Abstract
Background: Tremor is the most common movement disorder, with significant functional and psychosocial consequences. Oral medications have been disappointing or limited by side effects. Surgical techniques are effective but associated with risks and adverse events. Botulinum toxin (BT) represents a promising avenue but there is still no double-blind evidence of efficacy on upper limb function. A systematic review on the effects of BT in upper-limb tremor was conducted. Methods: A systematic search of the literature was conducted up to July 2023, including the keywords “botulinum toxin” and “tremor”. All randomized controlled trials (RCTs) and open-label studies were analyzed. Independent reviewers assessed their methodological quality. Results: There were only eight published RCTs and seven published open-label studies, with relatively small sample sizes. This review suggests that BT is more effective when injections are patient-tailored, with analyses based on clinical judgement or kinematics. Subjective and objective measures frequently improve but transient weakness may occur after injections, especially if wrist or fingers extensors are targeted. A number of studies had methodological limitations. Conclusions: The authors discuss how to optimize tremor assessments and effects of BT injection. Controlled evidence is still lacking but it is suggested that distal “asymmetric” BT injections (targeting flexors/pronators while sparing extensors/supinators) and proximal injections, involving shoulder rotators when indicated, may avoid excessive weakness while optimizing functional benefit.
Keywords:
tremor; botulinum toxin; ataxia; cerebellar; essential tremor; dystonic tremor; resting tremor; action tremor Key Contribution:
In a few published studies of patients with upper limb tremor, botulinum toxin appears to be associated with frequent improvement when injections are individualized, although transient weakness may occur. The authors discuss how to optimize tremor assessment and the effects of botulinum toxin injection.
1. Introduction
Tremor, defined as an involuntary and rhythmic oscillatory movement of any body part, is the most common movement disorder in adults, and typically predominates in upper limbs [1]. Tremors are classified into two main categories [2,3]. The first is resting tremor, which is not voluntarily activated and is assessed when the affected part of the body is relaxed, if possible with full support against gravity. The second is action tremor, which occurs during—and is triggered by—a voluntary action. Action tremor can be “postural” when tremor is triggered by the action of maintaining a position (zero speed) against gravity (postural tremor) or “kinetic” (with speed) when it is only triggered by movement (kinetic tremor) [2].
Essential tremor is a highly common action tremor. It is estimated to affect about 5% of the population over the age of 65 and 22% over the age of 95 [4,5]. Other action tremors may arise in all sorts of disorders involving cerebellar circuits [2,6], such as in multiple sclerosis where tremor is estimated to occur in 25% to 58% of cases [7,8,9]. Resting upper-limb tremor occurs in most patients with Parkinson’s disease, a condition estimated to affect 1% of individuals over the age of 60 [10,11]. Tremor that develops in a part of the body affected by dystonia may be called “dystonic tremor” [3].
Functional disturbances associated with tremor are profound: in a survey of more than 1500 patients with essential tremor, 89% reported difficulty with drinking, 85% with eating, and 75% with writing. In addition, 61% reported professional impact, with 13% having lost their job and 32% having changed their job [12]. Psychosocial hardship is also a serious issue: social phobia was found in 43% of participants and rates of antidepressant prescriptions were six times higher than in the general population [12]. In multiple sclerosis specifically, tremor is one of the most disabling symptoms, negatively affecting quality of life. Its severity correlates with disability and unemployment rate [7,13].
Given the above-mentioned frequency and functional impact of tremor, its treatment is of crucial importance. Avoidance of tremorogenic agents and a number of synaptic depressors has not been systematically evaluated to our knowledge. Promising physical techniques such as resistance or accuracy training programs have shown efficacy over very small series. This is an area of work that remains mostly unknown to the neurology community [14]. As for oral medications for essential tremor, there is a 30% non-response rate to drug treatment during the first year, and if treatment is pursued, a third of patients interrupt it during the second year due to lack of efficacy and/or adverse events (AEs) (notably sedation, falls with head trauma or fracture, cognitive impairment, bradycardia, syncope, severe anxiety, and suicidal ideation) [15,16]. It is worth noting that the two drugs that are still most commonly recommended (propranolol and primidone) were discovered in the 1970s and 1980s [15].
In Parkinson’s disease’s resting tremor, treatment is essentially based on dopaminergic agents, with the well-known fluctuations in efficacy and adverse events. Deep brain stimulation and functional lesions such as gamma-knife and focused ultrasounds may be highly effective but are also associated with risks. These procedures remain invasive and should be performed by well-trained teams [17].
In the past three decades, botulinum toxin injections have triggered growing interest as a treatment for tremor. Although patients might find this solution to be more satisfying than oral medications, it is still not widely offered in current practice. Indeed, 4% of patients with essential tremor benefit from botulinum toxin therapy in France, vs. 93% for systemic synaptic depressors such as propranolol, primidone, or benzodiazepines [12].
In this systematic review, we aimed to provide an update on the clinical trials that have focused on botulinum toxin injections to treat upper-limb tremor, and to discuss the perspectives to improve clinical practice.
2. Results
This systematic literature search resulted in 117 findings including 80 duplicates, yielding 37 publications. After screening, 21 manuscripts were excluded because they did not refer to tremor and/or did not target the upper limb or were not in the English or French language (Figure 1). After reviewing all 16 remaining manuscripts, 1 was excluded from the analyses because it was ultimately not considered relevant (a case series of two Parkinson’s disease and five essential tremor patients that was discussing the transition from a unilateral to a bilateral botulinum toxin injection pattern) [18]. In the end, 15 studies were collected for analysis.
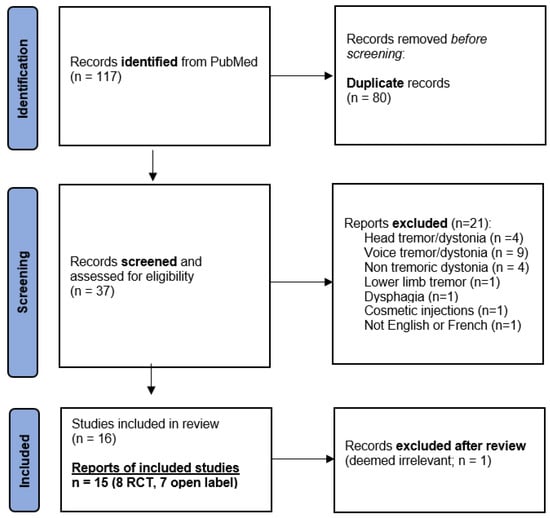
Figure 1.
