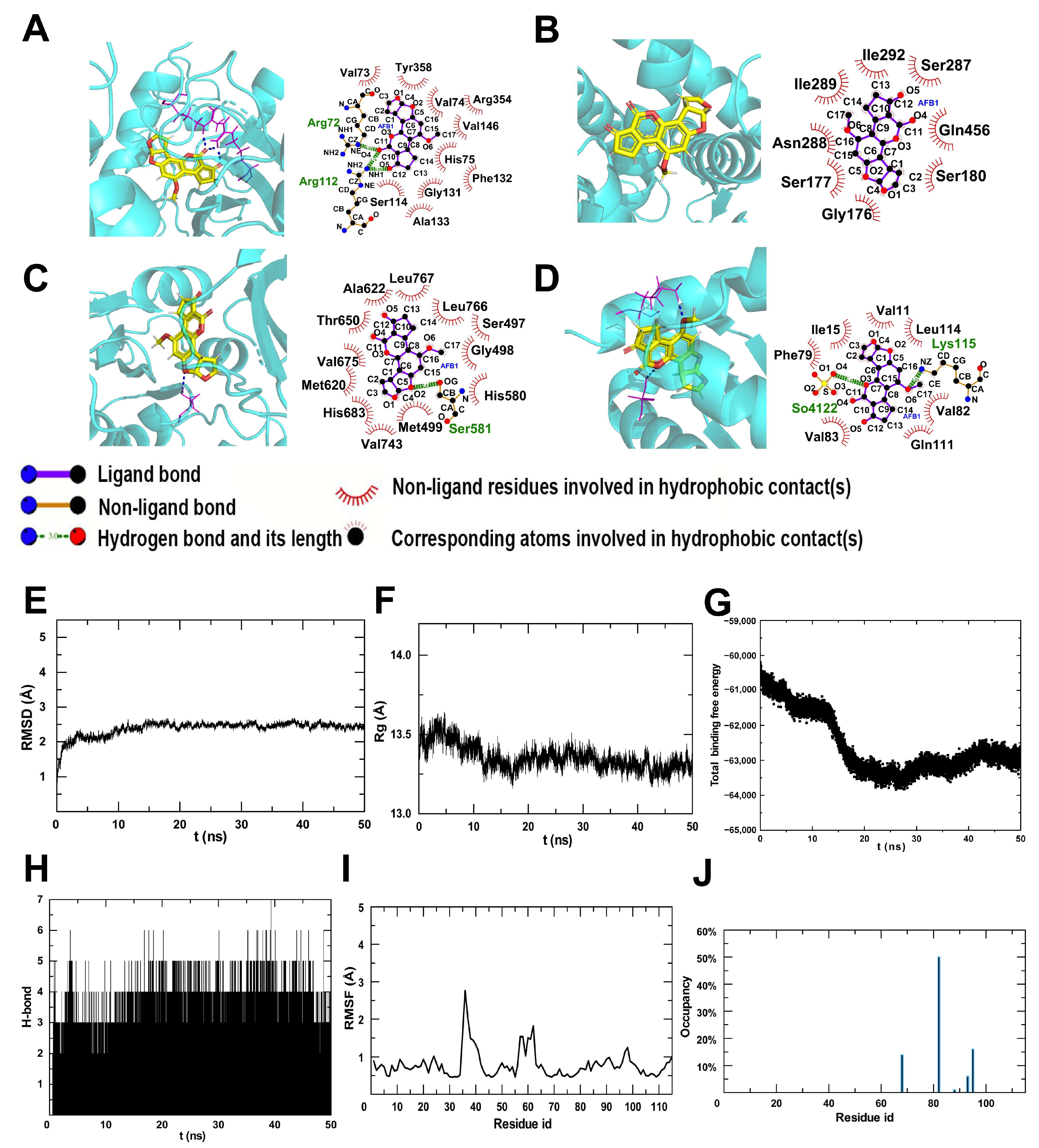
| Protein | PDB ID | Total Score | Crash | Polar | H-Bond Number | Residues Involved in H-Bond Formation | Hydrophobic Contact Number | Residues Involved in Hydrophobic Contacts |
|---|---|---|---|---|---|---|---|---|
| CAT | 8HID | 7.7967 | −0.2167 | 0 | 3 | Arg72, Arg112 (2 H-bonds) | 10 | Val73, Tyr358, Val74, Arg354, Val146, His75, Phe132, Gly131, Ser114, Ala133 |
| SEC61A1 | 8DNV | 5.6242 | −0.7089 | 2.9825 | 0 | − | 7 | Ile292, Ser287, Gln456, Ser180, Gly176, Ser177, Asn288 |
| ACSL1 | 2JFK | 5.5354 | −0.9486 | 1.9938 | 1 | Ser581 | 12 | Met620, His683, Val743, Met499, Thr650, Val675, Ala622, Leu767, His580, Ser497, Leu766, Gly498 |
| SCP2 | 1IKT | 5.1034 | −0.5708 | 1.8757 | 2 | So4122, Lys115, | 7 | Leu114, Val82, Gln111, Val83, Phe79, Ile15, Val11 |
| LPIN1 | 5WGB | 4.8557 | −0.6691 | 3.1075 | 2 | Tyr26, Arg34 | 5 | Val33, Thr415, Glu417, Thr30, Glu416 |
| NQO1 | 5EA2 | 4.24 | −0.6254 | 1.0619 | 2 | Lys113, Tyr126 | 4 | Tyr67, Phe120, Glu117, Phe178 |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| GRP78 | CAGTTGTTACTGTACCAGCCTA (F) | CATTTAGGCCAGCAATAGTTCC (R) |
| PERK | CCAGTTTTGTACTCCAATTGCA (F) | CAGATACAGCTGGCCTCTATAC (R) |
| IRE1α | CGTGAGCGACAGAATAGAAAAG (F) | GCTTCTTATTTCTCATGGCTCG (R) |
| XBP1 | CTTGTAGTTGAGAACCAGGAGT (F) | CCCAACAGGATATCAGACTCTG (R) |
| SEC61A1 | AATTTGAAGGTGCTATCATCGC (F) | ACGTATAGAAGAGCTTGATGGG (R) |
| CAT | GAGCACAGCATCCAATATTCTG (F) | CTCATTCAGCACGTTCACATAG (R) |
| GPX1 | GTTGCCTGGAACTTTGAGAAG (F) | CTCGATGTCAATGGTCTGGAAGG (R) |
| GSS | CTCTTTGACATCCACAAGCAAG (F) | AACATGTAGTCTGAGCGATTCA (R) |
| NQO1 | CAACCACGAGCCCAGCCAATC (F) | ACTCCACCACCTCCCATCCTTTC (R) |
| COX2 | TGTCAAAACCGAGGTGTATGTA (F) | AACGTTCCAAAATCCCTTGAAG (R) |
| HO-1 | CCTCCCTGTACCACATCTATGT (F) | GCTCTTCTGGGAAGTAGACAG (R) |
