Improvement in XIa Selectivity of Snake Venom Peptide Analogue BF9-N17K Using P2′ Amino Acid Replacements
Abstract
:1. Introduction
2. Results
2.1. Molecular Design of Four Single-Point Mutants with Snake Venom Kunitz-Type Peptide Scaffold BF9-N17K
2.2. Anticoagulation Functional Characterization of Four BF9-N17K Mutants
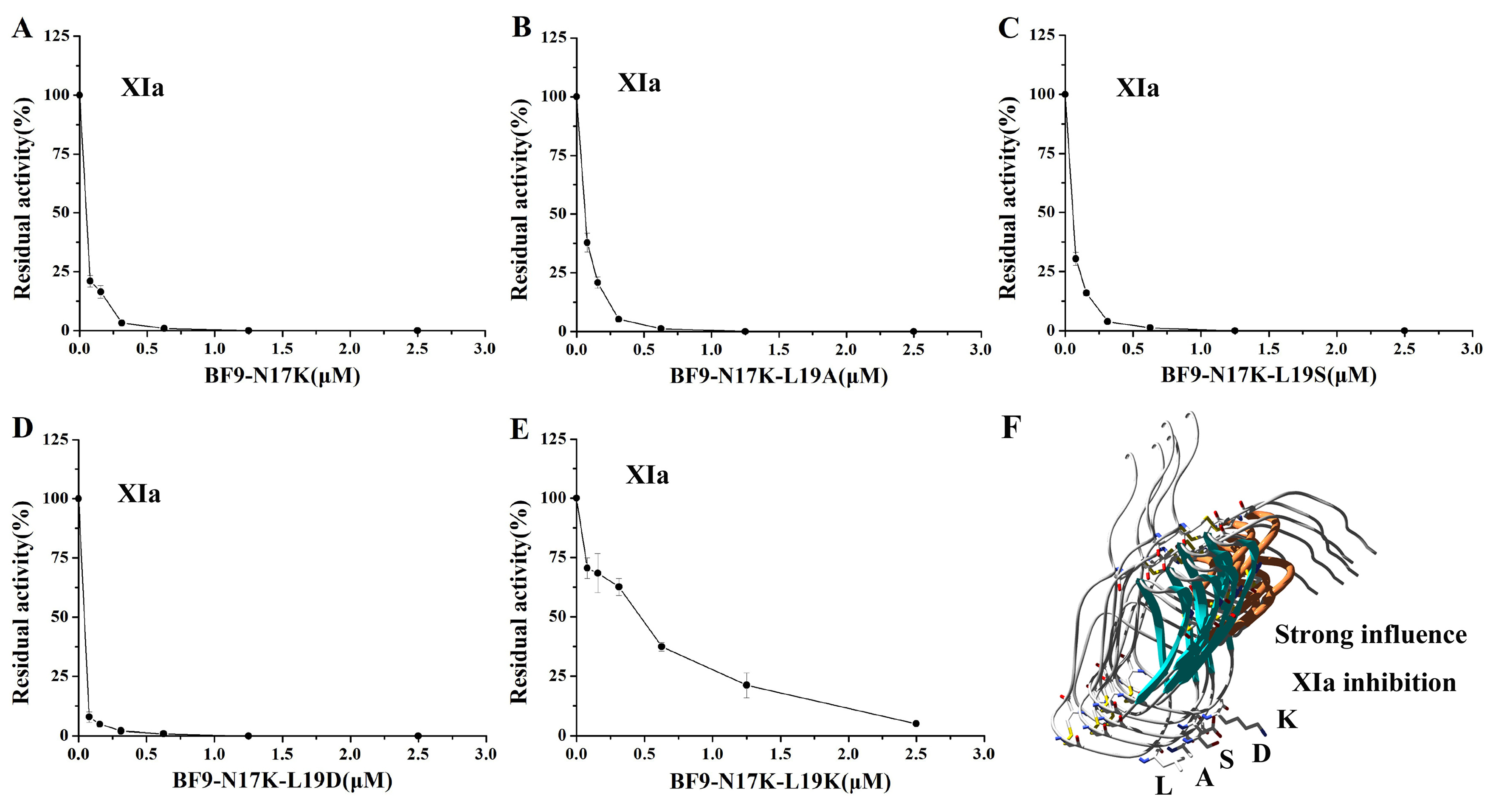
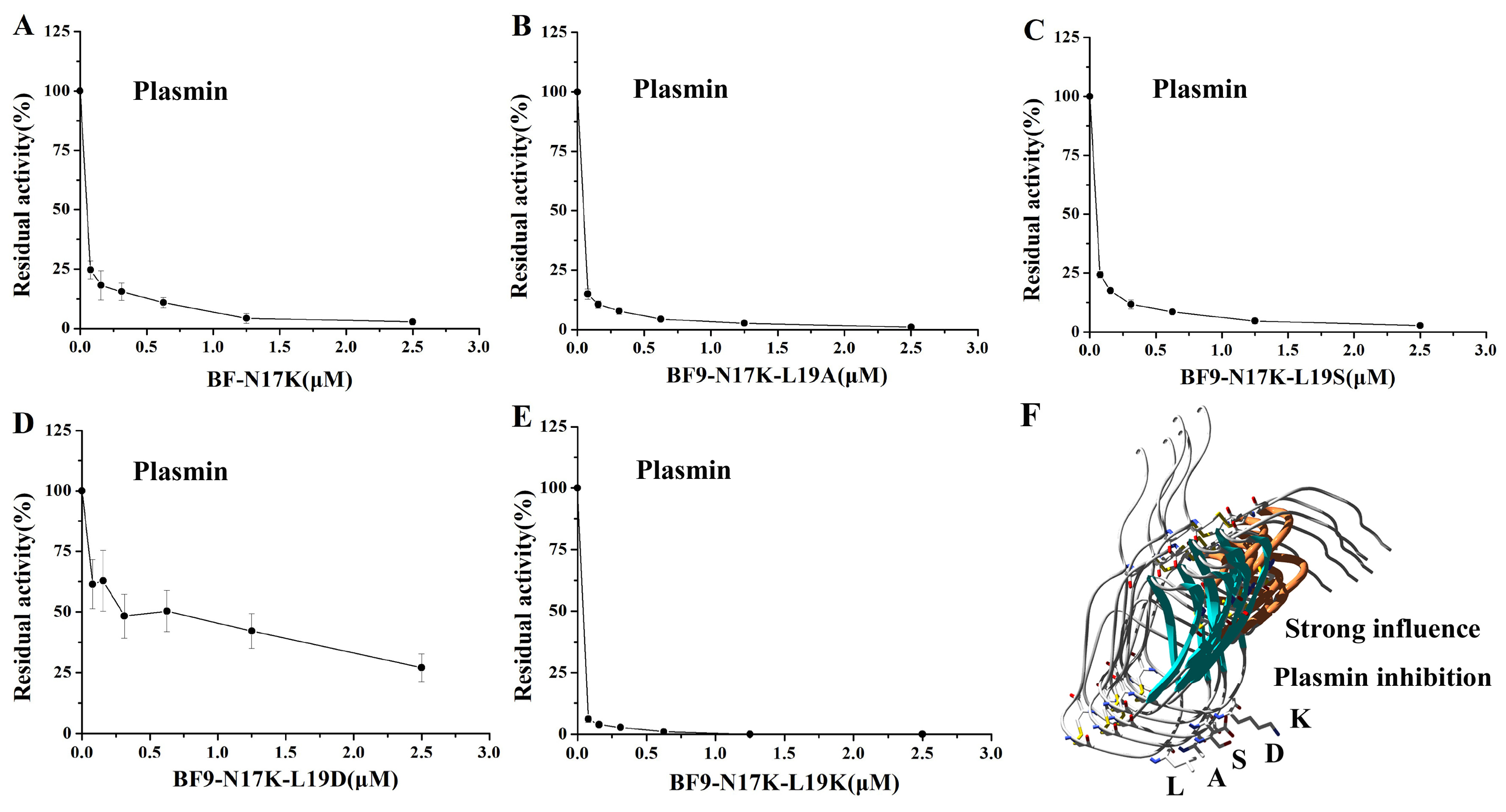
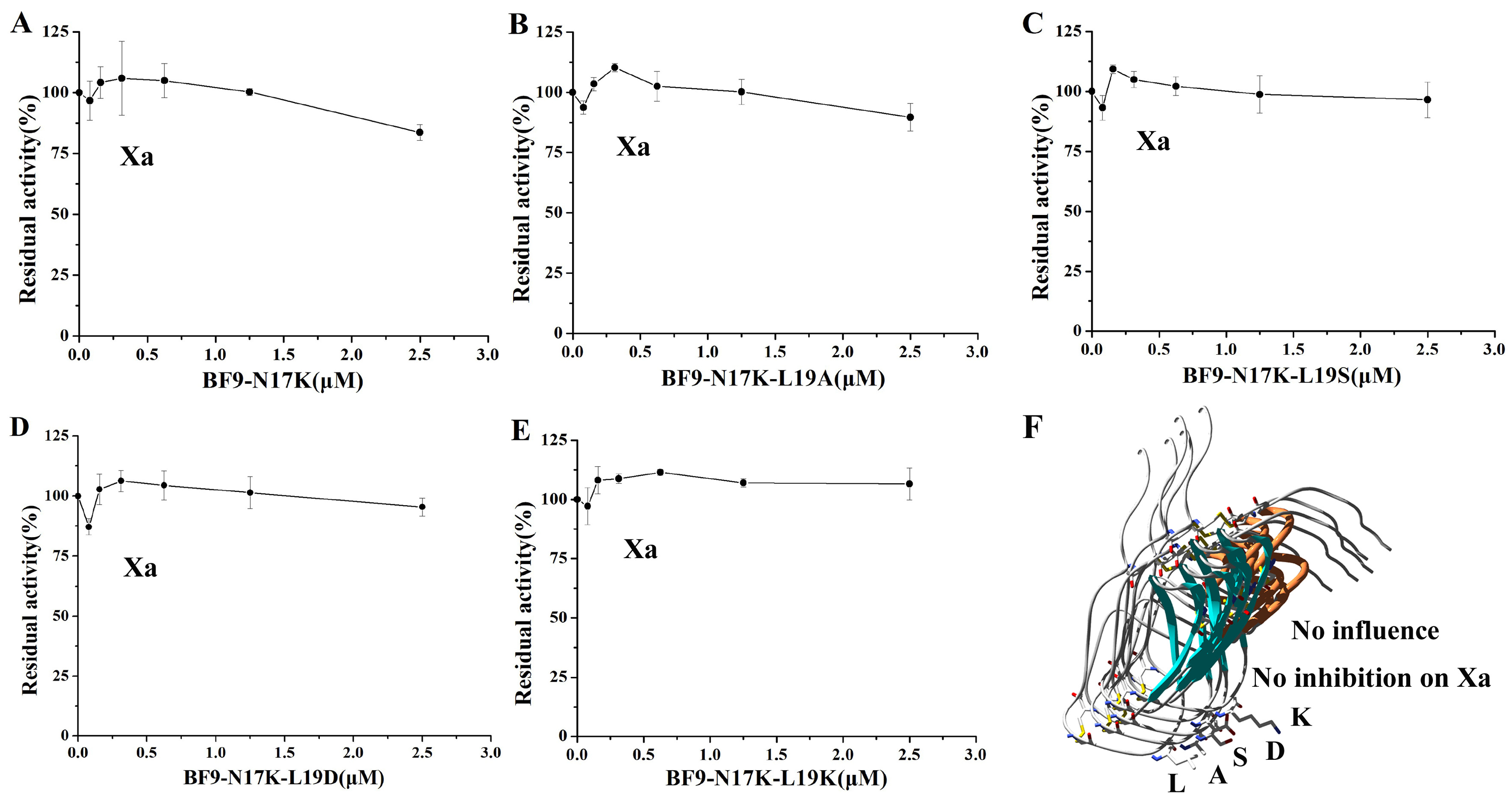
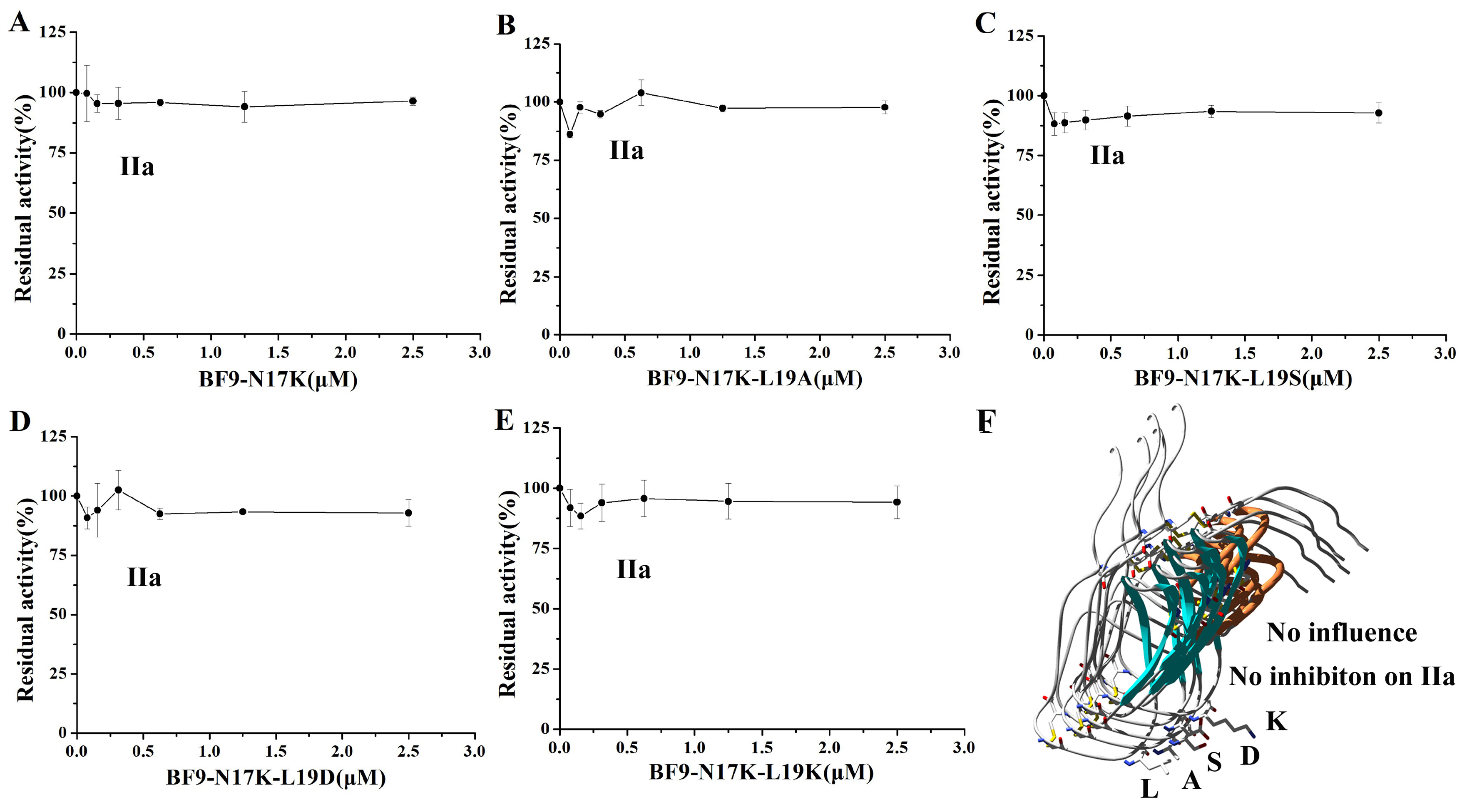
2.3. Evaluation of XIa, Plasmin, and Other Serine Protease Inhibitory Activity of Four BF9-N17K Mutants
2.4. Structure and Function Relationship of BF9-N17K-L19D with XIa
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Peptide Design, Recombinant Plasmids, and Recombinant Peptides
5.2. Serine Protease Inhibitory Activity Assay