Flowchart of study selection.
2.1. Data from RCTs
Findings from RCTs are presented in Table 1 and Table 2. In July 2023, only eight RCTs were found in the literature regarding botulinum toxin injections to treat upper-limb tremor.

Table 1.
Randomized controlled trials—part 1.

Table 2.
Randomized controlled trials—part 2.
Four studies referred to essential tremor. Among them, two early trials used a fixed-dose, fixed-muscle approach (both omitting pronators and using only wrist flexor and extensor injection) with mitigated results, particularly in terms of functional performance. Indeed, the first study found improvement in tremor severity on a four-point patient-reported subjective scale and in accelerometry, but no improvement in functional rating scales. The second study found a dose-dependent improvement in a patient-reported subjective scale, but not in writing, working, social embarrassment, or anxiety [19,20]. The other two studies used case-customized methodology, potentially guided by kinematic analyses. They had more compelling results on tremor severity scores such as the Fahn Tolosa Marin Clinical Rating Scale for Tremor (FTM) or the National Institutes of Health Collaborative Genetic Criteria for tremor severity (NIHCGC), patient global impression of change (PGIC), or accelerometric tremor amplitude measurements up to 8 weeks after botulinum toxin injections [21,22]. Of note, a minority (n = 30) of the enrolled participants were pre-evaluated using kinematics to analyze agonist/antagonist muscle patterns before injection [22].
All studies reported on excessive weakness, even though one report claimed that this was no greater than in the placebo group (although it should be noted that in that study at least one patient was excluded from the analyses because of “excessive weakness”) [21]. One study found weakness to be more prevalent in the “high dose” group compared with the “low dose” group [20].
In multiple sclerosis, two RCTs evaluated injection effects according to a customized approach, targeting agonist and antagonist muscles depending on the tremor pattern: the Bain score was improved for up to 12 weeks, as were writing and Archimedes spirals, but quality-of-life scores were not [23,24]. Interestingly, in one of these studies, functional MRI activation within two previously identified areas was measured at baseline and 6 weeks: activation was reduced in the ipsilateral inferior parietal cortex in the BT group but not in the placebo group, with correlation with the reduction in tremor severity; activation was unchanged in the premotor/supplementary motor cortex [24]. Excessive weakness was again found in both multiple sclerosis studies [23,24].
Only one RCT evaluated the effects on Parkinson’s disease’s resting tremor, with an individualized injection pattern based on clinical judgement showing an improvement in all assessment criteria (UPDRS III items for resting tremor and for action tremor, NIHCGC, PGIC, and quality of life) for up to 8 weeks [25]. Weakness was detected using an ergometer in 37% of patients with Parkinson’s disease compared with 22% of control subjects [25]. Lastly, a single clinical study was carried out on “dystonic” tremor, using injections based on clinical judgement, with mixed results: improvement was found in the FTM total score but not in its functional sub-score, with no improvement in the writer’s cramp rating scale or in accelerometry performed on the index finger. Cases of troublesome weakness were found despite the low doses used [26].
Of the eight RCTs, three were cross-over studies with wash-out periods of 12 to 16 weeks that could be viewed as short, as BT—which has been particularly examined for abobotulinumtoxinA—may retain efficacy beyond 24 weeks [27,28]. A total of 351 subjects were included in these RCTs, including 133 in a study, of arguable real-life relevance, in which a fixed dose was injected only into the wrist flexors and extensors of patients with essential tremor [20]. Levels of Evidence ranged from 2.5 (a well-designed RCT, but with few patients and not multi-site) to 3 (questionable randomization) [29]. The CASP for RCTs was considered “moderate” for all studies, as recruitment was adequate, but the results could not be considered robust due to questionable methods and/or the limited number of subjects included [30].
2.2. Data from Open-Label Studies
Findings from the seven open-label studies selected are presented in Table 3 and Table 4. Among the seven articles, five investigated the effects of botulinum injections in essential tremor. One of these studies used a fixed-dose, fixed-muscle injection pattern in wrist flexors and extensors [31]. The other studies used a more personalized approach, with EMG or kinematic analyses [32,33,34,35]. Among those, three targeted muscles from wrist to shoulder (thus avoiding finger flexors and extensors) [33,34,35], and one deliberately avoided wrist extensors (in order to “avoid exaggerated weakness”) [32]. Interestingly, the published kinematic analyses used motion sensor devices including three goniometers and one torsiometer placed over the forearm, wrist, elbow, and shoulder joints during two postural tasks and two weight-bearing tasks; at the shoulder level, these kinematic analyses assessed shoulder flexion–extension and abduction–adduction, but it is not clear whether they also considered shoulder rotation [33,34,35]. All of these studies reported worthy improvements in essential tremor, on various severity scores (mainly FTM) and also on function (ADLS), accelerometry, and quality-of-life scores (QUEST). Improvement duration reached 96 weeks in one long-term study in which injections were repeated every 16 weeks [35]. All of these investigations also reported weakness, particularly affecting finger and wrist muscles.

Table 3.
Open-label studies—part 1.

Table 4.
Open-label studies—part 2.
The two remaining reports addressed mixed populations of patients with essential tremor, Parkinson’s disease, and “cerebellar disorders” [36,37]. One early study used an injection pattern that has become uncommon today, involving “booster” injections every 10–14 days for the first month, and reported subjective improvement in patients (PGIC) but no improvement in quantitative tests, with 60% of patients showing “excessive” weakness [36]. The other more recent report used a customized approach guided by kinematics, with improvement in all parameters (FTM, kinematics, and quality-of-life scores) but excluded a number of cases from the analyses—from 8 to 14%—because of “lack of improvement” or “bothersome weakness” [37].
