Rab4b Promotes Cytolethal Distending Toxin from Glaesserella parasuis-Induced Cytotoxicity in PK-15 Cells
Abstract
1. Introduction
2. Results
2.1. Construction of the Rab4b Knockout and Overexpressing Cell Lines
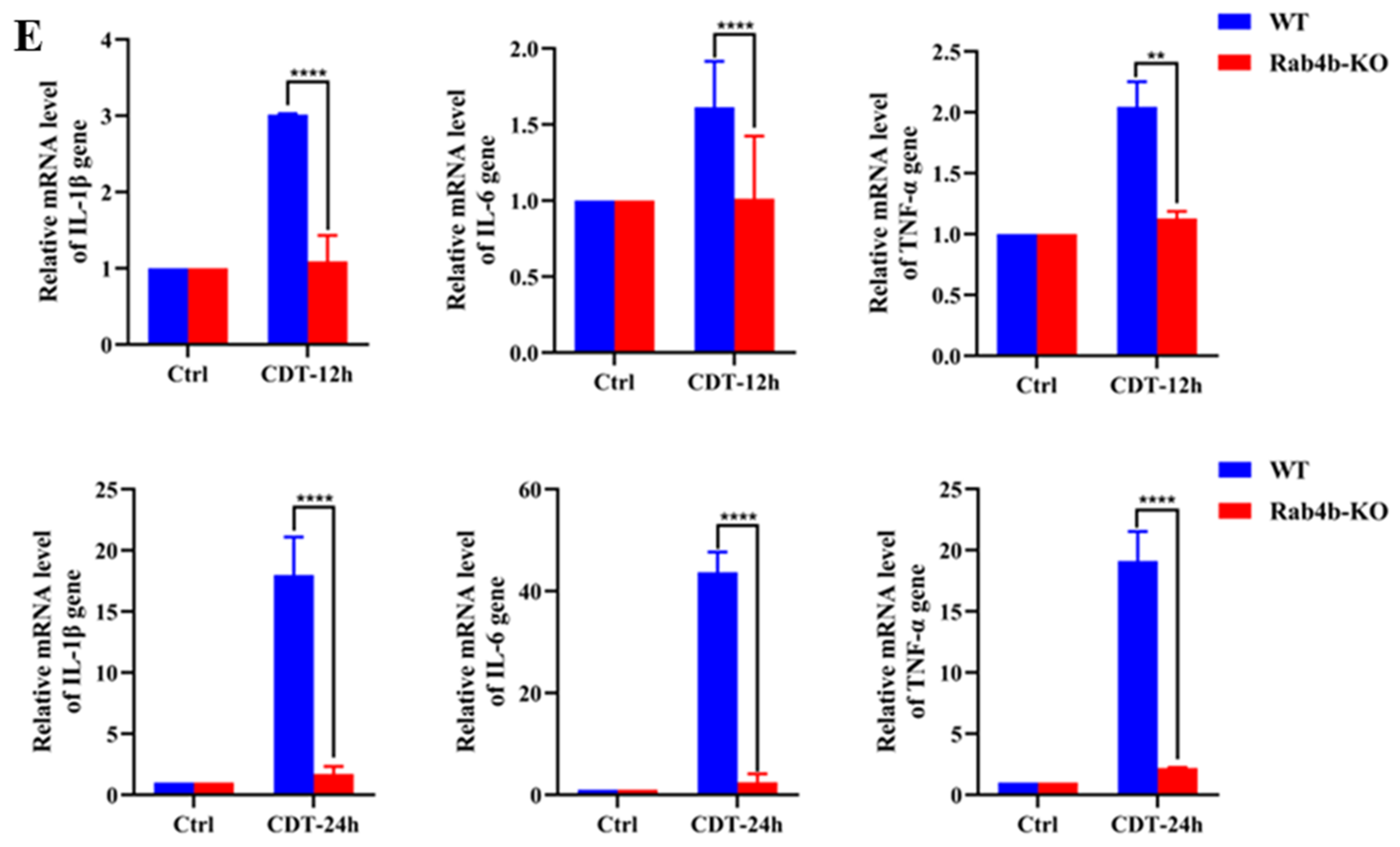
2.2. Rab4b Knockout Inhibits GpCDT-Induced Cytotoxicity in PK-15 Cells
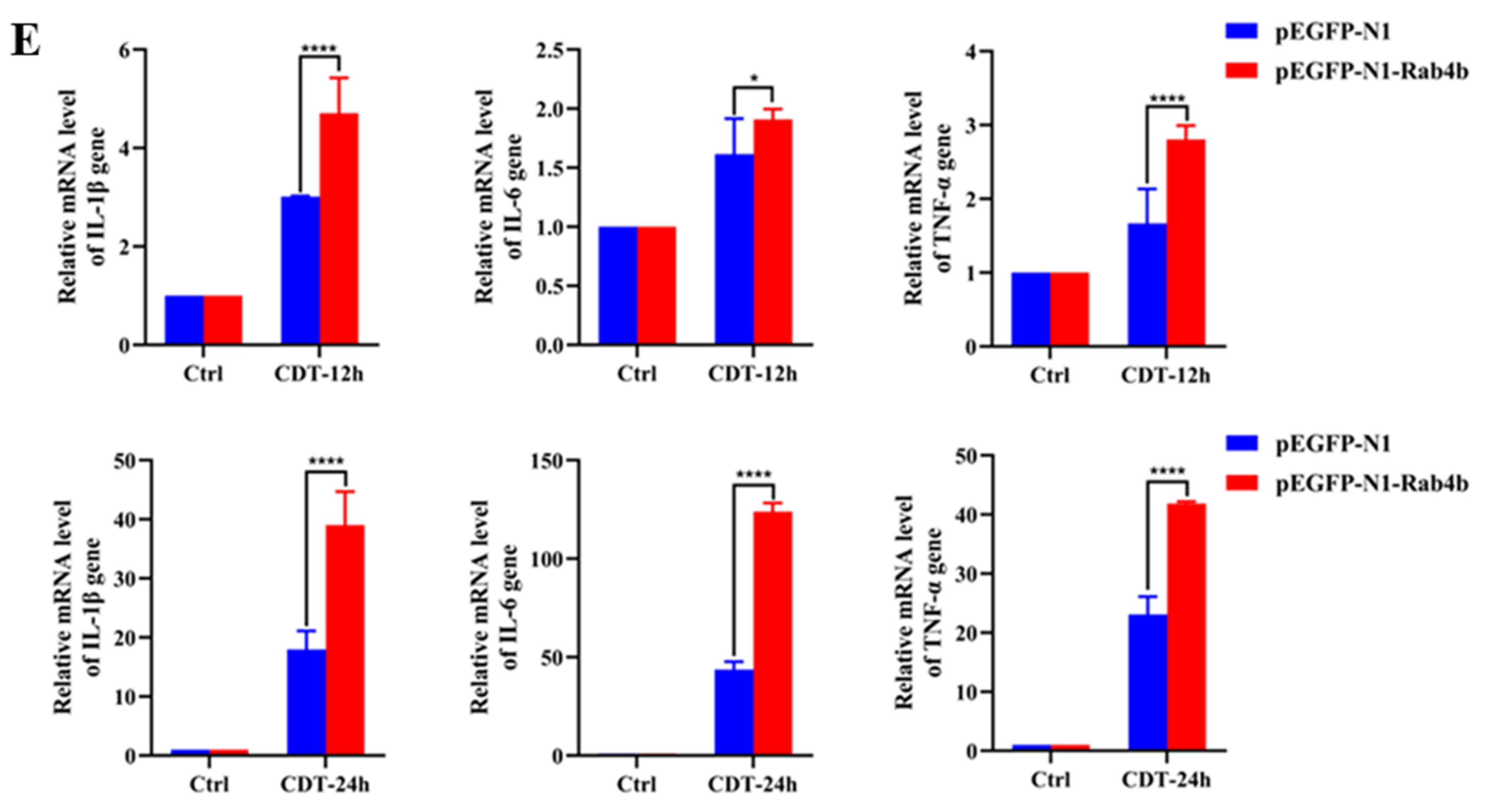
2.3. Rab4b Overexpression Enhances GpCDT-Induced Cytotoxicity in PK-15 Cells
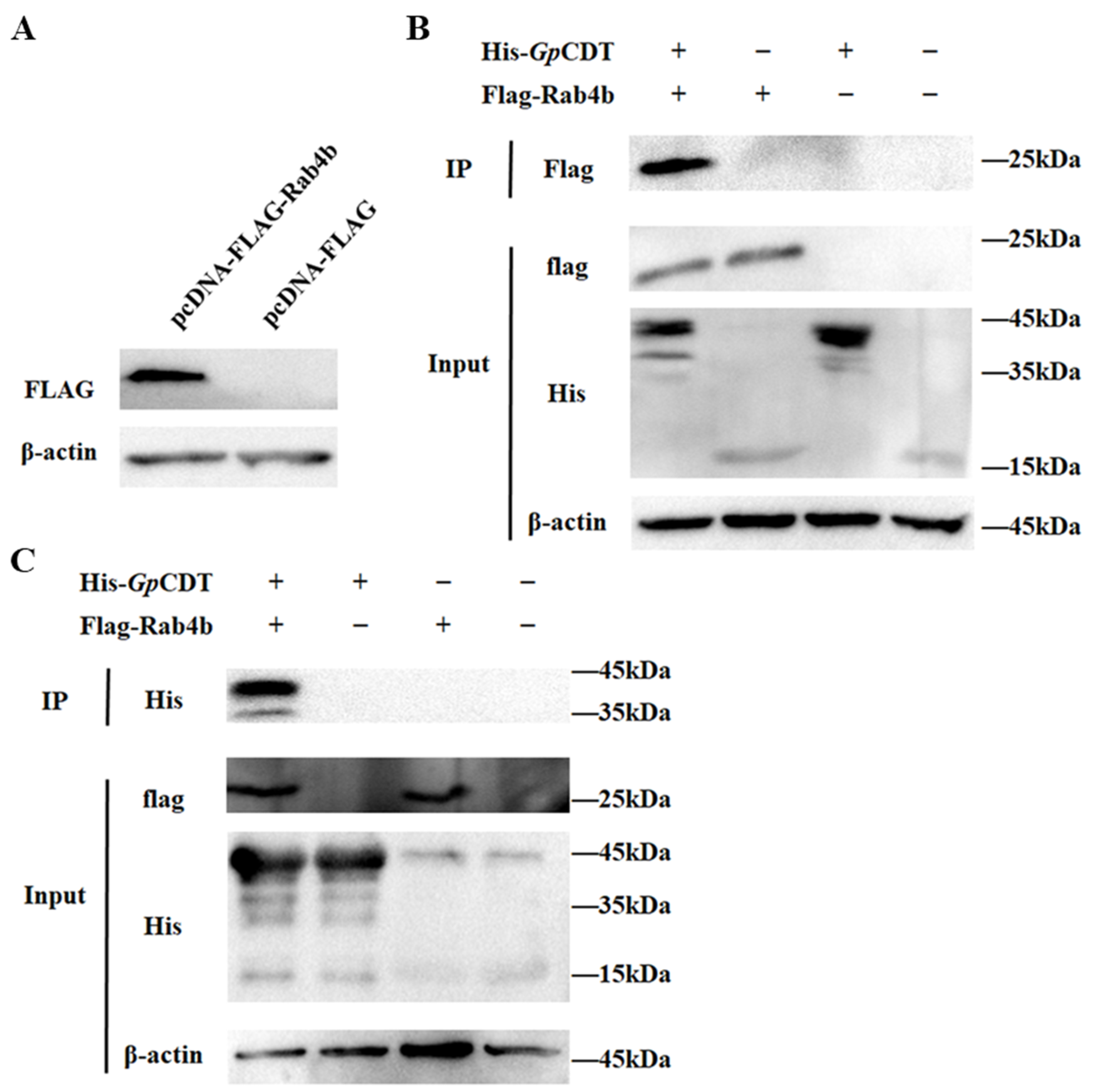
2.4. Further Validation of Interactions between Rab4b and GpCDT Proteins
2.5. Rab4b Affects the Vesicle Transport of GpCDT in PK-15 Cells
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cells, Plasmids, and Growth Conditions
5.2. Expression of GpCDT and Mouse Antiserum
5.3. Knockout of Rab4b Gene and Overexpression of Rab4b Protein
5.4. Microscopy Imaging
5.5. Cell Viability Assay
5.6. Western Blotting
5.7. Indirect Immunofluorescence
5.8. Quantitative Real-Time PCR
5.9. Creation of Plasmids and Coimmunoprecipitation Assay
5.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costa-Hurtado, M.; Barba-Vidal, E.; Maldonado, J.; Aragon, V. Update on Glässer’s disease: How to control the disease under restrictive use of antimicrobials. Veter. Microbiol. 2020, 242, 108595. [Google Scholar] [CrossRef] [PubMed]
- Brockmeier, S.L.; Loving, C.L.; Mullins, M.A.; Register, K.B.; Nicholson, T.L.; Wiseman, B.S.; Baker, R.B.; Kehrli, M.E. Virulence, Transmission, and Heterologous Protection of Four Isolates of Haemophilus parasuis. Clin. Vaccine Immunol. 2013, 20, 1466–1472. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, C.; Guo, L.; Ke, B.; Ke, C.; Zhang, B.; Deng, X.; Liao, M. A rapid pulsed-field gel electrophoresis method of genotyping Haemophilus parasuis isolates. Lett. Appl. Microbiol. 2011, 52, 589–595. [Google Scholar] [CrossRef]
