Caffeic Acid Phenethyl Ester Administration Reduces Enterotoxigenic Bacteroides fragilis-Induced Colitis and Tumorigenesis
Abstract
1. Introduction
2. Results
2.1. CAPE Administration Decreases ETBF-Induced Colitis
2.2. CAPE Administration Protects against ETBF-Induced Colonic Tumorigenesis
2.3. CAPE Treatment Attenuated Microadenoma Progression in ETBF-Mediated Tumorigenesis Model
2.4. CAPE-Mediated Reduction in Colitis from ETBF-Mediated Tumorigenesis Model
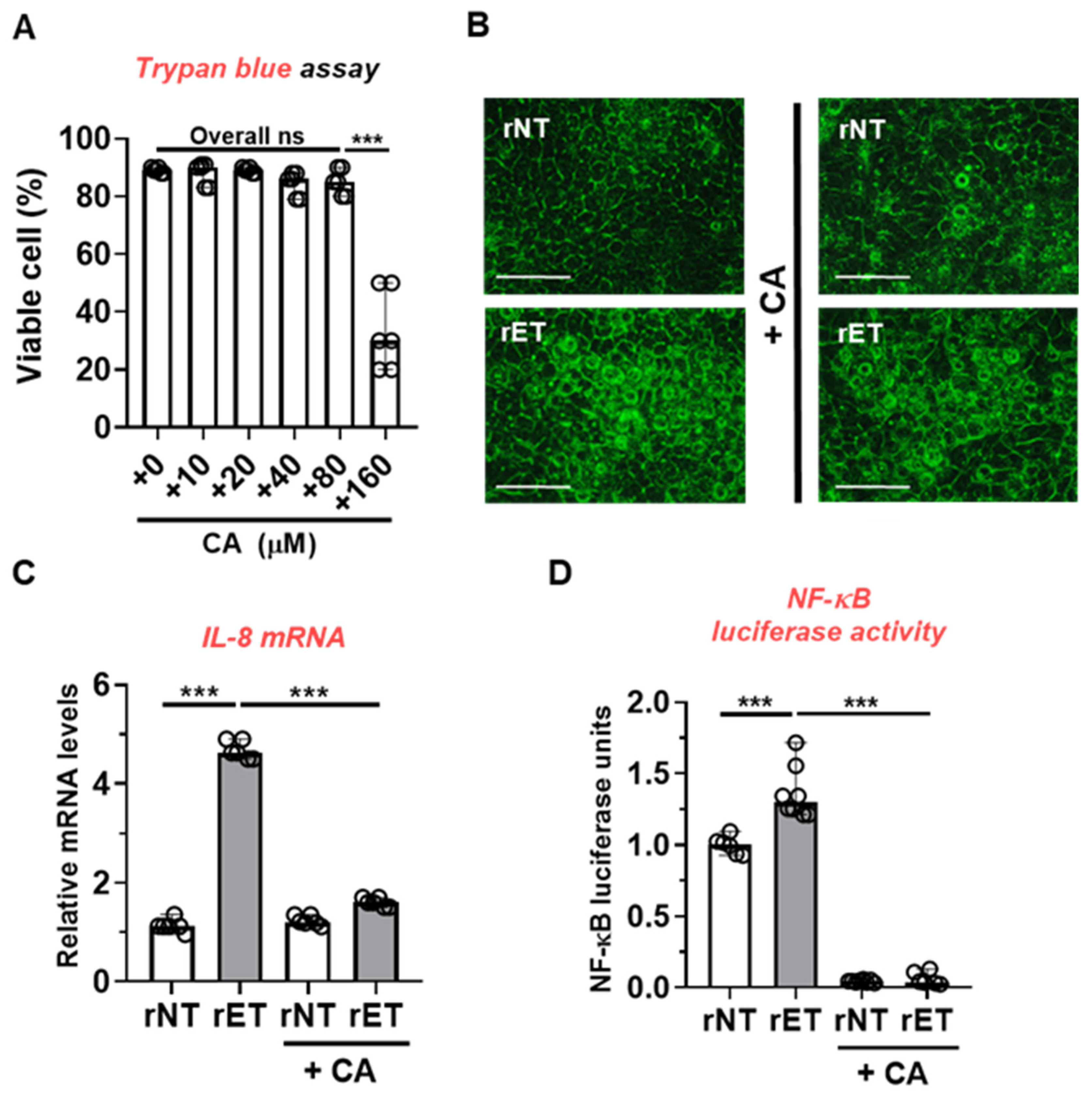
2.5. CAPE Reduced BFT-Induced IL-8 Expression and NF-κB Activity in Colon Epithelial Cells
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals
5.2. Human Model Intestinal Epithelial Cells
5.3. Cell Stimulation with Bacteroides fragilis Toxin
5.4. Treatment with CAPE in ETBF-Mediated Colitis Model
5.5. CAPE Treatment in ETBF-Promoted AOM/DSS-Induced Tumorigenesis
5.6. Tumor Enumeration and Histopathology
5.7. Quantification of Human Cytokine Expressions
5.8. Trypan Blue Exclusion Assay
5.9. ELISA
5.10. Histologic Assessment of Inflammation and Hyperplasia
5.11. Luciferase Reporter Assay
5.12. Structural Similarity Analysis
5.13. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Terzic, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Cytokines, IBD, and colitis-associated cancer. Inflamm. Bowel Dis. 2015, 21, 409–418. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730. [Google Scholar] [CrossRef]
- Sears, C.L.; Garrett, W.S. Microbes, microbiota, and colon cancer. Cell Host Microbe 2014, 15, 317–328. [Google Scholar] [CrossRef]
- Drewes, J.L.; Chen, J.; Markham, N.O.; Knippel, R.J.; Domingue, J.C.; Tam, A.J.; Chan, J.L.; Kim, L.; McMann, M.; Stevens, C.; et al. Human Colon Cancer-Derived Clostridioides difficile Strains Drive Colonic Tumorigenesis in Mice. Cancer Discov. 2022, 12, 1873–1885. [Google Scholar] [CrossRef]
- Rhee, K.J.; Wu, S.; Wu, X.; Huso, D.L.; Karim, B.; Franco, A.A.; Rabizadeh, S.; Golub, J.E.; Mathews, L.E.; Shin, J.; et al. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect. Immun. 2009, 77, 1708–1718. [Google Scholar] [CrossRef]
- Yim, S.; Gwon, S.Y.; Hwang, S.; Kim, N.H.; Jung, B.D.; Rhee, K.J. Enterotoxigenic Bacteroides fragilis causes lethal colitis in Mongolian gerbils. Anaerobe 2013, 21, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Wick, E.C.; Rabizadeh, S.; Albesiano, E.; Wu, X.; Wu, S.; Chan, J.; Rhee, K.J.; Ortega, G.; Huso, D.L.; Pardoll, D.; et al. Stat3 activation in murine colitis induced by enterotoxigenic Bacteroides fragilis. Inflamm. Bowel Dis. 2014, 20, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Mu, J.; Tseng, M.; Wattenberg, B.; Zhuang, X.; Egilmez, N.K.; Wang, Q.; Zhang, L.; Norris, J.; Guo, H.; et al. Enterobacteria-secreted particles induce production of exosome-like S1P-containing particles by intestinal epithelium to drive Th17-mediated tumorigenesis. Nat. Commun. 2015, 6, 6956. [Google Scholar] [CrossRef] [PubMed]
