Isolation and Pharmacological Characterisation of Pre-Synaptic Neurotoxins from Thai and Javanese Russell’s Viper (Daboia siamensis) Venoms
Abstract
1. Introduction
2. Results
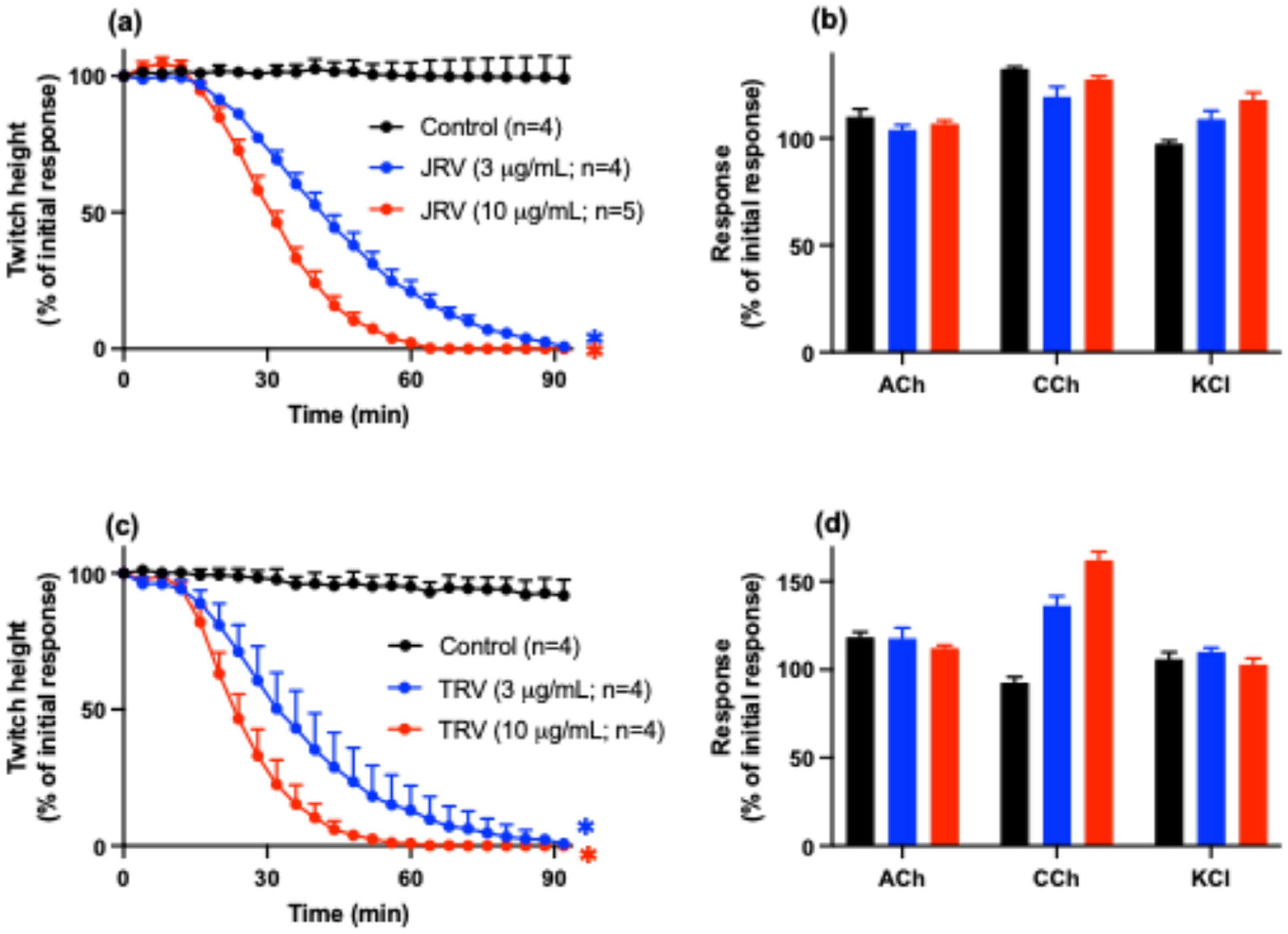
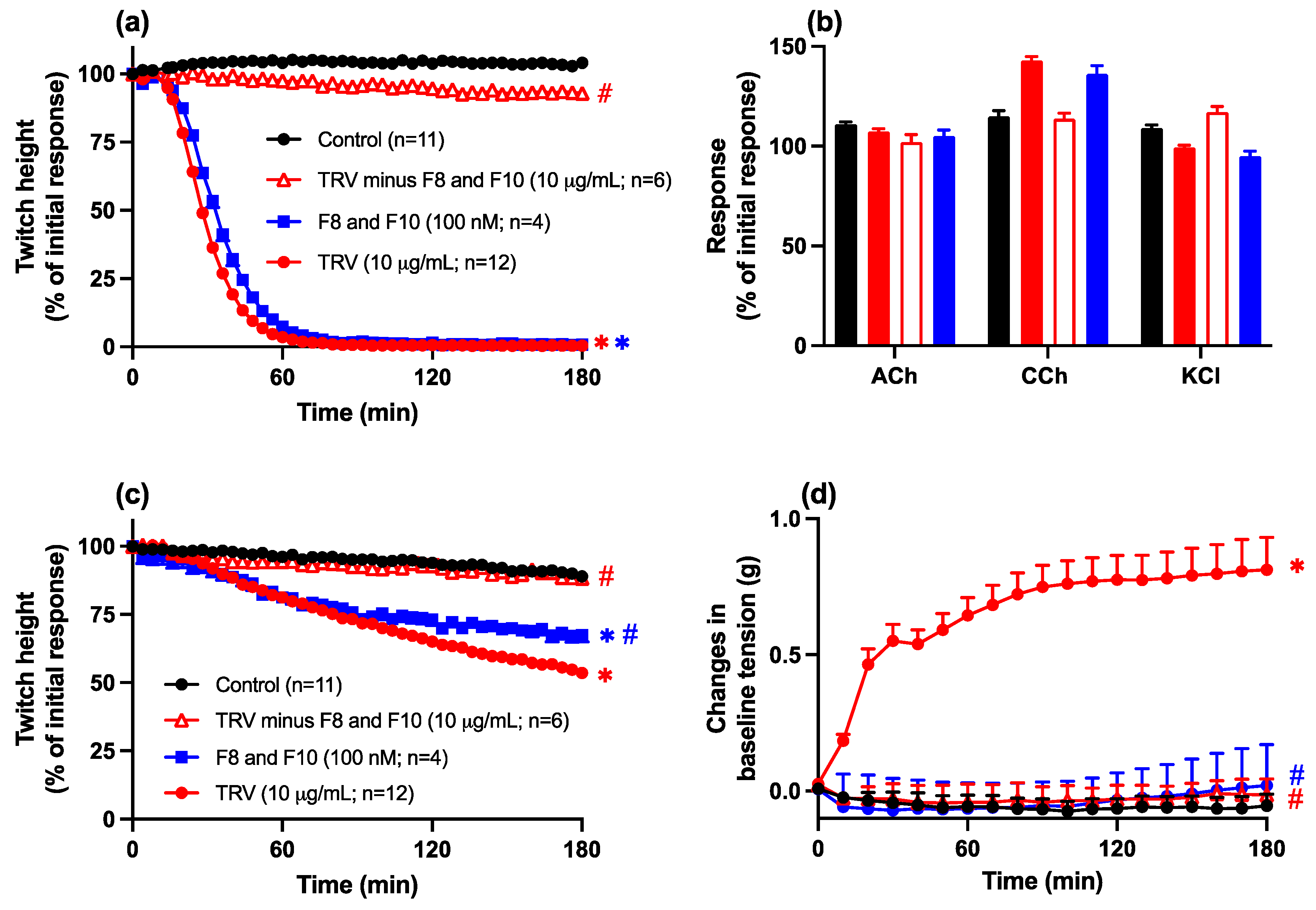
2.1. Whole D. siamensis Venom In Vitro Neurotoxicity and Myotoxicity
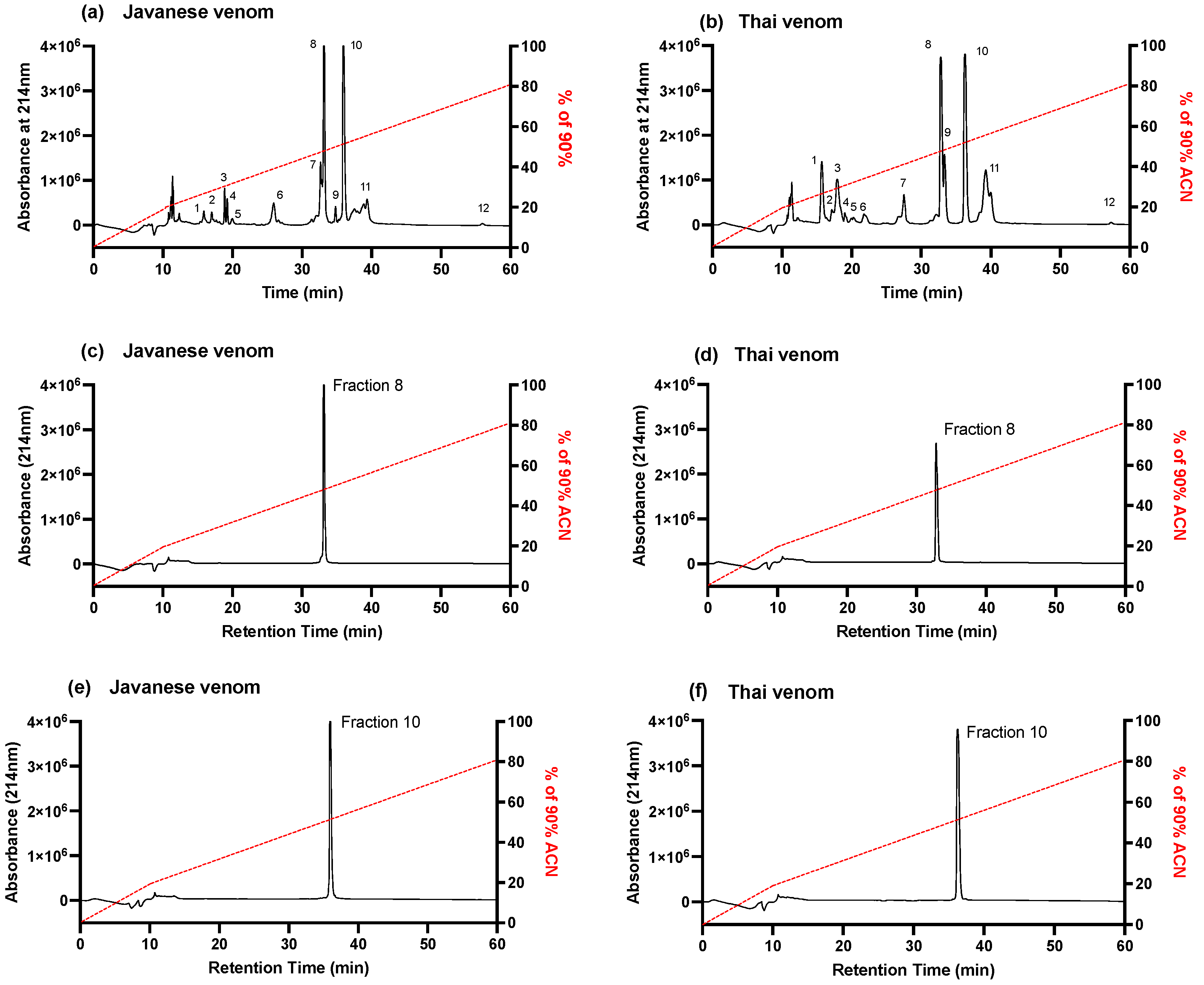
2.2. Isolation of Toxins
2.2.1. Fractionation of D. siamensis Venom via Reverse-Phase HPLC
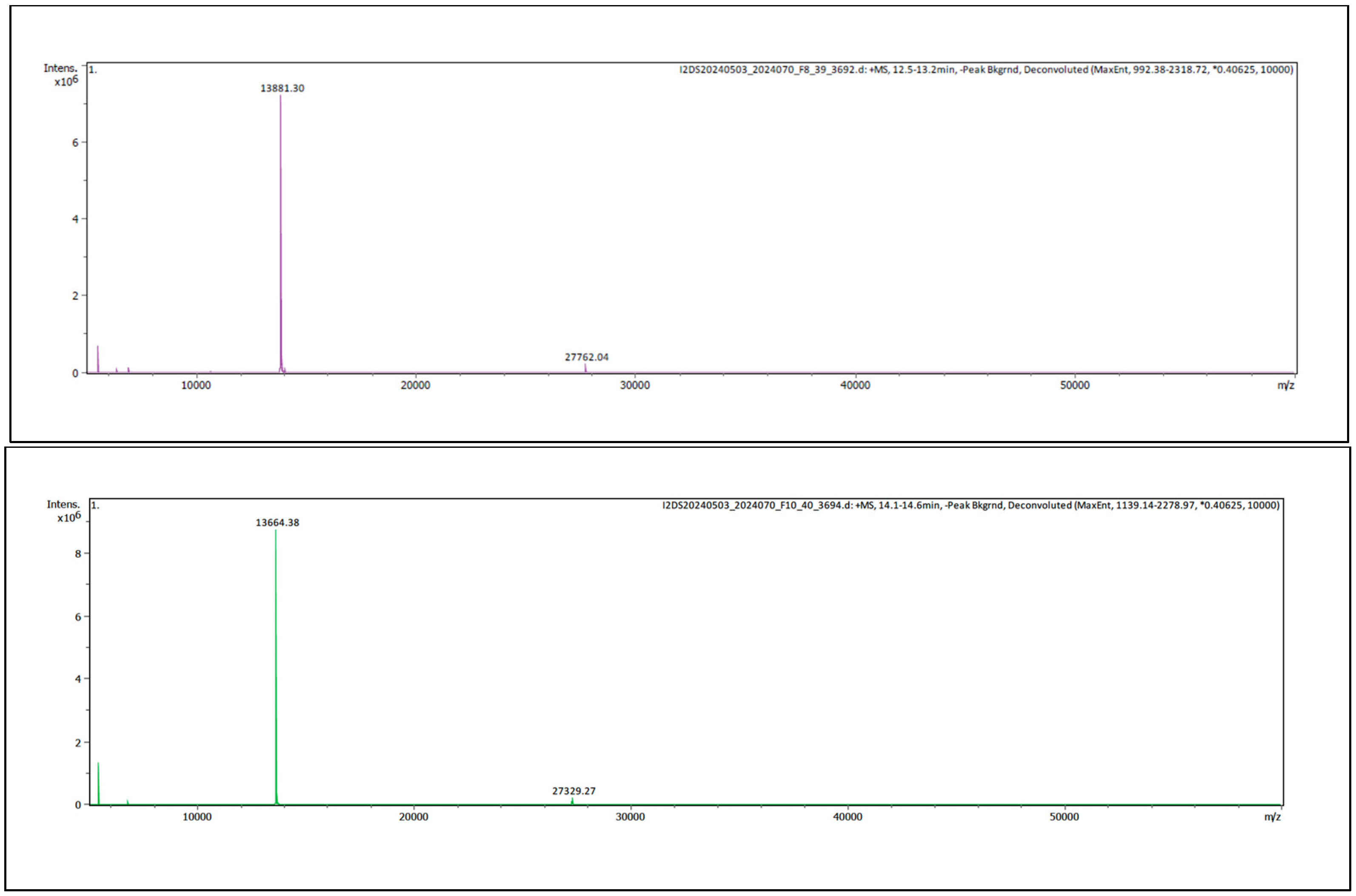
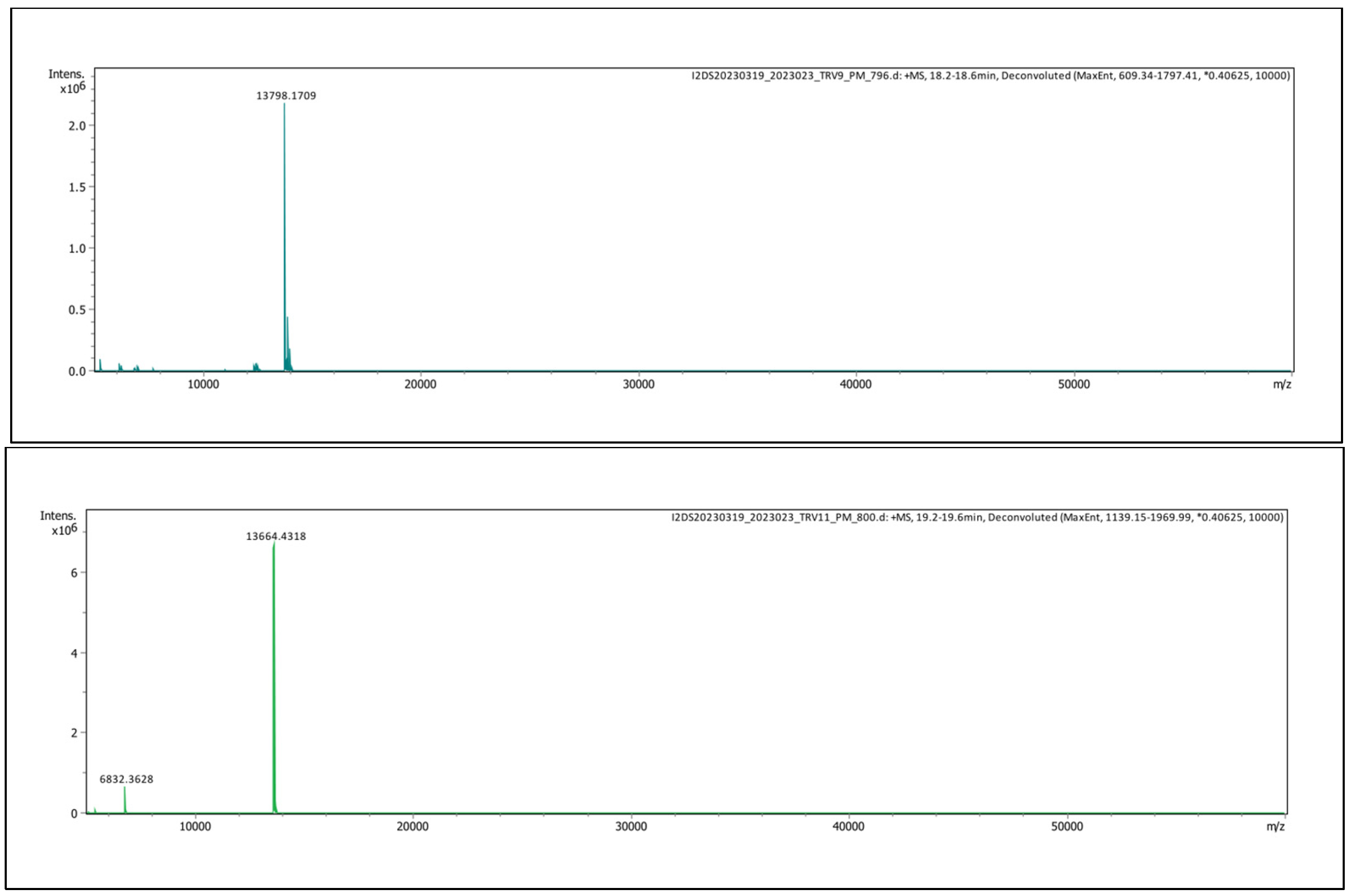
2.2.2. Intact Protein Analysis
2.3. In Vitro Neurotoxicity and Myotoxicity
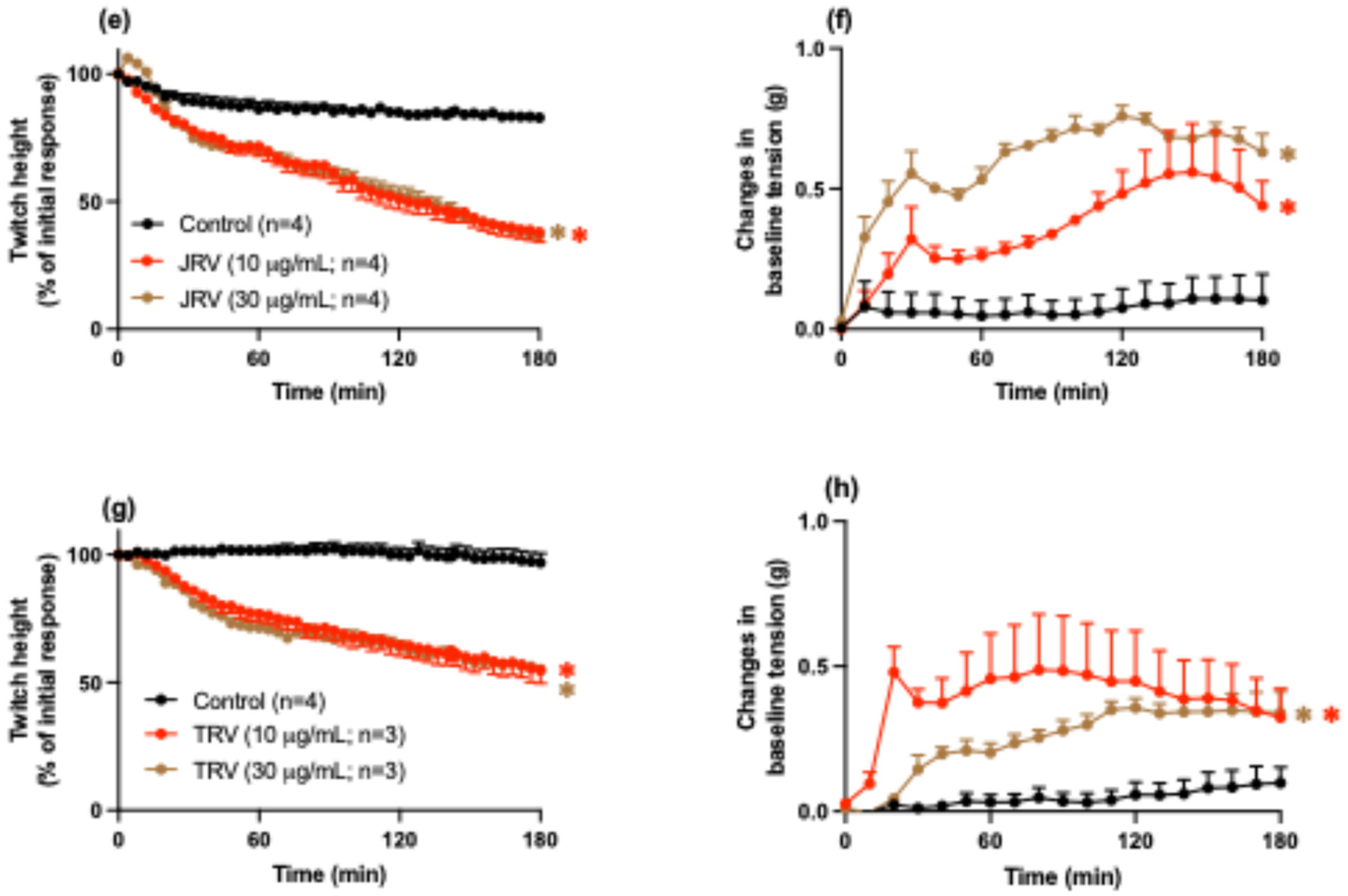
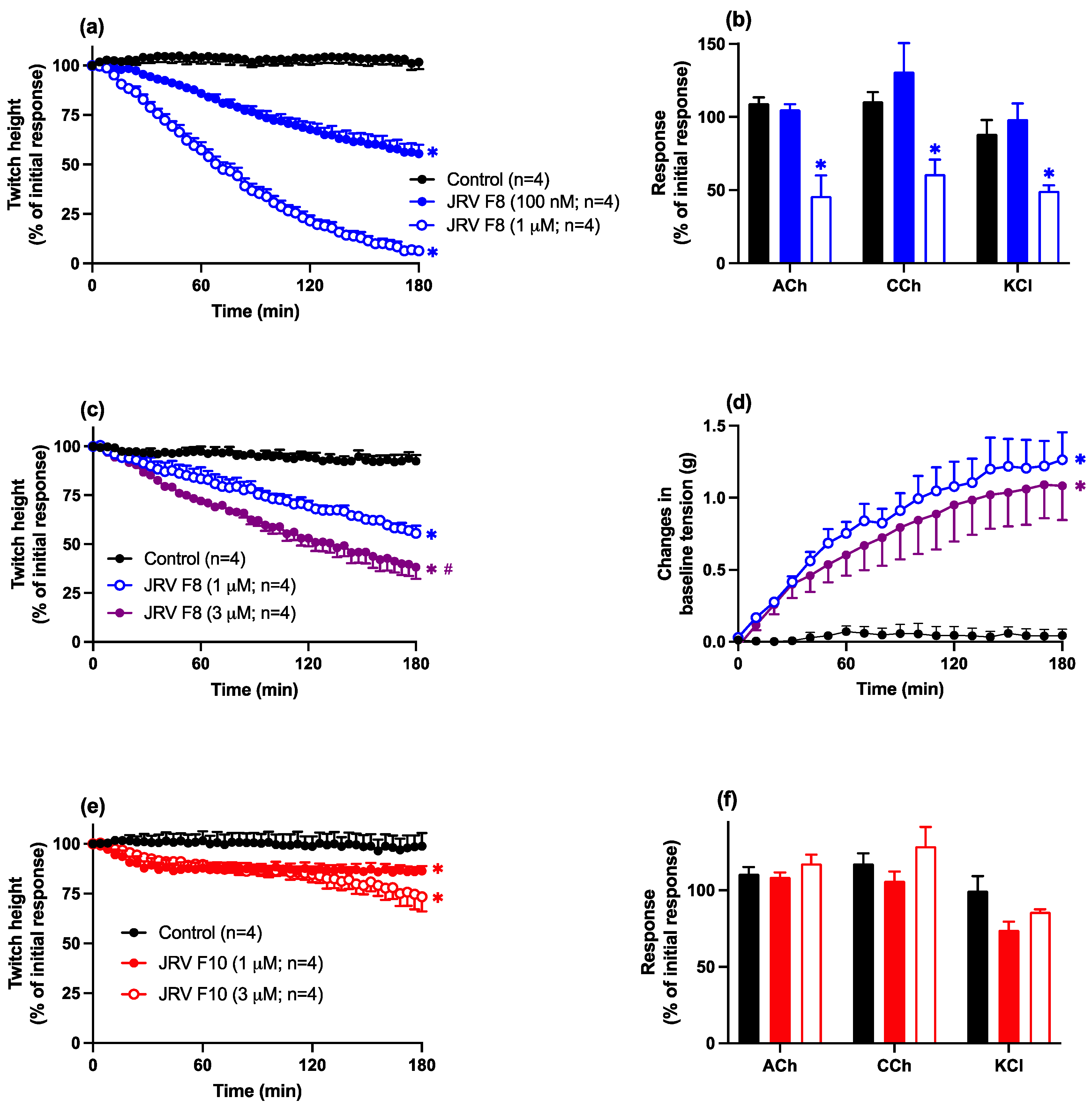
2.3.1. Javanese D. siamensis Venom Fractions
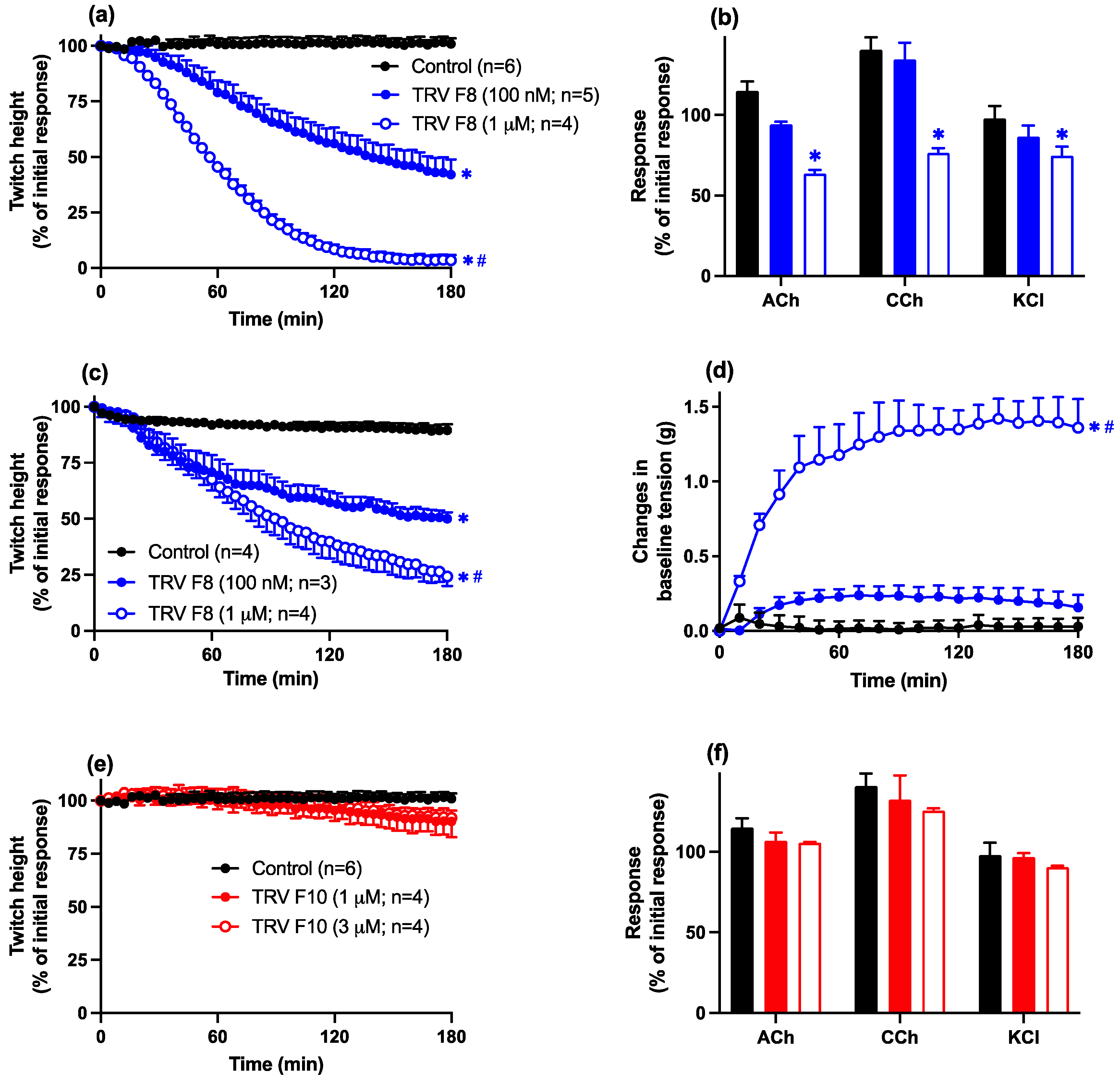
2.3.2. Thai D. siamensis Venom Fractions
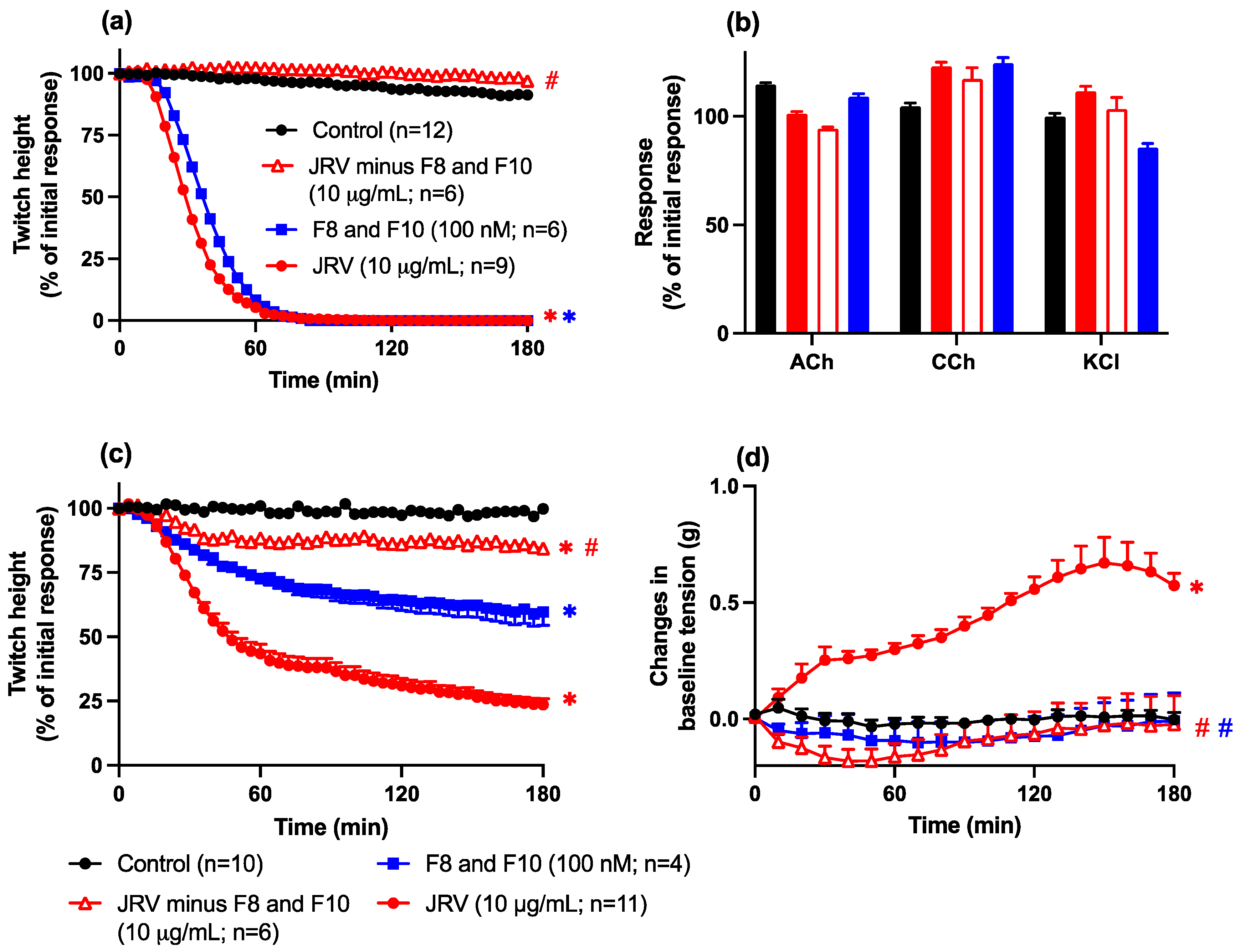
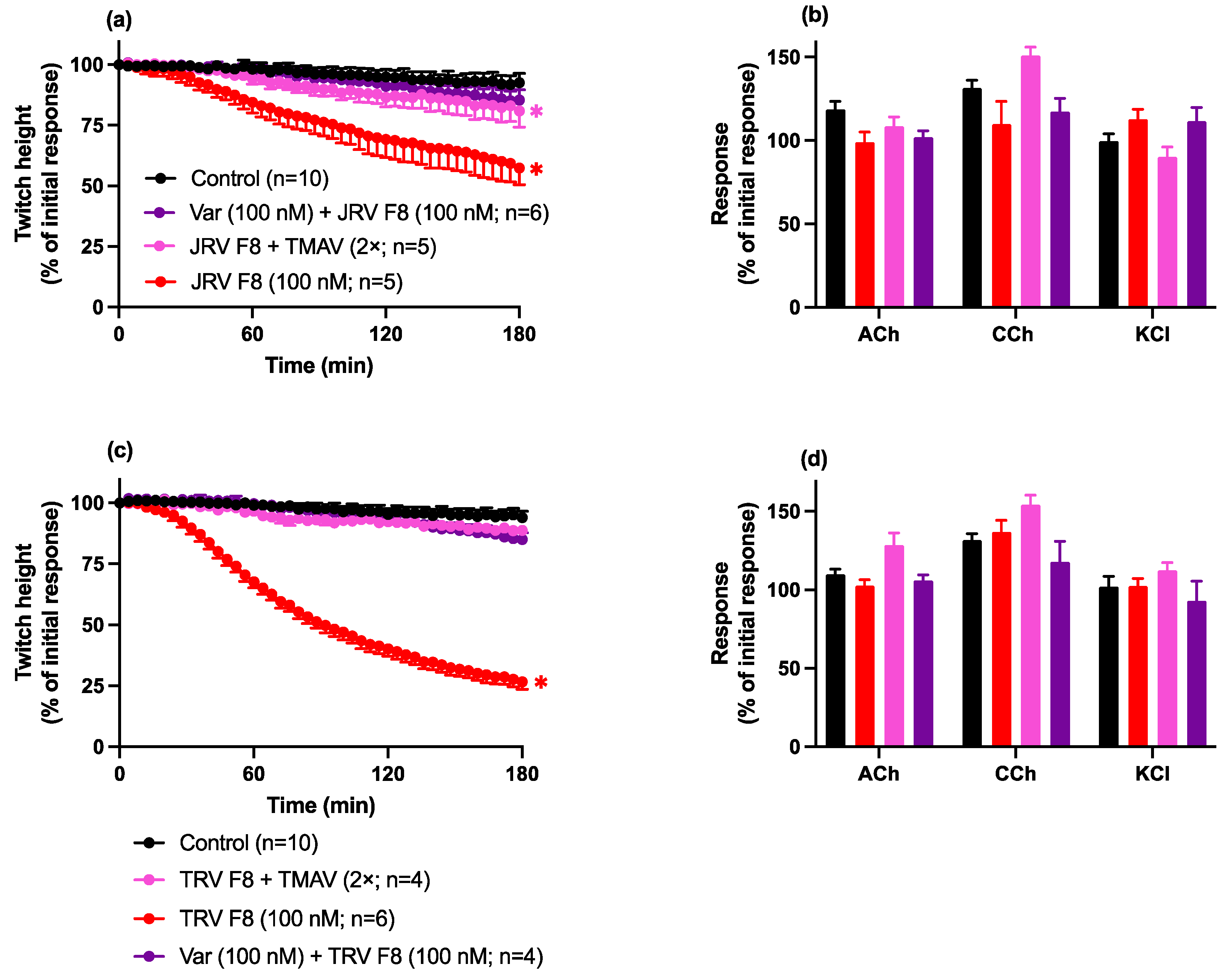
2.3.3. Removal, or Combined Activity, of Fractions 8 and 10 from Javanese and Thai D. siamensis Venoms
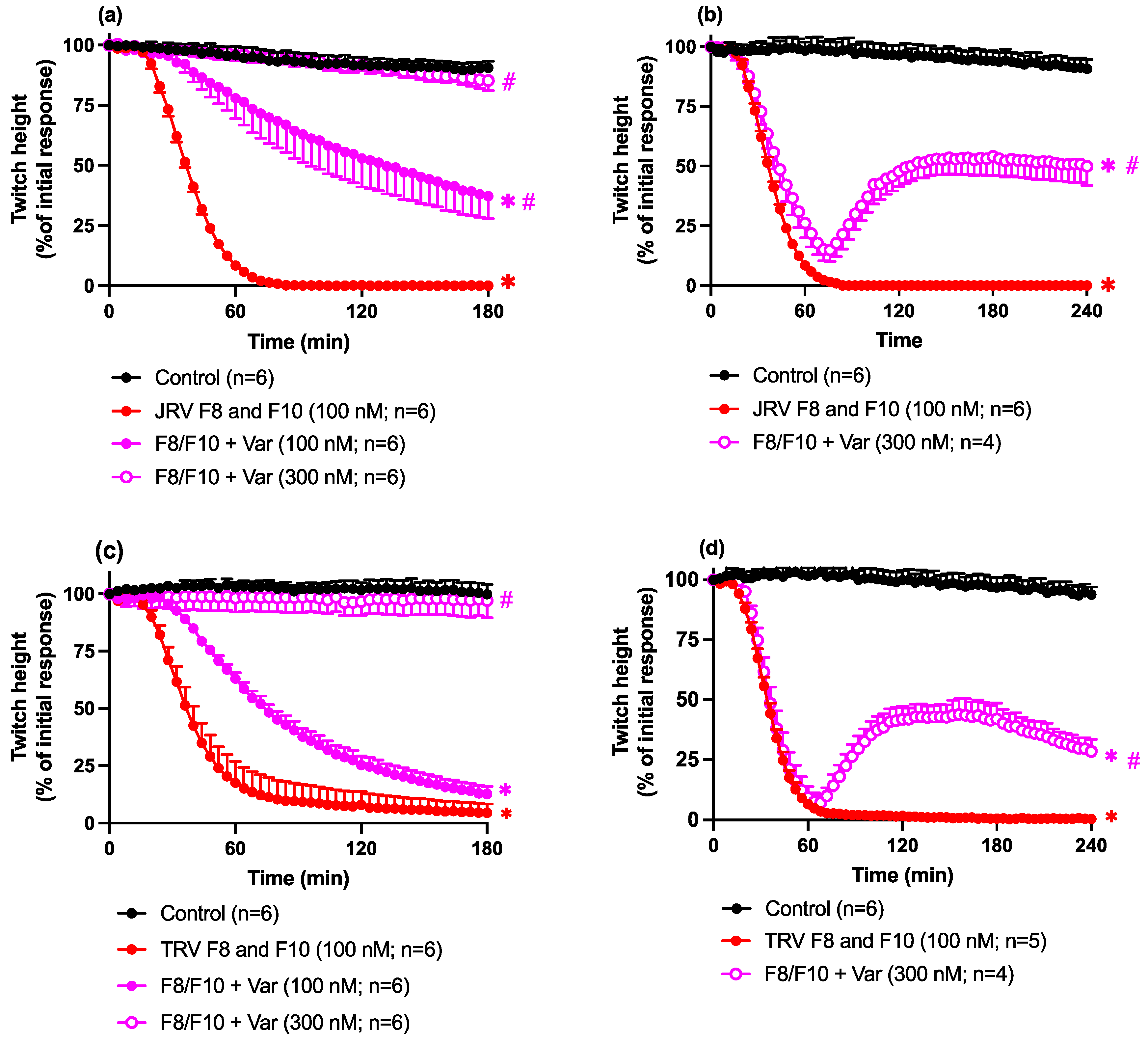
2.4. Antivenom or Varespladib Studies
Neurotoxicity: Prevention Studies
2.5. PLA2 Activity
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals
5.2. Chemicals and Drugs
5.3. Venoms and Antivenoms
5.4. Isolation and Purification of Toxins
5.4.1. Reverse Phase-High Performance Liquid Chromatography (RP-HPLC)
5.4.2. Liquid-Chromatography Electro-Spray Ionisation Intact Protein Analysis Coupled with Mass Spectrometry/Mass Spectrometry (ESI-LCMS/MS)
5.4.3. Intact Mass Spectrometry QTOF
5.5. Chick Biventer Cervicis Nerve-Muscle Preparation
5.6. PLA2 Activity