- Rafiee, M.; Blackall, P. Establishment, validation and use of the Kielstein-Rapp-Gabrielson serotyping scheme for Haemophilus parasuis. Aust. Veter. J. 2000, 78, 172–174. [Google Scholar] [CrossRef]
- Wang, Q.; Chang, X.; Liu, M.; Lu, Q.; Zhu, M.; Lin, H.; Fan, H. Glaesserella parasuis serotype 4 HPS4-YC disrupts the integrity of the swine tracheal epithelial barrier and facilitates bacterial translocation. Veter. Res. 2021, 52, 135. [Google Scholar] [CrossRef]
- Ni, H.-B.; Gong, Q.-L.; Zhao, Q.; Li, X.-Y.; Zhang, X.-X. Prevalence of Haemophilus parasuis “Glaesserella parasuis” in pigs in China: A systematic review and meta-analysis. Prev. Veter. Med. 2020, 182, 105083. [Google Scholar] [CrossRef]
- Pons, B.J.; Vignard, J.; Mirey, G. Cytolethal Distending Toxin Subunit B: A Review of Structure-Function Relationship. Toxins 2019, 11, 595. [Google Scholar] [CrossRef]
- Scuron, M.D.; Boesze-Battaglia, K.; Dlakić, M.; Shenker, B.J. The Cytolethal Distending Toxin Contributes to Microbial Virulence and Disease Pathogenesis by Acting as a Tri-Perditious Toxin. Front. Cell. Infect. Microbiol. 2016, 6, 168. [Google Scholar] [CrossRef]
- Sahni, A.; Pei, D. Bacterial Toxins Escape the Endosome by Inducing Vesicle Budding and Collapse. ACS Chem. Biol. 2021, 16, 2415–2422. [Google Scholar] [CrossRef]
- Matangkasombut, O.; Wattanawaraporn, R.; Tsuruda, K.; Ohara, M.; Sugai, M.; Mongkolsuk, S. Cytolethal Distending Toxin from Aggregatibacter actinomycetemcomitans Induces DNA Damage, S/G2Cell Cycle Arrest, and Caspase- Independent Death in a Saccharomyces cerevisiae Model. Infect. Immun. 2010, 78, 783–792. [Google Scholar] [CrossRef]
- Boesze-Battaglia, K.; Dhingra, A.; Walker, L.M.; Zekavat, A.; Shenker, B.J. Internalization and Intoxication of Human Macrophages by the Active Subunit of the Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin Is Dependent upon Cellugyrin (Synaptogyrin-2). Front. Immunol. 2020, 11, 1262. [Google Scholar] [CrossRef] [PubMed]