Across these seven open-label studies, the cumulative number of patients included was 185. Ten were not counted because of redundancy in two studies [35,37]. Levels of Evidence ranged from 4 (well-designed cohort studies) to 6 (single descriptive or qualitative studies).
3. Discussion
This systematic review of the literature available since the emergence of botulinum toxins in the 1980s found few trials and even fewer RCTs published on the topic of botulinum toxin injections for treating upper-limb tremor. This review suggests that botulinum toxin is more effective when injections are individualized to the patient, as opposed to a fixed dose injected to fixed muscles. Such individualization may rely on the clinical judgement of an experienced clinician, on kinematic analysis, on surface electromyography, or on a combination of the above. Overall, subjective and objective improvements are often reported on tremor severity scores, functional scores, impressions of clinical improvement and quality-of-life scores, and also on quantitative kinematic measurements. However, transient and troublesome weakness remains a common issue after injection in these studies, and it is reasonable to assume that this is one of the reasons why injections for tremor are not more commonly used in current practice. We may also assume that another reason might be an impression of technical difficulty that such injections could give to some injectors.
The level of evidence is suboptimal as there are few published studies (eight RCTs, seven open-label studies), and a moderate cumulative number of subjects included (546 analyzed in this review) suffering from at least four different disorders. Methodological limitations included low sample sizes; lack of standardized methods for assessing efficacy and for measuring weakness, precluding rigorous comparisons between toxins, doses, and dilutions; lack of multicentric protocols (only in one study); and few intention-to-treat analyses, often with patients being excluded from the analyses because of injections deemed “ineffective” or because there was “exaggerated weakness”. In addition, only a few studies directly evaluated the overall patient’s point of view, as in the Patient Global Impression of Change (PGIC) [21,22,25,26]. This is unfortunate as self-detection of tremor in daily life activities may be more readily captured by such a tool than by other assessment methods.
3.1. Botulinum Toxin in Parkinson’s Resting Tremor
Resting tremor likely occurs as a result of central oscillatory activity, particularly in the basal ganglia under the influence of dopamine [6,38]. In this type of central dysfunctional pattern, it might be challenging to address tremor without weakening the muscle. Only one RCT has been conducted in patients with Parkinson’s disease [25]. Although findings were encouraging (Table 1), many clinicians remain hesitant to use botulinum toxin in Parkinson’s disease resting tremor due to uncertain efficacy and to the risk of induced weakness. It is important to remember that parkinsonian tremor often predominates distally in forearm muscles (wrist and fingers), and that, compared to proximal arm or shoulder muscles, induced weakness in these muscles may be readily perceived by the patient when injected with botulinum toxin. Yet, some specialists continue to perform botulinum toxin injections for Parkinson’s disease tremor, with interesting anecdotal results, and the field certainly deserves more high-quality investigations.
3.2. Botulinum Toxin in Action “Cerebellar” Tremors
When active posture or action is required, the cerebellum receives information about the anticipated movement from descending commands elaborated in the premotor cortices and transmitted along corticopontine pathways. In parallel, the cerebellum receives feedback from the ascending spinocerebellar tracts about the actual movements currently processed. These two pieces of information are compared within the cerebellar cortex and then corrected via the efferent cerebellar pathways [6,39,40]. When aiming at a target, this command correction is responsible for movement slowing or braking through contraction of the stretched antagonist muscle to avoid overshooting. Malfunction in these circuits can delay antagonist correction, which delays deceleration and results in hypermetria [39]. The cerebellum finally corrects the movement in the opposite direction to return to the target. Delays in antagonist corrections reiterate, and new reverse hypermetric movements are produced. As a result, there may be continuous overshooting and limb oscillation around the target, typical for ataxia or “cerebellar tremor” [39,40].
There is now growing evidence in the literature that “essential tremor” is likely a degenerative disorder predominantly affecting the cerebellum [41,42,43,44,45,46,47,48]. As reviewed above, feedback from the muscle plays an important part in fostering and maintaining this tremor, and a muscle-specific procedure such as botulinum toxin injection may reduce spindle afferent activity, regardless of contraction reduction [49] and may find its best indication there.
Botulinum toxin injected into muscle may restore reciprocal inhibition from the injected muscle to the non-injected muscle, through Renshaw blockade at the origin of the injected axon and through likely concurrent action on the extrafusal and intrafusal motor end-plates, the latter resulting in decreased spindle afferent input [49,50,51,52,53]. It is also likely that botulinum toxin exerts additional central actions, possibly relevant in tremors to damp oscillatory activities [54]. Since muscle afferent input influences central motor structures, such as the motor cortex, thalamus, and cerebellum, it is reasonable to assume that reduced input to these structures might lead to a reduction in oscillatory activity, and therefore, in tremor [31,49,55]. Recently, a study on tremor in multiple sclerosis demonstrated significant reduction in neural activation within the ipsilateral inferior parietal lobule in fMRI, after botulinum toxin injection [24]. Notably, the ipsilateral inferior parietal lobule plays a role in sensorimotor prediction and is a target of cerebellar efferents [56,57,58].
3.3. Botulinum Toxin in Dystonic Tremors
The pathophysiology of dystonic tremor remains unclear. Since the new syndromic understanding of the term dystonia promoted by Marsden and Fahn in the late 1970s [59,60], the scientific community has struggled to come up with a straightforward and universally accepted definition of dystonia [61,62]. In that context, it would appear that up to 50% of patients with dystonia develop alternating movements in the dystonic body area, a condition that has been termed dystonic tremor [63]. Given the established efficacy of BT injections in focal dystonia and in the treatment of other tremor types, BT for dystonic tremor has received surprisingly little attention from the scientific community, with only one published placebo-controlled trial to date (referred to in Table 1 and Table 2) [26]. The effectiveness of BT injections was particularly marked in that trial.
3.4. Going Further
The authors of this review believe that botulinum toxin offers strong promise for patients with tremor and should attract greater interest from clinicians and the scientific community.