- Thiele Orberg, E.; Fan, H.; Tam, A.J.; Dejea, C.M.; Destefano Shields, C.E.; Wu, S.; Chung, L.; Finard, B.B.; Wu, X.; Fathi, P.; et al. The myeloid immune signature of enterotoxigenic Bacteroides fragilis-induced murine colon tumorigenesis. Mucosal Immunol. 2017, 10, 421–433. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Z.; Yan, Y.; Ji, L.; He, J.; Xuan, B.; Shen, C.; Ma, Y.; Jiang, S.; Ma, D.; et al. Enterotoxigenic Bacteroidesfragilis Promotes Intestinal Inflammation and Malignancy by Inhibiting Exosome-Packaged miR-149-3p. Gastroenterology 2021, 161, 1552–1566. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Hu, T.; Huang, H.; Chen, G.; Jin, B.; Zeng, G.; Liu, J. Entero-toxigenic Bacteroides fragilis contributes to intestinal barrier injury and colorectal cancer progression by mediating the BFT/STAT3/ZEB2 pathway. Cell Cycle 2024, 23, 70–82. [Google Scholar] [CrossRef]
- Toprak, N.U.; Yagci, A.; Gulluoglu, B.M.; Akin, M.L.; Demirkalem, P.; Celenk, T.; Soyletir, G. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin. Microbiol. Infect. 2006, 12, 782–786. [Google Scholar] [CrossRef]
- Boleij, A.; Hechenbleikner, E.M.; Goodwin, A.C.; Badani, R.; Stein, E.M.; Lazarev, M.G.; Ellis, B.; Carroll, K.C.; Albesiano, E.; Wick, E.C.; et al. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin. Infect. Dis. 2015, 60, 208–215. [Google Scholar] [CrossRef]
- Purcell, R.V.; Pearson, J.; Aitchison, A.; Dixon, L.; Frizelle, F.A.; Keenan, J.I. Colonization with enterotoxigenic Bacteroides fragilis is associated with early-stage colorectal neoplasia. PLoS ONE 2017, 12, e0171602. [Google Scholar] [CrossRef]
- Haghi, F.; Goli, E.; Mirzaei, B.; Zeighami, H. The association between fecal enterotoxigenic Bacteroides fragilis with colorectal cancer. BMC Cancer 2019, 19, 879. [Google Scholar] [CrossRef]
- Chung, L.; Orberg, E.T.; Geis, A.L.; Chan, J.L.; Fu, K.; DeStefano Shields, C.E.; Dejea, C.M.; Fathi, P.; Chen, J.; Finard, B.B.; et al. Bacteroides fragilis Toxin Coordinates a Pro-carcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells. Cell Host Microbe 2018, 23, 421. [Google Scholar] [CrossRef] [PubMed]