- Tsuruda, K.; Matangkasombut, O.; Ohara, M.; Sugai, M. CdtC-Induced Processing of Membrane-Bound CdtA Is a Crucial Step in Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin Holotoxin Formation. Infect. Immun. 2018, 86, e00731-17. [Google Scholar] [CrossRef] [PubMed]
- Shenker, B.J.; Boesze-Battaglia, K.; Scuron, M.D.; Walker, L.P.; Zekavat, A.; Dlakić, M. The toxicity of the Aggregatibacter actinomycetemcomitans cytolethal distending toxin correlates with its phosphatidylinositol-3,4,5-triphosphate phosphatase activity. Cell. Microbiol. 2016, 18, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-P.; Li, L.; Chen, X.; Yang, M.-F.; Ye, Y.; Wang, X.-Q.; Xu, Y. The mechanism of Jurkat cells apoptosis induced by Aggregatibacter actinomycetemcomitans cytolethal distending toxin. Apoptosis Int. J. Program. Cell Death 2017, 22, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Huhn, G.R.; Sparkes, C.; Silva, I.; Reyes, C.; Perez, G.; Khondker, F.; Jones, T.; Fragoso, A.; Contreras, P.; Alvarez, M.; et al. Acid-induced disassembly of the Haemophilus ducreyi cytolethal distending toxin. Biochem. Biophys. Res. Commun. 2022, 636, 57–63. [Google Scholar] [CrossRef]
- Dixon, S.D.; Huynh, M.M.; Tamilselvam, B.; Spiegelman, L.M.; Son, S.B.; Eshraghi, A.; Blanke, S.R.; Bradley, K.A. Distinct Roles for CdtA and CdtC during Intoxication by Cytolethal Distending Toxins. PLoS ONE 2015, 10, e0143977. [Google Scholar] [CrossRef]
- Gargi, A.; Reno, M.; Blanke, S.R. Bacterial toxin modulation of the eukaryotic cell cycle: Are all cytolethal distending toxins created equally? Front. Cell. Infect. Microbiol. 2012, 2, 124. [Google Scholar] [CrossRef]