3.4.1. Comments and Suggestions for Best Practice
The following represents advice from the experience of the authors following this literature review:
- –
- Injecting finger muscles may cause troublesome weakness of grip and should be avoided whenever possible.
- –
- Movements of elbow pronation–supination seem to be those most often found in all activity tremors (essential tremors and other cerebellar tremors) and pronator muscles should thus not be omitted when planning for the injection.
- –
- Involvement of proximal muscles should not be underestimated, particularly in action tremors. Rotation movements of the shoulder are frequent in cerebellar tremors. Tests to assess this issue include bringing a glass from the table to the mouth, “finger-to-nose” movements, or the posture with elbows bent and fingertips close in the opposition. Unfortunately, there are no studies in the literature targeting these muscles, although anecdotal experience of the authors has shown promising results when targeting the pectoralis major, subscapularis, and/or teres major. In addition, patients with deep brain stimulation (DBS) often have a better outcome with respect to the distal components of tremor and may keep a persistent, troublesome proximal tremor, which could be a good indication for combining DBS with injections of botulinum toxin.
- –
- When deciding on the dose of botulinum toxin, caution should be used as weakness is dose-dependent [20].
- –
- To avoid diffusion to adjacent muscles, especially the muscles of the forearm and hands, it is better to not overdilute the toxin (e.g., one may use 100–200 U/mL for incobotulinumtoxin or onabotulinumtoxin; 300–500 U/mL for abobotulinumtoxin).
- –
- Doses: The total dose used per muscle and per injection session likely plays a role in the outcome but these differed among investigators. There are currently no consensus recommendations regarding the doses to be used in the treatment of the various types of tremor. From the overall literature in the field cited here and from our experience, we recommend using the following ranges of doses for a first injection, depending on the muscle size and trophic state: for abobotulinumtoxin, 60–100 U/forearm muscle, 140–200 U/arm or shoulder muscle, without exceeding 300–500 U/whole of a single upper limb; for incobotulinumtoxin or onabotulinumtoxin, 30–50 U/forearm muscle, 70–100 U/arm or shoulder muscle, without exceeding 150–200 U/whole of a single upper limb.
- –
- Injection techniques: As each has its advantages and limitations, a combination of the following BT injection techniques may be an ideal approach:
- –
- Electromyography-guided injection makes it possible to hear the bursts of muscle contraction when the tremor occurs [64,65];
- –
- Electrical stimulation is precious as this is the sole technique ensuring that the functional effect of the stimulated muscle indeed corresponds to the tremor movements; however, it can be difficult to distinguish between contractions due to stimulation and those due to the tremor [64,65];
- –
- Ultrasound helps in ensuring that the needle is in the targeted muscle and sometimes makes it possible to see the contraction during the tremor, but it does not make it possible to know if the BT is injected to a muscle area actually causing the tremor movements, which could mitigate its effectiveness [66].
Using anatomical landmarks is the least effective strategy, as it has been shown to be overall less effective than the above-mentioned techniques [64]. Finally and importantly, the success of each of these techniques is highly dependent on the experience and skill of the clinician using it; therefore, all the published comparisons have been biased by the respective skills of the individual investigators in mastering each of the techniques [64,65,66].
3.4.2. An “Asymmetric” Injection Pattern?
Currently, both in clinical practice and in the literature, botulinum toxin injections often target agonist and antagonist muscles. When injections are guided by kinematic analysis or surface electromyography, injections tend to be more “asymmetric” (targeting the flexor/pronator more than the extensor/supinator compartment), although studies to date have not established this strategy [67,68].
The authors of this review inject botulinum toxin asymmetrically, targeting agonist pronators or flexors only, in the forearm, as extensors or supinators might be particularly disabling when they become weaker [67,68]. Following the above-mentioned concept of the muscle acting in a neuro-muscular loop both as the origin of sensory afferents towards antagonist motoneurons and as the motor effector, it does not seem necessary to interrupt the loop from both sites (agonist and antagonist). Obviously, this concept of “asymmetric” injections needs to be demonstrated in well-designed clinical trials.
3.4.3. Neurorehabilitation Techniques as an Adjunct to Botulinum Toxin Injections
Neurorehabilitation techniques are currently not widely used for tremor. Yet, when patients with essential tremor are asked the question: “If you were to design a comprehensive approach/ideal clinical center for the treatment of tremor, what problems other than tremor would you focus on?”, the second most common answer is “physiotherapy or occupational therapy in order to help with self or personal care or personal hygiene” (29%) [16]. Considering the “potential importance of specialists in the treatment of patients with essential tremor”, 62% of respondents considered the physiatrist or physical therapist to be “very important” or “essential” [16].
We here suggest that adding a neurorehabilitation protocol to toxin injections may prove useful, particularly for patients with essential or other cerebellar types of tremor. Two rehabilitation techniques have shown promise in that population: accuracy training and motor-strengthening techniques. Accuracy training is effective for cerebellar disorders, including essential tremor [69,70,71]. Simple accuracy exercises performed regularly, even daily, as part of a self-rehabilitation program could significantly improve movement control if more widely prescribed [72]. Motor-strengthening programs have also produced encouraging results in essential tremor and certainly deserve to be investigated in larger controlled trials [14,73,74,75,76]. Evidence for the rehabilitation of dystonic tremor is still lacking.
Taking all these aspects into account, much still needs to be done to improve knowledge about BT injections for tremor. There is a need for some form of uniform upper-limb tremor botulinum injection protocol usable for high-quality RCTs, with the objective to improve muscle targeting and the selection of dose and dilution to maximize efficacy and avoid exaggerated weakness, while ensuring an individualized approach for each patient. Improving these aspects could involve the use of kinematics. By analyzing angular movements applied to each joint in different tasks, these tools may allow objective identification of the muscles to be targeted, as well as tremor monitoring after BT injection [33,34,35]. This could be supported by automated algorithms, albeit under investigator control. While visual assessment remains key and the experience of the examiner plays a major role, they may be enhanced by kinematics for diagnostic accuracy and objectivity.
4. Methods
The research methodology followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [77].