5.3. Anticoagulation Activity Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaiser, R.; Dewender, R.; Mulkers, M.; Stermann, J.; Rossaro, D.; Di Fina, L.; Li, L.; Gold, C.; Schmid, M.; Kaab, L.; et al. Procoagulant platelet activation promotes venous thrombosis. Blood 2024, 144, 2546–2553. [Google Scholar] [CrossRef]
- Seanoon, K.; Payongsri, P.; Vivithanaporn, P.; Sirachainan, N.; Chuansumrit, A.; Hongeng, S.; Tanratana, P. Mutations of TFPI-binding exosites on factor VII cause bleeding phenotypes in factor VII deficiency. Blood Adv. 2022, 6, 5887–5897. [Google Scholar] [CrossRef] [PubMed]
- Naudin, C.; Burillo, E.; Blankenberg, S.; Butler, L.; Renne, T. Factor XII Contact Activation. Semin. Thromb. Hemost. 2017, 43, 814–826. [Google Scholar] [CrossRef]
- Galli, M.; Gragnano, F.; Berteotti, M.; Marcucci, R.; Gargiulo, G.; Calabro, P.; Terracciano, F.; Andreotti, F.; Patti, G.; De Caterina, R.; et al. Antithrombotic Therapy in High Bleeding Risk, Part II: Noncardiac Percutaneous Interventions. JACC. Cardiovasc. Interv. 