- Guerra, L.; Nemec, K.N.; Massey, S.; Tatulian, S.A.; Thelestam, M.; Frisan, T.; Teter, K. A novel mode of translocation for cytolethal distending toxin. Biochim. Biophys. Acta Mol. Cell Res. 2009, 1793, 489–495. [Google Scholar] [CrossRef]
- Jordens, I.; Marsman, M.; Kuijl, C.; Neefjes, J. Rab Proteins, Connecting Transport and Vesicle Fusion. Traffic 2005, 6, 1070–1077. [Google Scholar] [CrossRef]
- He, H.; Dai, F.; Yu, L.; She, X.; Zhao, Y.; Jiang, J.; Chen, X.; Zhao, S. Identification and Characterization of Nine Novel Human Small GTPases Showing Variable Expressions in Liver Cancer Tissues. Gene Expr. 2002, 10, 231–242. [Google Scholar] [CrossRef]
- Pereira-Leal, J.B.; Seabra, M.C. Evolution of the rab family of small GTP-binding proteins. J. Mol. Biol. 2001, 313, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Perrin, L.; Lacas-Gervais, S.; Gilleron, J.; Ceppo, F.; Prodon, F.; Benmerah, A.; Tanti, J.-F.; Cormont, M. Rab4b controls an early endosome sorting event by interacting with the γ subunit of the clathrin adaptor complex. J. Cell Sci. 2013, 126, 4950–4962. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Leimgruber, E.; Seguín-Estévez, Q.; Dunand-Sauthier, I.; Barras, E.; Reith, W. Expression of RAB4B, a protein governing endocytic recycling, is co-regulated with MHC class II genes. Nucleic Acids Res. 2006, 35, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Spanò, S.; Galán, J.E. Taking control: Hijacking of Rab GTPases by intracellular bacterial pathogens. Small GTPases 2018, 9, 182–191. [Google Scholar] [CrossRef]
- Gilleron, J.; Chafik, A.; Lacas-Gervais, S.; Tanti, J.-F.; Cormont, M. Golgi-associated retrograde protein (GARP) complex-dependent endosomes to trans Golgi network retrograde trafficking is controlled by Rab4b. Cell. Mol. Biol. Lett. 2024, 29, 54. [Google Scholar] [CrossRef]