4.1. Research Methodology and Inclusion Criteria
A search was carried out on PubMed until July 2023 with the following terms: “botulinum toxin” AND “tremor” AND/OR “essential tremor”, “Parkinson”, “multiple sclerosis”, “Wilson”, “dystonic”, “rubral”, “writing”, “hand”, “upper limb”, “proximal”, “distal”, “cerebellar”, and “action”. Article type was filtered to retain all “clinical trials” and “randomized clinical trials” (RCTs). Duplicates were removed by the primary investigator (DM) using the “sorting” function in Excel software (2016, v.16.0).
4.2. Screening Methodology and Exclusion Criteria
Two investigators (DM and CD) independently screened all titles and abstracts of the remaining studies. They excluded irrelevant articles, i.e., those not pertaining to botulinum toxin injections in upper limbs, those not intended to treat tremor of any type, and those that were not in the English or French language. Careful reading of all the selected studies was then carried out jointly by the same two investigators. Articles could still be excluded if the content was considered irrelevant.
4.3. Data Collection
The following data were collected and entered into an Excel database: first author, journal, year of publication, type of study (open-label or RCT), disease studied, number of groups, type of analyses (cross-over or longitudinal, intention-to-treat or per protocol), number of patients (total and per group), presence of placebo, botulinum toxin used, method of muscle selection, dose used, all reported outcomes including the clinical scales used, adverse effects (weakness and others), and identified bias.
4.4. Quality Assessment Method
The methodological quality of each study was assessed using the Levels of Evidence and the Critical Appraisal Skills Programme (CASP) checklist for RCTs [29,30]. Concerning CASP, quality assessments were categorized as “low” (no appropriate recruitment), “moderate” (appropriate recruitment but low or intermediate results), or “high” (appropriate recruitment and strong results). Any disagreements regarding screening or quality assessment could be settled by discussion with a third investigator (ER).
Author Contributions
Conceptualization, D.M., M.B., J.-M.G. and E.R.; methodology, D.M., M.B. and C.D.; software, D.M., C.D. and N.B.; validation, M.B., J.-M.G. and E.R.; formal analysis, D.M and C.D.; investigation, D.M., C.D., N.B. and E.R.; resources, D.M., N.B., M.B. and J.-M.G.; data curation, D.M., C.D. and E.R.; writing—original draft preparation, D.M.; writing—review and editing, C.D., N.B., J.-M.G., E.R. and M.B.; visualization, D.M., C.D., N.B., J.-M.G., E.R. and M.B.; supervision, M.B., J.-M.G. and E.R.; project administration, D.M. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All available data are provided in the figure and tables published in this manuscript.
Conflicts of Interest
All the authors of the manuscript are or have been consultants for Merz Pharma, Ipsen and Abbvie, but received no specific compensation for this manuscript. J.-M.G. received consulting honoraria by Ipsen, Ltd., Fastox, and Merz and Abbvie. E.R. declares that he also received speech honoraria from Orkyn, Aguettant, Elivie, Merz Pharma, and Janssen and for participating in advisory boards from Merz Pharma, Elivie, Teva, and BIAL. He received research support from Merz Pharma, Orkyn, Aguettant, Elivie, Ipsen, Everpharma, Enjoysharing, Fondation Desmarest, AMADYS, ADCY5.org, Fonds de dotation Patrick Brou de Laurière, Agence Nationale de la Recherche, Dystonia Medical Reasearch Foundation, Hope For Annabel, Cure Alternating Hemiplegia of Childhood Alternating Hemiplegia of Childhood Foundation, Alternating Hemiplegia of Childhood Association of Iceland, Association française de l’hémiplégie alternante, and Alternating Hemiplegia of Childhood Kids of Netherlands.
References
- Elias, W.J.; Shah, B.B. Tremor. JAMA 2014, 311, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Velickovic, M.; Gracies, J.M. Keys to identifying and treating tremor. Mov. Disord. 2002, 57, 32–36. [Google Scholar]
- Bhatia, K.P.; Bain, P.; Bajaj, N.; Elble, R.J.; Hallett, M.; Louis, E.D.; Raethjen, J.; Stamelou, M.; Testa, C.M.; Deuschl, G.; et al. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov. Disord. Off. J. Mov. Disord. Soc. 2018, 33, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.D. Essential tremors: A family of neurodegenerative disorders? Arch. Neurol. 2009, 66, 1202–1208. [Google Scholar] [CrossRef]
- Deuschl, G.; Raethjen, J.; Hellriegel, H.; Elble, R. Treatment of patients with essential tremor. Lancet Neurol. 2011, 10, 148–161. [Google Scholar] [CrossRef]
- Buijink, A.W.G.; van Rootselaar, A.F.; Helmich, R.C. Connecting tremors-a circuits perspective. Curr. Opin. Neurol. 2022, 35, 518–524. [Google Scholar] [CrossRef]
- Feys, P.; Romberg, A.; Ruutiainen, J.; Ketelaer, P. Interference of upper limb tremor on daily life activities in people with multiple sclerosis. Occup. Ther. Health Care 2004, 17, 81–95. [Google Scholar] [CrossRef]
- Alusi, S.H.; Bain, P.G. Tremor: Natural Behaviour, Trial Design and Physiological Outcome Measures. In Clinical Trials in Neurology; Guiloff, R.J., Ed.; Springer: London, UK, 2001. [Google Scholar]
- Koch, M.; Mostert, J.; Heersema, D.; De Keyser, J. Tremor in multiple sclerosis. J. Neurol. 2007, 254, 133–145. [Google Scholar] [CrossRef]
- Baumann, C.R. Epidemiology, diagnosis and differential diagnosis in Parkinson’s disease tremor. Park. Relat. Disord. 2012, 18 (Suppl. S1), S90–S92. [Google Scholar] [CrossRef]
- Marras, C.; Beck, J.C.; Bower, J.H.; Roberts, E.; Ritz, B.; Ross, G.W.; Abbott, R.D.; Savica, R.; Van Den Eeden, S.K.; Willis, A.W.; et al. Prevalence of Parkinson’s disease across North America. NPJ Park. Dis. 2018, 4, 21. [Google Scholar] [CrossRef]
- Enquête Aptes-Aptes. Available online: https://www.aptes.org/communaute/enquete (accessed on 1 July 2015).