- Housseau, F.; Wu, S.; Wick, E.C.; Fan, H.; Wu, X.; Llosa, N.J.; Smith, K.N.; Tam, A.; Ganguly, S.; Wanyiri, J.W.; et al. Redundant Innate and Adaptive Sources of IL17 Production Drive Colon Tumorigenesis. Cancer Res. 2016, 76, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Rhee, K.J.; Albesiano, E.; Rabizadeh, S.; Wu, X.; Yen, H.R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009, 15, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Rhee, K.J.; Zhang, M.; Franco, A.; Sears, C.L. Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and gamma-secretase-dependent E-cadherin cleavage. J. Cell Sci. 2007, 120, 1944–1952. [Google Scholar] [CrossRef]
- Kim, J.M.; Cho, S.J.; Oh, Y.K.; Jung, H.Y.; Kim, Y.J.; Kim, N. Nuclear factor-kappa B activation pathway in intestinal epithelial cells is a major regulator of chemokine gene expression and neutrophil migration induced by Bacteroides fragilis enterotoxin. Clin. Exp. Immunol. 2002, 130, 59–66. [Google Scholar] [CrossRef]
- Wu, S.; Powell, J.; Mathioudakis, N.; Kane, S.; Fernandez, E.; Sears, C.L. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-kappaB pathway. Infect. Immun. 2004, 72, 5832–5839. [Google Scholar] [CrossRef]
- Jeon, J.I.; Ko, S.H.; Kim, J.M. Intestinal Epithelial Cells Exposed to Bacteroides fragilis Enterotoxin Regulate NF-kappaB Activation and Inflammatory Responses through beta-Catenin Expression. Infect. Immun. 2019, 87, e00312-19. [Google Scholar] [CrossRef]
- Lee, C.G.; Hwang, S.; Gwon, S.Y.; Park, C.; Jo, M.; Hong, J.E.; Rhee, K.J. Bacteroides fragilis Toxin Induces Intestinal Epithelial Cell Secretion of Interleukin-8 by the E-Cadherin/beta-Catenin/NF-kappaB Dependent Pathway. Biomedicines 2022, 10, 827. [Google Scholar] [CrossRef]
- DeStefano Shields, C.E.; Van Meerbeke, S.W.; Housseau, F.; Wang, H.; Huso, D.L.; Casero, R.A., Jr.; O’Hagan, H.M.; Sears, C.L. Reduction of Murine Colon Tumorigenesis Driven by Enterotoxigenic Bacteroides fragilis Using Cefoxitin Treatment. J. Infect. Dis. 2016, 214, 122–129. [Google Scholar] [CrossRef]
- Jung, P.Y.; Ryu, H.; Rhee, K.J.; Hwang, S.; Lee, C.G.; Gwon, S.Y.; Kim, J.; Kim, J.; Yoo, B.S.; Baik, S.K.; et al. Adipose tissue-derived mesenchymal stem cells cultured at high density express IFN-beta and TRAIL and suppress the growth of H460 human lung cancer cells. Cancer Lett. 2019, 440–441, 202–210. [Google Scholar] [CrossRef]
- Byun, C.S.; Hwang, S.; Woo, S.H.; Kim, M.Y.; Lee, J.S.; Lee, J.I.; Kong, J.H.; Bae, K.S.; Park, I.H.; Kim, S.H.; et al. Adipose Tissue-Derived Mesenchymal Stem Cells Suppress Growth of Huh7 Hepatocellular Carcinoma Cells via Interferon (IFN)-beta-Mediated JAK/STAT1 Pathway in vitro. Int. J. Med. Sci. 2020, 17, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, Q.; Hong, H.; Qian, Z.; Wan, B.; Xia, M. Caffeic acid phenethyl ester inhibits neuro-inflammation and oxidative stress following spinal cord injury by mitigating mitochondrial dysfunction via the SIRT1/PGC1alpha/DRP1 signaling pathway. J. Transl. Med. 2024, 22, 304. [Google Scholar] [CrossRef] [PubMed]