- Mohrmann, K.; Gerez, L.; Oorschot, V.; Klumperman, J.; van der Sluijs, P. rab4 Function in Membrane Recycling from Early Endosomes Depends on a Membrane to Cytoplasm Cycle. J. Biol. Chem. 2002, 277, 32029–32035. [Google Scholar] [CrossRef]
- Taieb, F.; Petit, C.; Nougayrède, J.-P.; Oswald, E. The Enterobacterial Genotoxins: Cytolethal Distending Toxin and Colibactin. EcoSal Plus 2016, 7, 10.1128/ecosalplus.ESP-0008-2016. [Google Scholar] [CrossRef]
- Guerra, L.; Cortes-Bratti, X.; Guidi, R.; Frisan, T. The Biology of the Cytolethal Distending Toxins. Toxins 2011, 3, 172–190. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Liu, S.; Li, C.; Sun, L.; Bao, L.; Feng, J.; Liu, Z. Analysis of 52 Rab GTPases from channel catfish and their involvement in immune responses after bacterial infections. Dev. Comp. Immunol. 2014, 45, 21–34. [Google Scholar] [CrossRef]
- Chen, M.-X.; Chen, Y.; Fu, R.; Mao, G.-Q.; Liu, S.-Y.; Shen, T.-B. Rab5a Promotes Cytolethal Distending Toxin B-Induced Cytotoxicity and Inflammation. Infect. Immun. 2020, 88, e00132-20. [Google Scholar] [CrossRef]
- Gerding, D.N.; Johnson, S.; Rupnik, M.; Aktories, K. Clostridium difficile binary toxin CDT: Mechanism, epidemiology, and potential clinical importance. Gut Microbes 2014, 5, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-K.; Chen, Y.-A.; Lin, C.-J.; Lin, H.-J.; Kao, M.-C.; Huang, M.-Z.; Lin, Y.-H.; Chuan, C.-N.; Chen, C.-J.; Lo, U.-G.; et al. Molecular Mechanisms and Potential Clinical Applications of Campylobacter jejuni Cytolethal Distending Toxin. Front. Cell. Infect. Microbiol. 2016, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Kaddai, V.; Gonzalez, T.; Keslair, F.; Grémeaux, T.; Bonnafous, S.; Gugenheim, J.; Tran, A.; Gual, P.; Le Marchand-Brustel, Y.; Cormont, M. Rab4b Is a Small GTPase Involved in the Control of the Glucose Transporter GLUT4 Localization in Adipocyte. PLoS ONE 2009, 4, e5257. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, M.W.; Bielli, A.; Cantalupo, G.; Mora, S.; Roberti, V.; Santillo, M.; Drummond, F.; Bucci, C. Rab4 affects both recycling and degradative endosomal trafficking. FEBS Lett. 2001, 495, 21–30. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Du, S.; Zhao, Q.; Huang, X.; Wu, R.; Yan, Q.; Han, X.; Cao, S.; Chang, Y.-F.; et al. Upregulation of occludin by cytolethal distending toxin facilitates Glaesserella parasuis adhesion to respiratory tract cells. Infect. Immun. 2023, 91, e0035123. [Google Scholar] [CrossRef]
- Tang, X.; Xu, S.; Yang, Z.; Wang, K.; Dai, K.; Zhang, Y.; Hu, B.; Wang, Y.; Cao, S.; Huang, X.; et al. EspP2 Regulates the Adhesion of Glaesserella parasuis via Rap1 Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 4570. [Google Scholar] [CrossRef]






| Strain or Plasmid | Relevant Characteristics | Source |
|---|---|---|
| E. coli DH5α | F− endA1 glnV44 thi-1 recA1 relA1 gyrA96 deoR nupG purB20 φ80dlacZΔM15 Δ(lacZYA-argF) U169, hsdR17(rK−mK+), λ− | Biomed |
| pET-cdtA | A 624 bp cdtA CDS in pET-32a (+) | Laboratory collection |
| pET-cdtB | A 768 bp cdtB CDS in pET-32a (+) | Laboratory collection |
| pET-cdtC | A 471 bp cdtC CDS in pET-32a (+) | Laboratory collection |
| pET-32a (+) | Prokaryotic expression vector | Laboratory collection |
| pMD2.G | Lentivirus envelope plasmid | Laboratory collection |
| pSPAX2 | Lentivirus envelope plasmid | Laboratory collection |
| pLentiCRISPR V2 | sgRNA carrier plasmid | Laboratory collection |
| pEGFP-N1 | Overexpression plasmid | Laboratory collection |
| pcDNA3.1-Flag | Eukaryotic expression vector | Laboratory collection |
| pcDNA3.1-Flag-Rab4b | Overexpression plasmid | Laboratory collection |
| Gene | Primer Direction | Sequence (5′–3′) | Size (bp) |
|---|---|---|---|
| Rab4b-sgRNA | Forward | CACCGTGACGCGGAGTTATTACCG | 24 bp |