5.7. Data Analysis and Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casewell, N.R.; Jackson, T.N.W.; Laustsen, A.H.; Sunagar, K. Causes and Consequences of Snake Venom Variation. Trends Pharmacol. Sci. 2020, 41, 570–581. [Google Scholar] [CrossRef]
- Gopalan, G.; Thwin, M.M.; Gopalakrishnakone, P.; Swaminathan, K. Structural and pharmacological comparison of daboiatoxin from Daboia russelli siamensis with viperotoxin F and vipoxin from other vipers. Acta Crystallogr. D Biol. Crystallogr. 2007, 63, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Lingam, T.M.C.; Tan, K.Y.; Tan, C.H. Proteomics and antivenom immunoprofiling of Russell’s viper (Daboia siamensis) venoms from Thailand and Indonesia. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190048. [Google Scholar] [CrossRef]
- Tan, K.Y.; Tan, N.H.; Tan, C.H. Venom proteomics and antivenom neutralization for the Chinese eastern Russell’s viper, Daboia siamensis from Guangxi and Taiwan. Sci. Rep. 2018, 8, 8545. [Google Scholar] [CrossRef]
- Warrell, D.A. Snake venoms in science and clinical medicine. 1. Russell’s viper: Biology, venom and treatment of bites. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Huang, C. Synergistic strategies of predominant toxins in snake venoms. Toxicol. Lett. 2018, 287, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Warrell, D.A. Guidelines for the Management of Snake-Bites; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Chanhome, L.; Cox, M.J.; Wilde, H.; Jintakoon, P.; Chaiyabutr, N.; Sitprija, V. Venomous Snakebite in Thailand I: Medically Important Snakes. Mil. Med. 1998, 163, 310–317. [Google Scholar] [CrossRef]