- Salikha, K.; Narmada, I.B.; Nugraha, A.P.; Sari, A.F.; Riawan, W.; Ahmad, T.N.E.B.T. Anti-inflammatory effect of caffeic acid phenethyl ester supplementation on TNF-? and NF-KB expressions throughout experimental tooth movement in vivo. J. Pharm. Pharmacogn. R. 2022, 10, 1037–1045. [Google Scholar] [CrossRef]
- Semis, H.S.; Gur, C.; Ileriturk, M.; Kaynar, O.; Kandemir, F.M. Investigation of the anti-inflammatory effects of caffeic acid phenethyl ester in a model of lambda-Carrageenan-induced paw edema in rats. Hum. Exp. Toxicol. 2021, 40, S721–S738. [Google Scholar] [CrossRef]
- Al-Hariri, M.; Alsunni, A.; Shaikh, M.H.; Gamal Eldin, T.; Al Ghamdi, K.; Alharbi, A.F.; Alhawaj, H.; Chathoth, S. Caffeic Acid Phenethyl Ester reduces Pro Inflammatory Cytokines in Moderate Swimming Test in Growing Rats Model. J. Inflamm. Res. 2021, 14, 5653–5657. [Google Scholar] [CrossRef]
- Shao, B.; Wang, M.; Chen, A.; Zhang, C.; Lin, L.; Zhang, Z.; Chen, A. Protective effect of caffeic acid phenethyl ester against imidacloprid-induced hepatotoxicity by attenuating oxidative stress, endoplasmic reticulum stress, inflammation and apoptosis. Pestic. Biochem. Physiol. 2020, 164, 122–129. [Google Scholar] [CrossRef]
- Stahli, A.; Maheen, C.U.; Strauss, F.J.; Eick, S.; Sculean, A.; Gruber, R. Caffeic acid phenethyl ester protects against oxidative stress and dampens inflammation via heme oxygenase 1. Int. J. Oral. Sci. 2019, 11, 6. [Google Scholar] [CrossRef]
- Armutcu, F.; Akyol, S.; Ustunsoy, S.; Turan, F.F. Therapeutic potential of caffeic acid phenethyl ester and its anti-inflammatory and immunomodulatory effects (Review). Exp. Ther. Med. 2015, 9, 1582–1588. [Google Scholar] [CrossRef]
- Zhao, W.X.; Wang, L.; Yang, J.L.; Li, L.Z.; Xu, W.M.; Li, T. Caffeic acid phenethyl ester attenuates pro-inflammatory and fibrogenic phenotypes of LPS-stimulated hepatic stellate cells through the inhibition of NF-κB signaling. Int. J. Mol. Med. 2014, 33, 687–694. [Google Scholar] [CrossRef]
- Toyoda, T.; Tsukamoto, T.; Takasu, S.; Shi, L.; Hirano, N.; Ban, H.; Kumagai, T.; Tatematsu, M. Anti-inflammatory effects of caffeic acid phenethyl ester (CAPE), a nuclear factor-κB inhibitor, on Helicobacter pylori-induced gastritis in Mongolian gerbils. Int. J. Cancer 2009, 125, 1786–1795. [Google Scholar] [CrossRef]