| Reverse | AAACCGGTAATAACTCCGCGTCAC | ||
| Rab4b-KO | Forward | CACAATCGGCGTGGAGTT | 176 bp |
| Reverse | AGTTGTAAGTCTCCCGGCTGT | ||
| Rab4b-KO | Forward | CACAATCGGCGTGGAGTT | 176 bp |
| Reverse | AGTTGTAAGTCTCCCGGCTGT | ||
| pEGFP-N1-Rab4b | Forward | GACTCAGATCTCGAGGCCACCATGGCTGAGACCTACGACTTCC | 682 bp |
| Reverse | GTACCGTCGACTGCAGAATTCCGCAGCCACAGGGCTGAGG | ||
| IL-6-sus | Forward | GGGACTGATGCTGGTGACAA | 147 bp |
| Reverse | TCCACGATTTCCCAGAGAACA | ||
| TNF-α-sus | Forward | CGTCAGCCGATTTGCTATCT | 184 bp |
| Reverse | CTTGGGCAGATTGACCTCAG | ||
| β-actin-sus | Forward | CTTCCTGGGCATGGAGTCC | 201 bp |
| Reverse | GGCGCGATGATCTTGATCTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yang, Z.; Dai, K.; Hu, B.; Xu, S.; Wang, Y.; Lei, L.; Du, S.; Zhao, Q.; Huang, X.; et al. Rab4b Promotes Cytolethal Distending Toxin from Glaesserella parasuis-Induced Cytotoxicity in PK-15 Cells. Toxins 2024, 16, 407. https://doi.org/10.3390/toxins16090407
Zhang Y, Yang Z, Dai K, Hu B, Xu S, Wang Y, Lei L, Du S, Zhao Q, Huang X, et al. Rab4b Promotes Cytolethal Distending Toxin from Glaesserella parasuis-Induced Cytotoxicity in PK-15 Cells. Toxins. 2024; 16(9):407. https://doi.org/10.3390/toxins16090407
Chicago/Turabian StyleZhang, Yiwen, Zhen Yang, Ke Dai, Bangdi Hu, Shiyu Xu, Yu Wang, Li Lei, Senyan Du, Qin Zhao, Xiaobo Huang, and et al. 2024. "Rab4b Promotes Cytolethal Distending Toxin from Glaesserella parasuis-Induced Cytotoxicity in PK-15 Cells" Toxins 16, no. 9: 407. https://doi.org/10.3390/toxins16090407
APA StyleZhang, Y., Yang, Z., Dai, K., Hu, B., Xu, S., Wang, Y., Lei, L., Du, S., Zhao, Q., Huang, X., Wu, R., Yan, Q., Wang, Y., Cao, S., & Wen, Y. (2024). Rab4b Promotes Cytolethal Distending Toxin from Glaesserella parasuis-Induced Cytotoxicity in PK-15 Cells. Toxins, 16(9), 407. https://doi.org/10.3390/toxins16090407