- Looareesuwan, S.; Viravan, C.; Warrell, D.A. Factors contributing to fatal snakebite in the rural tropics: Analysis of 46 cases in Thailand. Trans. R. Soc. Trop. Med. Hyg. 1988, 82, 930–934. [Google Scholar] [CrossRef]
- Lertsakulbunlue, S.; Suebtuam, R.; Eamchotchawalit, T.; Chantkran, W.; Chaisakul, J. Clinical Profile and Pharmacological Management of Snakebites in Community Care Units: A Retrospective Study Using Two Military Hospital Databases in South Thailand. Trop. Med. Infect. Dis. 2023, 8, 346. [Google Scholar] [CrossRef]
- Adiwinata, R.; Nelwan, E.J. Snakebite in Indonesia. Acta Med. Indones 2015, 47, 358–365. [Google Scholar]
- Belt, P.J.; Malhotra, A.; Thorpe, R.S.; Warrell, D.A.; Wüster, W. Russell’s viper in Indonesia: Snakebite and systematics. Symp. Zool. Soc. Lond. 1997, 70, 219–234. [Google Scholar]
- Kini, R.M. Excitement ahead: Structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon 2003, 42, 827–840. [Google Scholar] [CrossRef]
- Sanz, L.; Quesada-bernat, S.; Chen, P.Y.; Lee, C.D.; Chiang, J.R.; Calvete, J.J. Translational Venomics: Third-Generation Antivenomics of Anti-Siamese Russell’s Viper, Daboia siamensis, Antivenom Manufactured in Taiwan CDC’s Vaccine Center. Trop. Med. Infect. Dis. 2018, 3, 66. [Google Scholar] [CrossRef]
- Tsai, I.H.; Lu, P.J.; Su, J.C. Two types of Russell’s viper revealed by variation in Phospholipases A2 from venom of the subspecies. Toxicon 1996, 34, 99–109. [Google Scholar] [CrossRef]