- Ilhan, A.; Koltuksuz, U.; Ozen, S.; Uz, E.; Ciralik, H.; Akyol, O. The effects of caffeic acid phenethyl ester (CAPE) on spinal cord ischemia/reperfusion injury in rabbits. Eur. J. Cardiothorac. Surg. 1999, 16, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, K.; Singh, S.; Burke, T.R.; Grunberger, D.; Aggarwal, B.B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc. Natl. Acad. Sci. USA 1996, 93, 9090–9095. [Google Scholar] [CrossRef] [PubMed]
- Orban, Z.; Mitsiades, N.; Burke, T.R., Jr.; Tsokos, M.; Chrousos, G.P. Caffeic acid phenethyl ester induces leukocyte apoptosis, modulates nuclear factor-kappa B and suppresses acute inflammation. Neuroimmunomodulation 2000, 7, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Márquez, N.; Sancho, R.; Macho, A.; Calzado, M.A.; Fiebich, B.L.; Muñoz, E. Caffeic acid phenethyl ester inhibits T-cell activation by targeting both nuclear factor of activated T-cells and NF-κB transcription factors. J. Pharmacol. Exp. Ther. 2004, 308, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Ek, R.O.; Serter, M.; Ergin, K.; Yildiz, Y.; Cecen, S.; Kavak, T.; Yenisey, C. The effects of caffeic acid phenethyl ester (CAPE) on TNBS-induced colitis in ovariectomized rats. Dig. Dis. Sci. 2008, 53, 1609–1617. [Google Scholar] [CrossRef]
- Dai, G.; Jiang, Z.; Sun, B.; Liu, C.; Meng, Q.; Ding, K.; Jing, W.; Ju, W. Caffeic acid phenethyl ester prevents colitis-associated cancer by inhibiting NLRP3 inflammasome. Front. Oncol. 2020, 10, 721. [Google Scholar] [CrossRef]
- Hwang, S.; Yi, H.C.; Hwang, S.; Jo, M.; Rhee, K.J. Dietary salt administration decreases enterotoxigenic Bacteroides fragilis (ETBF)-promoted tumorigenesis via inhibition of colonic inflammation. Int. J. Mol. Sci. 2020, 21, 8034. [Google Scholar] [CrossRef]
- Papanikolaou, A.; Wang, Q.S.; Papanikolaou, D.; Whiteley, H.E.; Rosenberg, D.W. Sequential and morphological analyses of aberrant crypt foci formation in mice of differing susceptibility to azoxymethane-induced colon carcinogenesis. Carcinogenesis 2000, 21, 1567–1572. [Google Scholar] [CrossRef]
- Rosenberg, D.W.; Giardina, C.; Tanaka, T. Mouse models for the study of colon carcinogenesis. Carcinogenesis 2009, 30, 183–196. [Google Scholar] [CrossRef]
- Washington, M.K.; Powell, A.E.; Sullivan, R.; Sundberg, J.P.; Wright, N.; Coffey, R.J.; Dove, W.F. Pathology of rodent models of intestinal cancer: Progress report and recommendations. Gastroenterology 2013, 144, 705–717. [Google Scholar] [CrossRef]