- Rinker, J.R.; Salter, A.R.; Walker, H.; Amara, A.; Meador, W.; Cutter, G.R. Prevalence and characteristics of tremor in the NARCOMS multiple sclerosis registry: A cross-sectional survey. BMJ Open 2015, 5, e006714. [Google Scholar] [CrossRef] [PubMed]
- Keogh, J.W.L.; O’Reilly, S.; O’Brien, E.; Morrison, S.; Kavanagh, J.J. Can Resistance Training Improve Upper Limb Postural Tremor, Force Steadiness and Dexterity in Older Adults? A Systematic Review. Sports Med. Auckl. NZ 2019, 49, 1199–1216. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Deuschl, G. Therapeutic advances in tremor: Therapeutic Advances in Tremor. Mov. Disord. 2015, 30, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.D.; Rohl, B.; Rice, C. Defining the Treatment Gap: What Essential Tremor Patients Want That They Are Not Getting. Tremor Hyperkinetic Mov. N. Y N. 2015, 5, 331. [Google Scholar] [CrossRef]
- Shashkin, C. Complications of Deep Brain Stimulation for Movement Disorders: Literature Review and Personal Experience. Acta Neurochir. Suppl. 2023, 130, 121–126. [Google Scholar]
- Samotus, O.; Lee, J.; Jog, M. Transitioning from Unilateral to Bilateral Upper Limb Tremor Therapy for Parkinson’s Disease and Essential Tremor Using Botulinum Toxin: Case Series. Case Series. Toxins 2018, 10, 394. [Google Scholar] [CrossRef]
- Jankovic, J.; Schwartz, K.; Clemence, W.; Aswad, A.; Mordaunt, J. A randomized, double-blind, placebo-controlled study to evaluate botulinum toxin type A in essential hand tremor. Mov. Disord. Off. J. Mov. Disord. Soc. 1996, 11, 250–256. [Google Scholar] [CrossRef]
- Brin, M.; Lyons, K.; Doucette, J.; Adler, C.; Caviness, J.; Comella, C.; Dubinsky, R.; Friedman, J.; Manyam, B.; Matsumoto, J.; et al. A randomized, double masked, controlled trial of botulinum toxin type A in essential hand tremor. Neurology 2001, 56, 1523–1528. [Google Scholar] [CrossRef]
- Mittal, S.O.; Machado, D.; Richardson, D.; Dubey, D.; Jabbari, B. Botulinum toxin in essential hand tremor-A randomized double-blind placebo-controlled study with customized injection approach. Park. Relat. Disord. 2018, 56, 65–69. [Google Scholar] [CrossRef]
- Jog, M.; Lee, J.; Scheschonka, A.; Chen, R.; Ismail, F.; Boulias, C.; Hobson, D.; King, D.; Althaus, M.; Simon, O.; et al. Tolerability and Efficacy of Customized IncobotulinumtoxinA Injections for Essential Tremor: A Randomized, Double-Blind, Placebo-Controlled Study. Toxins 2020, 12, 807. [Google Scholar] [CrossRef]
- Van Der Walt, A.; Sung, S.; Spelman, T.; Marriott, M.; Kolbe, S.; Mitchell, P.; Evans, A.; Butzkueven, H. A double-blind, randomized, controlled study of botulinum toxin type A in MS-related tremor. Neurology 2012, 79, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, F.M.; Evans, A.; Noffs, G.; Perera, T.; Jokubaitis, V.; Stankovich, J.; Vogel, A.P.; Moffat, B.A.; Butzkueven, H.; Kolbe, S.C.; et al. OnabotulinumtoxinA treatment for MS-tremor modifies fMRI tremor response in central sensory-motor integration areas. Mult. Scler. Relat. Disord. 2020, 40, 101984. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.O.; Machado, D.; Richardson, D.; Dubey, D.; Jabbari, B. Botulinum Toxin in Parkinson Disease Tremor: A Randomized, Double-Blind, Placebo-Controlled Study With a Customized Injection Approach. Mayo Clin. Proc. 2017, 92, 1359–1367. [Google Scholar] [CrossRef]
- Rajan, R.; Srivastava, A.K.; Anandapadmanabhan, R.; Saini, A.; Upadhyay, A.; Gupta, A.; Vishnu, V.Y.; Pandit, A.K.; Vibha, D.; Singh, M.B.; et al. Assessment of Botulinum Neurotoxin Injection for Dystonic Hand Tremor: A Randomized Clinical Trial. JAMA Neurol. 2021, 78, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Esquenazi, A.; Delgado, M.R.; Hauser, R.A.; Picaut, P.; Foster, K.; Lysandropoulos, A.; Gracies, J.-M. Duration of Symptom Relief Between Injections for AbobotulinumtoxinA (Dysport®) in Spastic Paresis and Cervical Dystonia: Comparison of Evidence From Clinical Studies. Front. Neurol. 2020, 11, 576117. [Google Scholar] [CrossRef] [PubMed]
- Ojardias, E.; Ollier, E.; Lafaie, L.; Celarier, T.; Giraux, P.; Bertoletti, L. Time course response after single injection of botulinum toxin to treat spasticity after stroke: Systematic review with pharmacodynamic model-based meta-analysis. Ann. Phys. Rehabil. Med. 2022, 65, 101579. [Google Scholar] [CrossRef]
- Evidence-Based Practice in Nursing Healthcare. Wolters. Available online: https://shop.lww.com/evidence-based-practice-in-nursing-healthcare/p/9781975185725 (accessed on 4 January 2023).