- Wang, Y.M.; Lu, P.J.; Ho, C.L.; Tsai, I.H. Characterization and molecular cloning of neurotoxic phospholipases A2 from Taiwan viper (Vipera russelli formosensis). Eur. J. Biochem. 1992, 209, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.B.; Scott-Davey, T. Secreted phospholipases A2 of snake venoms: Effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry. Toxins 2013, 5, 2533–2571. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, R.S.; Pook, C.E.; Malhotra, A. Phylogeography of the Russell’s viper (Daboia russelii) complex in relation to variation in the colour pattern and symptoms of envenoming. Herpetol. J. 2007, 17, 209–217. [Google Scholar]
- Lay, M.; Hodgson, W.C. A Comparison of the Efficacy of Antivenoms and Varespladib against the In Vitro Pre-Synaptic Neurotoxicity of Thai and Javanese Russell’s Viper (Daboia spp.) Venoms. Toxins 2024, 16, 124. [Google Scholar] [CrossRef]
- Bustillo, S.; Gay, C.C.; García Denegri, M.E.; Ponce-Soto, L.A.; Bal de Kier Joffé, E.; Acosta, O.; Leiva, L.C. Synergism between baltergin metalloproteinase and Ba SPII RP4 PLA2 from Bothrops alternatus venom on skeletal muscle (C2C12) cells. Toxicon 2012, 59, 338–343. [Google Scholar] [CrossRef]
- Cintra-Francischinelli, M.; Pizzo, P.; Rodrigues-Simioni, L.; Ponce-Soto, L.A.; Rossetto, O.; Lomonte, B.; Gutiérrez, J.M.; Pozzan, T.; Montecucco, C. Calcium imaging of muscle cells treated with snake myotoxins reveals toxin synergism and presence of acceptors. Cell Mol. Life Sci. 2009, 66, 1718–1728. [Google Scholar] [CrossRef]
- Ghazaryan, N.A.; Ghulikyan, L.; Kishmiryan, A.; Andreeva, T.V.; Utkin, Y.N.; Tsetlin, V.I.; Lomonte, B.; Ayvazyan, N.M. Phospholipases A2 from Viperidae snakes: Differences in membranotropic activity between enzymatically active toxin and its inactive isoforms. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Mora-Obando, D.; Fernández, J.; Montecucco, C.; Gutiérrez, J.M.; Lomonte, B. Synergism between basic Asp49 and Lys49 phospholipase A2 myotoxins of viperid snake venom in vitro and in vivo. PLoS ONE 2014, 9, e109846. [Google Scholar] [CrossRef] [PubMed]
- Doley, R.; Kini, R.M. Protein complexes in snake venom. Cell Mol. Life Sci. 2009, 66, 2851–2871. [Google Scholar] [CrossRef] [PubMed]
- Choumet, V.; Saliou, B.; Fideler, L.; Chen, Y.C.; Gubensek, F.; Bon, C.; Delot, E. Snake-venom phospholipase A2 neurotoxins. Potentiation of a single-chain neurotoxin by the chaperone subunit of a two-component neurotoxin. Eur. J. Biochem. 1993, 211, 57–62. [Google Scholar] [CrossRef]
- Fernandes, C.A.; Pazin, W.M.; Dreyer, T.R.; Bicev, R.N.; Cavalcante, W.L.; Fortes-Dias, C.L.; Ito, A.S.; Oliveira, C.L.; Fernandez, R.M.; Fontes, M.R. Biophysical studies suggest a new structural arrangement of crotoxin and provide insights into its toxic mechanism. Sci. Rep. 2017, 7, 43885. [Google Scholar] [CrossRef]
- Silva, A.; Kuruppu, S.; Othman, I.; Goode, R.J.; Hodgson, W.C.; Isbister, G.K. Neurotoxicity in Sri Lankan Russell’s Viper (Daboia russelii) Envenoming is Primarily due to U1-viperitoxin-Dr1a, a Pre-Synaptic Neurotoxin. Neurotox. Res. 2017, 31, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Maung Maung, T.; Gopalakrishnakone, P.; Yuen, R.; Tan, C.H. A major lethal factor of the venom of Burmese Russell’s viper (Daboia russelli siamensis): Isolation, N-terminal sequencing and biological activities of daboiatoxin. Toxicon 1995, 33, 63–76. [Google Scholar] [CrossRef]