- Janakiram, N.B.; Rao, C.V. Chemoprevention of colon aancer by iNOS-selective inhibitors. For. Immunopathol. Dis. Ther. 2012, 3, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, H.; Xu, H.; Li, Y.; Li, Z.; Du, Y.; He, J.; Zhou, Y.; Wang, H.; Nie, Y. Association of oncogenic bacteria with colorectal cancer in South China. Oncotarget 2016, 7, 80794–80802. [Google Scholar] [CrossRef] [PubMed]
- Dejea, C.M.; Fathi, P.; Craig, J.M.; Boleij, A.; Taddese, R.; Geis, A.L.; Wu, X.; DeStefano Shields, C.E.; Hechenbleikner, E.M.; Huso, D.L.; et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 2018, 359, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Lee, C.G.; Jo, M.; Park, C.O.; Gwon, S.Y.; Hwang, S.; Yi, H.C.; Lee, S.Y.; Eom, Y.B.; Karim, B.; et al. Enterotoxigenic Bacteroides fragilis infection exacerbates tumorigenesis in AOM/DSS mouse model. Int. J. Med. Sci. 2020, 17, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lim, K.C.; Huang, J.; Saidi, R.F.; Sears, C.L. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc. Natl. Acad. Sci. USA 1998, 95, 14979–14984. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Chang, Y.; Li, R.; Sun, X.; Peng, L.; Zheng, W.; Qiu, W. Caffeic acid phenethyl ester protects against experimental autoimmune encephalomyelitis by regulating T cell activities. Oxid. Med. Cell Longev. 2020, 2020, 7274342. [Google Scholar] [CrossRef]
- Yang, X.O.; Panopoulos, A.D.; Nurieva, R.; Chang, S.H.; Wang, D.; Watowich, S.S.; Dong, C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007, 282, 9358–9363. [Google Scholar] [CrossRef]
- Wang, X.; Feng, S.; Song, H. Caffeic acid phenethyl ester encapsulated in self-assemble rice peptides nanoparticles: Storage stability, in vitro release, and their interaction mechanisms. Foods 2024, 13, 755. [Google Scholar] [CrossRef]
- Wei, X.; Dai, J.; Liu, R.; Wan, G.; Gu, S.; Du, Y.; Yang, X.; Wang, L.; Huang, Y.; Chen, P.; et al. S/O/W Emulsion with CAPE ameliorates DSS-induced colitis by regulating NF-kappaB pathway, gut microbiota and fecal metabolome in C57BL/6 mice. Nutrients 2024, 16, 1145. [Google Scholar] [CrossRef]
- Demestre, M.; Messerli, S.M.; Celli, N.; Shahhossini, M.; Kluwe, L.; Mautner, V.; Maruta, H. CAPE (caffeic acid phenethyl ester)-based propolis extract (Bio 30) suppresses the growth of human neurofibromatosis (NF) tumor xenografts in mice. Phytother. Res. 2009, 23, 226–230. [Google Scholar] [CrossRef]