- Haile, Z.T. Critical Appraisal Tools and Reporting Guidelines. J. Hum. Lact. 2022, 38, 21–27. [Google Scholar] [CrossRef]
- Modugno, N.; Priori, A.; Berardelli, A.; Vacca, L.; Mercuri, B.; Manfredi, M. Botulinum toxin restores presynaptic inhibition of group Ia afferents in patients with essential tremor. Muscle Nerve 1998, 21, 1701–1705. [Google Scholar] [CrossRef]
- Pacchetti, C.; Mancini, F.; Bulgheroni, M.; Zangaglia, R.; Cristina, S.; Sandrini, G.; Nappi, G. Botulinum toxin treatment for functional disability induced by essential tremor. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2000, 21, 349–353. [Google Scholar] [CrossRef]
- Samotus, O.; Rahimi, F.; Lee, J.; Jog, M. Functional Ability Improved in Essential Tremor by IncobotulinumtoxinA Injections Using Kinematically Determined Biomechanical Patterns-A New Future. PLoS ONE 2016, 11, e0153739. [Google Scholar] [CrossRef]
- Samotus, O.; Lee, J.; Jog, M. Personalized Bilateral Upper Limb Essential Tremor Therapy with Botulinum Toxin Using Kinematics. Toxins 2019, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Samotus, O.; Kumar, N.; Rizek, P.; Jog, M. Botulinum Toxin Type A Injections as Monotherapy for Upper Limb Essential Tremor Using Kinematics. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2018, 45, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Pullman, S.L.; Greene, P.; Fahn, S.; Pedersen, S.F. Approach to the treatment of limb disorders with botulinum toxin A. Experience with 187 patients. Arch. Neurol. 1996, 53, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Samotus, O.; Lee, J.; Jog, M. Long-term tremor therapy for Parkinson and essential tremor with sensor-guided botulinum toxin type A injections. PLoS ONE 2017, 12, e0178670. [Google Scholar] [CrossRef]
- Weinberger, M.; Mahant, N.; Hutchison, W.D.; Lozano, A.M.; Moro, E.; Hodaie, M.; Lang, A.E.; Dostrovsky, J.O. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson’s disease. J. Neurophysiol. 2006, 96, 3248–3256. [Google Scholar] [CrossRef]
- Diener, H.C.; Dichgans, J. Pathophysiology of cerebellar ataxia. Mov. Disord. 1992, 7, 95–109. [Google Scholar] [CrossRef]
- Marsden, J.; Harris, C. Cerebellar ataxia: Pathophysiology and rehabilitation. Clin. Rehabil. 2011, 25, 195–216. [Google Scholar] [CrossRef]
- Grimaldi, G.; Manto, M. Is essential tremor a Purkinjopathy? The role of the cerebellar cortex in its pathogenesis. Mov. Disord. Off. J. Mov. Disord. Soc. 2013, 28, 1759–1761. [Google Scholar] [CrossRef]
- Louis, E.D.; Lee, M.; Babij, R.; Ma, K.; Cortés, E.; Vonsattel, J.-P.G.; Faust, P.L. Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain 2014, 137, 3142–3148. [Google Scholar] [CrossRef]
- Lin, C.Y.; Louis, E.D.; Faust, P.L.; Koeppen, A.H.; Vonsattel, J.P.G.; Kuo, S.H. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain J. Neurol. 2014, 137 Pt 12, 3149–3159. [Google Scholar] [CrossRef]
- Lee, D.; Gan, S.R.; Faust, P.L.; Louis, E.D.; Kuo, S.H. Climbing fiber-Purkinje cell synaptic pathology across essential tremor subtypes. Park. Relat. Disord. 2018, 51, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Pandey, U.; Saini, J.; Ingalhalikar, M.; Pal, P.K. Atrophy of cerebellar peduncles in essential tremor: A machine learning-based volumetric analysis. Eur. Radiol. 2019, 29, 7037–7046. [Google Scholar] [CrossRef] [PubMed]
- Gionco, J.T.; Hartstone, W.G.; Martuscello, R.T.; Kuo, S.H.; Faust, P.L.; Louis, E.D. Essential Tremor versus “ET-plus”: A Detailed Postmortem Study of Cerebellar Pathology. Cerebellum 2021, 20, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Salinas, M.; Salinas, V.H.; McGurn, M.; Hernandez, N.; Louis, E.D. Serum Neurofilament Light-Chain Concentrations in Essential Tremor: A Case-Control Study. Cerebellum 2023, 23, 951–956. [Google Scholar] [CrossRef]
- Tsapanou, A.; Ghanem, A.; Chapman, S.; Stern, Y.; Huey, E.D.; Cosentino, S.; Louis, E.D. Sleep problems as predictors of cognitive decline in essential tremor: A prospective longitudinal cohort study. Sleep. Med. 2024, 116, 13–18. [Google Scholar] [CrossRef]
- Rosales, R.L.; Arimura, K.; Takenaga, S.; Osame, M. Extrafusal and intrafusal muscle effects in experimental botulinum toxin-A injection. Muscle Nerve 1996, 19, 488–496. [Google Scholar] [CrossRef]
- Gracies, J.M.; Lugassy, M.; Weisz, D.J.; Vecchio, M.; Flanagan, S.; Simpson, D.M. Botulinum toxin dilution and endplate targeting in spasticity: A double-blind controlled study. Arch. Phys. Med. Rehabil. 2009, 90, 9–16.e2. [Google Scholar] [CrossRef]
- Kaňovský, P.; Rosales, R.L. Debunking the pathophysiological puzzle of dystonia--with special reference to botulinum toxin therapy. Park. Relat. Disord. 2011, 17 (Suppl. S1), S11–S14. [Google Scholar] [CrossRef]
- Vinti, M.; Costantino, F.; Bayle, N.; Simpson, D.M.; Weisz, D.J.; Gracies, J.M. Spastic cocontraction in hemiparesis: Effects of botulinum toxin. Muscle Nerve 2012, 46, 926–931. [Google Scholar] [CrossRef]
- Hok, P.; Veverka, T.; Hluštík, P.; Nevrlý, M.; Kaňovský, P. The Central Effects of Botulinum Toxin in Dystonia and Spasticity. Toxins 2021, 13, 155. [Google Scholar] [CrossRef]