- Perbandt, M.; Tsai, I.H.; Fuchs, A.; Banumathi, S.; Rajashankar, K.R.; Georgieva, D.; Kalkura, N.; Singh, T.P.; Genov, N.; Betzel, C. Structure of the heterodimeric neurotoxic complex viperotoxin F (RV-4/RV-7) from the venom of Vipera russelli formosensis at 1.9 A resolution. Acta Crystallogr. D Biol. Crystallogr. 2003, 59, 1679–1687. [Google Scholar] [CrossRef]
- Chaisakul, J.; Khow, O.; Wiwatwarayos, K.; Rusmili, M.R.A.; Prasert, W.; Othman, I.; Abidin, S.A.Z.; Charoenpitakchai, M.; Hodgson, W.C.; Chanhome, L.; et al. A Biochemical and Pharmacological Characterization of Phospholipase A2 and Metalloproteinase Fractions from Eastern Russell’s Viper (Daboia siamensis) Venom: Two Major Components Associated with Acute Kidney Injury. Toxins 2021, 13, 521. [Google Scholar] [CrossRef]
- Lingam, T.M.C.; Tan, K.Y.; Tan, C.H. Capillary leak syndrome induced by the venoms of Russell’s Vipers (Daboia russelii and Daboia siamensis) from eight locales and neutralization of the differential toxicity by three snake antivenoms. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 250, 109186. [Google Scholar] [CrossRef]
- Saikia, D.; Majumdar, S.; Mukherjee, A.K. Mechanism of in vivo anticoagulant and haemolytic activity by a neutral phospholipase A2 purified from Daboia russelii russelii venom: Correlation with clinical manifestations in Russell’s Viper envenomed patients. Toxicon 2013, 76, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Ponce-Soto, L.A.; Marangoni, S.; Lomonte, B. Systemic and local myotoxicity induced by snake venom group II phospholipases A2: Comparison between crotoxin, crotoxin B and a Lys49 PLA2 homologue. Toxicon 2008, 51, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, M.; Gowda, V. Synergistically acting PLA2: Peptide hemorrhagic complex from Daboia russelii venom. Toxicon 2013, 73, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Pochanugool, C.; Wilde, H.; Bhanganada, K.; Chanhome, L.; Cox, M.; Chaiyabutr, N.; Sitprija, V. Venomous Snakebite in Thailand II: Clinical Experience. Mil. Med. 1998, 163, 318–323. [Google Scholar] [CrossRef]
- Saethang, T.; Somparn, P.; Payungporn, S.; Sriswasdi, S.; Yee, K.T.; Hodge, K.; Knepper, M.A.; Chanhome, L.; Khow, O.; Chaiyabutr, N.; et al. Identification of Daboia siamensis venome using integrated multi-omics data. Sci. Rep. 2022, 12, 13140. [Google Scholar] [CrossRef]
- Tan, N.H.; Fung, S.Y.; Tan, K.Y.; Yap, M.K.K.; Gnanathasan, C.A.; Tan, C.H. Functional venomics of the Sri Lankan Russell’s viper (Daboia russelii) and its toxinological correlations. J. Proteom. 2015, 128, 403–423. [Google Scholar] [CrossRef]
- Oliveira, A.; Bleicher, L.; Schrago, C.G.; Silva Junior, F.P. Conservation analysis and decomposition of residue correlation networks in the phospholipase A2 superfamily (PLA2s): Insights into the structure-function relationships of snake venom toxins. Toxicon 2018, 146, 50–60. [Google Scholar] [CrossRef]
- Sai-Ngam, A.; Phongtananant, S.; Nuchprayoon, I. Phospholipase A2 genes and their expressions in Thai Russell’s viper venom glands. Toxicon 2008, 52, 395–399. [Google Scholar] [CrossRef]
- Tsai, I.H.; Wang, Y.M. Geographic Variations of Venom from Daboia siamensis, Based on the Phospholipase Variants. Available online: https://www.uniprot.org/uniprotkb/B3RFI7/entry (accessed on 9 September 2024).