- Taira, N.; Nguyen, B.C.; Be Tu, P.T.; Tawata, S. Effect of Okinawa propolis on PAK1 Activity, Caenorhabditis elegans longevity, melanogenesis, and growth of cancer cells. J. Agric. Food Chem. 2016, 64, 5484–5489. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, P.; Mahanta, D.; Kaul, A.; Ishida, Y.; Terao, K.; Wadhwa, R.; Kaul, S.C. Experimental evidence for therapeutic potentials of propolis. Nutrients 2021, 13, 2528. [Google Scholar] [CrossRef] [PubMed]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An update on its chemistry and pharmacological applications. Chin. Med. 2022, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Moskwa, J.; Naliwajko, S.K.; Markiewicz-Zukowska, R.; Gromkowska-Kepka, K.J.; Soroczynska, J.; Puscion-Jakubik, A.; Borawska, M.H.; Isidorov, V.; Socha, K. Polish and New Zealand propolis as sources of antioxidant compounds inhibit glioblastoma (T98G, LN-18) cell lines and astrocytoma cells derived from patient. Antioxidants 2022, 11, 1305. [Google Scholar] [CrossRef]
- Huang, Y.; Jin, M.; Pi, R.; Zhang, J.; Chen, M.; Ouyang, Y.; Liu, A.; Chao, X.; Liu, P.; Liu, J.; et al. Protective effects of caffeic acid and caffeic acid phenethyl ester against acrolein-induced neurotoxicity in HT22 mouse hippocampal cells. Neurosci. Lett. 2013, 535, 146–151. [Google Scholar] [CrossRef]
- Wen, X.; Wan, F.; Wu, Y.; Liu, L.; Liu, Y.; Zhong, R.; Chen, L.; Zhang, H. Caffeic acid supplementation ameliorates intestinal injury by modulating intestinal microbiota in LPS-challenged piglets. Food Funct. 2023, 14, 7705–7717. [Google Scholar] [CrossRef]
- Backman, T.W.; Cao, Y.; Girke, T. ChemMine tools: An online service for analyzing and clustering small molecules. Nucleic Acids Res. 2011, 39, W486–W491. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.; Jo, M.; Hong, J.-E.; Kim, W.-S.; Kang, D.-H.; Yoo, S.-H.; Kang, K.; Rhee, K.-J. Caffeic Acid Phenethyl Ester Administration Reduces Enterotoxigenic Bacteroides fragilis-Induced Colitis and Tumorigenesis. Toxins 2024, 16, 403. https://doi.org/10.3390/toxins16090403
Hwang S, Jo M, Hong J-E, Kim W-S, Kang D-H, Yoo S-H, Kang K, Rhee K-J. Caffeic Acid Phenethyl Ester Administration Reduces Enterotoxigenic Bacteroides fragilis-Induced Colitis and Tumorigenesis. Toxins. 2024; 16(9):403. https://doi.org/10.3390/toxins16090403
Chicago/Turabian StyleHwang, Soonjae, Minjeong Jo, Ju-Eun Hong, Woo-Seung Kim, Da-Hye Kang, Sang-Hyeon Yoo, Kyungsu Kang, and Ki-Jong Rhee. 2024. "Caffeic Acid Phenethyl Ester Administration Reduces Enterotoxigenic Bacteroides fragilis-Induced Colitis and Tumorigenesis" Toxins 16, no. 9: 403. https://doi.org/10.3390/toxins16090403
APA StyleHwang, S., Jo, M., Hong, J.-E., Kim, W.-S., Kang, D.-H., Yoo, S.-H., Kang, K., & Rhee, K.-J. (2024). Caffeic Acid Phenethyl Ester Administration Reduces Enterotoxigenic Bacteroides fragilis-Induced Colitis and Tumorigenesis. Toxins, 16(9), 403. https://doi.org/10.3390/toxins16090403