- Gracies, J.M. Physiological effects of botulinum toxin in spasticity. Mov. Disord. Off. J. Mov. Disord. Soc. 2004, 19 (Suppl. S8), S120–S128. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, B.; Berardelli, A.; Modugno, N.; Vacca, L.; Ruggieri, S.; Manfredi, M. Reciprocal inhibition in forearm muscles in patients with essential tremor. Muscle Nerve 1998, 21, 796–799. [Google Scholar] [CrossRef]
- Blakemore, S.J.; Sirigu, A. Action prediction in the cerebellum and in the parietal lobe. Exp. Brain Res. 2003, 153, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Clower, D.M.; West, R.A.; Lynch, J.C.; Strick, P.L. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 6283–6291. [Google Scholar] [CrossRef]
- Shum, M.; Shiller, D.M.; Baum, S.R.; Gracco, V.L. Sensorimotor integration for speech motor learning involves the inferior parietal cortex. Eur. J. Neurosci. 2011, 34, 1817–1822. [Google Scholar] [CrossRef]
- Marsden, C.D.; Harrison, M.J. Idiopathic torsion dystonia (dystonia musculorum deformans). A Rev. Forty Two Patients. Brain J. Neurol. 1974, 97, 793–810. [Google Scholar] [CrossRef]
- Burke, R.E.; Fahn, S.; Marsden, C.D.; Bressman, S.B.; Moskowitz, C.; Friedman, J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology 1985, 35, 73–77. [Google Scholar] [CrossRef]
- Grütz, K.; Klein, C. Dystonia updates: Definition, nomenclature, clinical classification, and etiology. J. Neural Transm. 2021, 128, 395–404. [Google Scholar] [CrossRef]
- Albanese, A.; Bhatia, K.; Bressman, S.B.; DeLong, M.R.; Fahn, S.; Fung, V.S.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Klein, C.; et al. Phenomenology and classification of dystonia: A consensus update. Mov. Disord. Off. J. Mov. Disord. Soc. 2013, 28, 863–873. [Google Scholar] [CrossRef]
- Erro, R.; Rubio-Agusti, I.; Saifee, T.A.; Cordivari, C.; Ganos, C.; Batla, A.; Bhatia, K.P. Rest and other types of tremor in adult-onset primary dystonia. J. Neurol. Neurosurg. Psychiatry 2014, 85, 965–968. [Google Scholar] [CrossRef]
- Walker, H.W.; Lee, M.Y.; Bahroo, L.B.; Hedera, P.; Charles, D. Botulinum toxin injection techniques for the management of adult spasticity. PMR 2015, 7, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.M.; Simpson, D.M. Chapter 41: Focal injection therapy. In Handbook of Clinical Neurophysiology; Hallett, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 651–695. [Google Scholar]
- Hauret, I.; Dobija, L.; Givron, P.; Goldstein, A.; Pereira, B.; Coudeyre, E. Effectiveness of Ultrasound-guided vs. Electrical-stimulation-guided Botulinum Toxin Injections in Triceps Surae Spasticity after Stroke: A Randomized Controlled Study. J. Rehabil. Med. 2023, 55, jrm11963. [Google Scholar] [CrossRef] [PubMed]
- Le Fur, C.; Vielotte, J.; Mardale, V.; Guihard, M.; Hutin, E.; Gracies, J.M. Treatment of hypermetric tremors using motor strengthening combined with muscle weakening with botulinum toxin. Ann. Phys. Rehabil. Med. 2017, 60, e24. [Google Scholar] [CrossRef]
- Domzał, T.M. Botulinum toxin in the treatment of tremor. Neurol. Neurochir. Pol. 1998, 32 (Suppl. S1), 51–56. [Google Scholar]
- Budini, F.; McManus, L.M.; Berchicci, M.; Menotti, F.; Macaluso, A.; Di Russo, F.; Lowery, M.M.; De Vito, G. Alpha band cortico-muscular coherence occurs in healthy individuals during mechanically-induced tremor. PLoS ONE 2014, 9, e115012. [Google Scholar] [CrossRef]
- Synofzik, M.; Ilg, W. Motor Training in Degenerative Spinocerebellar Disease: Ataxia-Specific Improvements by Intensive Physiotherapy and Exergames. BioMed Res. Int. 2014, 2014, 583507. [Google Scholar] [CrossRef]
- Lanza, G.; Casabona, J.A.; Bellomo, M.; Cantone, M.; Fisicaro, F.; Bella, R.; Pennisi, G.; Bramanti, P.; Pennisi, M.; Bramanti, A. Update on intensive motor training in spinocerebellar ataxia: Time to move a step forward? J. Int. Med. Res. 2019, 48, 0300060519854626. [Google Scholar] [CrossRef]
- Kamm, C.P.; Mattle, H.P.; Müri, R.M.; Heldner, M.R.; Blatter, V.; Bartlome, S.; Lüthy, J.; Imboden, D.; Pedrazzini, G.; Bohlhalter, S.; et al. Home-based training to improve manual dexterity in patients with multiple sclerosis: A randomized controlled trial. Mult. Scler. Houndmills Basingstoke Engl. 2015, 21, 1546–1556. [Google Scholar] [CrossRef]
- Semmler, J.G.; Nordstrom, M.A. Motor unit discharge and force tremor in skill- and strength-trained individuals. Exp. Brain Res. 1998, 119, 27–38. [Google Scholar] [CrossRef]
- Bilodeau, M.; Keen, D.A.; Sweeney, P.J.; Shields, R.W.; Enoka, R.M. Strength training can improve steadiness in persons with essential tremor. Muscle Nerve 2000, 23, 771–778. [Google Scholar] [CrossRef]
- Özer, G.; Kirmaci, Z.İ.K.; Adigüzel, H.; Ergun, N. Correlation of proximal and distal muscle strength with upper limb functional ability in patients with essential tremor. Acta Neurol. Belg. 2020, 120, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Keogh, J.W.L.; Morrison, S.; Barrett, R. Strength and coordination training are both effective in reducing the postural tremor amplitude of older adults. J. Aging Phys. Act. 2010, 18, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).