- Laustsen, A.H. Toxin synergism in snake venoms. Toxin Rev. 2016, 35, 165–170. [Google Scholar] [CrossRef]
- de Oliveira, A.L.N.; Lacerda, M.T.; Ramos, M.J.; Fernandes, P.A. Viper Venom Phospholipase A2 Database: The Structural and Functional Anatomy of a Primary Toxin in Envenomation. Toxins 2024, 16, 71. [Google Scholar] [CrossRef]
- Buranasin, P. Snakebites at Maharat Nakhon Ratchasima Regional Hospital. Southeast Asian J. Trop. Med. Public Health 1993, 24, 186–192. [Google Scholar] [PubMed]
- Sontichai, W.; Reungrongrat, S.; Narongchai, P.; Natesirinilkul, R. Neurological Involvement and Hepatocellular Injury Caused by a Snake With Hematotoxin Envenomation. Wilderness Environ. Med. 2015, 26, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Viravan, C.; Looareesuwan, S.; Kosakarn, W.; Wuthiekanun, V.; McCarthy, C.J.; Stimson, A.F.; Bunnag, D.; Harinasuta, T.; Warrell, D.A. A national hospital-based survey of snakes responsible for bites in Thailand. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 100–106. [Google Scholar] [CrossRef]
- Chaiyabutr, N.; Chanhome, L.; Vasaruchapong, T.; Laoungbua, P.; Khow, O.; Rungsipipat, A.; Reamtong, O.; Sitprija, V. Comparative compositional and functional venomic profiles among venom specimens from juvenile, subadult and adult Russell’s viper (Daboia siamensis): Correlation with renal pathophysiology in experimental rabbits. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, 20210111. [Google Scholar] [CrossRef]
- Barber, C.M.; Isbister, G.K.; Hodgson, W.C. Solving the ‘Brown snake paradox’: In vitro characterisation of Australasian snake presynaptic neurotoxin activity. Toxicol. Lett. 2012, 210, 318–323. [Google Scholar] [CrossRef]
- Silva, A.; Johnston, C.; Kuruppu, S.; Kneisz, D.; Maduwage, K.; Kleifeld, O.; Smith, A.I.; Siribaddana, S.; Buckley, N.A.; Hodgson, W.C.; et al. Clinical and Pharmacological Investigation of Myotoxicity in Sri Lankan Russell’s Viper (Daboia russelii) Envenoming. PLoS Negl. Trop. Dis. 2016, 10, e0005172. [Google Scholar] [CrossRef]
- Yuniasih, D.; Tejosukmono, A.; Heriyanto, J. Snakebite as a Neglected Tropical Diseases in Indonesia: A Review. Int. J. Sci. Technol. Res. 2021, 9, 6180–6185. [Google Scholar]
- Chaisakul, J.; Rusmili, M.R.A.; Alsolaiss, J.; Albulescu, L.-O.; Harrison, R.A.; Othman, I.; Casewell, N.R. In Vitro Immunological Cross-Reactivity of Thai Polyvalent and Monovalent Antivenoms with Asian Viper Venoms. Toxins 2020, 12, 766. [Google Scholar] [CrossRef] [PubMed]
- Maciel, F.V.; Ramos Pinto, Ê.K.; Valério Souza, N.M.; Gonçalves de Abreu, T.A.; Ortolani, P.L.; Fortes-Dias, C.L.; Garrido Cavalcante, W.L. Varespladib (LY315920) prevents neuromuscular blockage and myotoxicity induced by crotoxin on mouse neuromuscular preparations. Toxicon 2021, 202, 40–45. [Google Scholar] [CrossRef]
- Salvador, G.H.M.; Borges, R.J.; Lomonte, B.; Lewin, M.R.; Fontes, M.R.M. The synthetic varespladib molecule is a multi-functional inhibitor for PLA2 and PLA2-like ophidic toxins. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129913. [Google Scholar] [CrossRef]
- Salvador, G.H.M.; Gomes, A.A.S.; Bryan-Quirós, W.; Fernández, J.; Lewin, M.R.; Gutiérrez, J.M.; Lomonte, B.; Fontes, M.R.M. Structural basis for phospholipase A2-like toxin inhibition by the synthetic compound Varespladib (LY315920). Sci. Rep. 2019, 9, 17203. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, W.C.; Wickramaratna, J.C. In vitro neuromuscular activity of snake venoms. Clin. Exp. Pharmacol. Physiol. 2002, 29, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Prasarnpun, S.; Walsh, J.; Harris, J.B. Beta-bungarotoxin-induced depletion of synaptic vesicles at the mammalian neuromuscular junction. Neuropharmacology 2004, 47, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.C.F.; Gutiérrez, J.M.; Lewin, M.R.; Oshima-Franko, Y. Varespladib (LY315920) inhibits neuromuscular blockade induced by Oxyuranus scutellatus venom in nerve-muscle preparation. Toxicon 2020, 187, 101–104. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lay, M.; Hodgson, W.C. Isolation and Pharmacological Characterisation of Pre-Synaptic Neurotoxins from Thai and Javanese Russell’s Viper (Daboia siamensis) Venoms. Toxins 2024, 16, 405. https://doi.org/10.3390/toxins16090405
Lay M, Hodgson WC. Isolation and Pharmacological Characterisation of Pre-Synaptic Neurotoxins from Thai and Javanese Russell’s Viper (Daboia siamensis) Venoms. Toxins. 2024; 16(9):405. https://doi.org/10.3390/toxins16090405
Chicago/Turabian StyleLay, Mimi, and Wayne C. Hodgson. 2024. "Isolation and Pharmacological Characterisation of Pre-Synaptic Neurotoxins from Thai and Javanese Russell’s Viper (Daboia siamensis) Venoms" Toxins 16, no. 9: 405. https://doi.org/10.3390/toxins16090405
APA StyleLay, M., & Hodgson, W. C. (2024). Isolation and Pharmacological Characterisation of Pre-Synaptic Neurotoxins from Thai and Javanese Russell’s Viper (Daboia siamensis) Venoms. Toxins, 16(9), 405. https://doi.org/10.3390/toxins16090405