| ACSBG1 | AAACAAGATGGCCAATGTGTAC (F) | CGTGATATTGTCTTGACTCAGC (R) |
| ACSL5 | ATATGATGCTGAGAACCTAGGC (F) | CATGCTCCACACATTTGAGAAA (R) |
| CYP4F3 | AGAAGAAGGGGAGAGGAGGTTGTG (F) | GAGGCGGCAGCAGTTGTCATAG (R) |
| CYP4F12 | AAGGCATTGTCAGATGAGGATA (F) | TTCAATCTCTTTAGGATCGCGG (R) |
| LPIN1 | GAGCAGCAGAACTCTTCCTAAT (F) | CTTTTGCAATCTACCAGGCTAC (R) |
| LDLR | CTGTAGGGGTCTTTACGTGTTC (F) | GTTTTCCTCGTCAGATTTGTCC (R) |
| ACSL1 | CTGTGTCATGGAGCTAAAATCG (F) | GTGTTTGCTTGTCCGAAAATTC (R) |
| SCP2 | GGAGCTGAGAATTCAAGAGACT (F) | AGTCACCAAAAACATAGCCAAC (R) |
| β-ACTIN | GCACTCTTCCAGCCTTCCTTCC (F) | GCGGATGTCCACGTCACACTTC (R) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Wen, J.; Qiu, Y.; Gao, X.; Zhang, J.; Lin, Y.; Wu, Z.; Lin, X.; Zhu, A. Combining Multiple Omics with Molecular Dynamics Reveals SCP2-Mediated Cytotoxicity Effects of Aflatoxin B1 in SW480 Cells. Toxins 2024, 16, 375. https://doi.org/10.3390/toxins16090375
Chen M, Wen J, Qiu Y, Gao X, Zhang J, Lin Y, Wu Z, Lin X, Zhu A. Combining Multiple Omics with Molecular Dynamics Reveals SCP2-Mediated Cytotoxicity Effects of Aflatoxin B1 in SW480 Cells. Toxins. 2024; 16(9):375. https://doi.org/10.3390/toxins16090375
Chicago/Turabian StyleChen, Mengting, Jiaxin Wen, Yiyan Qiu, Xinyue Gao, Jian Zhang, Yifan Lin, Zekai Wu, Xiaohuang Lin, and An Zhu. 2024. "Combining Multiple Omics with Molecular Dynamics Reveals SCP2-Mediated Cytotoxicity Effects of Aflatoxin B1 in SW480 Cells" Toxins 16, no. 9: 375. https://doi.org/10.3390/toxins16090375
APA StyleChen, M., Wen, J., Qiu, Y., Gao, X., Zhang, J., Lin, Y., Wu, Z., Lin, X., & Zhu, A. (2024). Combining Multiple Omics with Molecular Dynamics Reveals SCP2-Mediated Cytotoxicity Effects of Aflatoxin B1 in SW480 Cells. Toxins, 16(9), 375. https://doi.org/10.3390/toxins16090375