2024, 17, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Gragnano, F.; Berteotti, M.; Marcucci, R.; Gargiulo, G.; Calabro, P.; Terracciano, F.; Andreotti, F.; Patti, G.; De Caterina, R.; et al. Antithrombotic Therapy in High Bleeding Risk, Part I: Percutaneous Cardiac Interventions. JACC. Cardiovasc. Interv. 2024, 17, 2197–2215. [Google Scholar] [CrossRef] [PubMed]
- Frunt, R.; El Otmani, H.; Smits, S.; Clark, C.C.; Maas, C. Factor XII contact activation can be prevented by targeting 2 unique patches in its epidermal growth factor-like 1 domain with a nanobody. J. Thromb. Haemost. JTH 2024, 22, 2562–2575. [Google Scholar] [CrossRef] [PubMed]
- Heestermans, M.; Naudin, C.; Mailer, R.K.; Konrath, S.; Klaetschke, K.; Jamsa, A.; Frye, M.; Deppermann, C.; Pula, G.; Kuta, P.; et al. Identification of the factor XII contact activation site enables sensitive coagulation diagnostics. Nat. Commun. 2021, 12, 5596. [Google Scholar] [CrossRef] [PubMed]
- Puy, C.; Moellmer, S.A.; Pang, J.; Vu, H.H.; Melrose, A.R.; Lorentz, C.U.; Tucker, E.I.; Shatzel, J.J.; Keshari, R.S.; Lupu, F.; et al. Coagulation factor XI regulates endothelial cell permeability and barrier function in vitro and in vivo. Blood 2024, 144, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Moellmer, S.A.; Puy, C.; McCarty, O.J.T. Biology of factor XI. Blood 2024, 143, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Gailani, D.; Gruber, A. Targeting factor XI and factor XIa to prevent thrombosis. Blood 2024, 143, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.; Piccini, J.P.; Alexander, J.H.; Granger, C.B.; Patel, M.R. Clinical Evaluation of Factor XIa Inhibitor Drugs: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2023, 81, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Meng, Z.; Yang, X.; Duan, Y.; Wang, Q.; Liao, C. Factor XIa Inhibitors in Anticoagulation Therapy: Recent Advances and Perspectives. J. Med. Chem. 2023, 66, 5332–5363. [Google Scholar] [CrossRef] [PubMed]
- Decrem, Y.; Rath, G.; Blasioli, V.; Cauchie, P.; Robert, S.; Beaufays, J.; Frere, J.M.; Feron, O.; Dogne, J.M.; Dessy, C.; et al. Ir-CPI, a coagulation contact phase inhibitor from the tick Ixodes ricinus, inhibits thrombus formation without impairing hemostasis. J. Exp. Med. 2009, 206, 2381–2395. [Google Scholar] [CrossRef]
- Ma, D.; Mizurini, D.M.; Assumpcao, T.C.; Li, Y.; Qi, Y.; Kotsyfakis, M.; Ribeiro, J.M.; Monteiro, R.Q.; Francischetti, I.M. Desmolaris, a novel factor XIa anticoagulant from the salivary gland of the vampire bat (Desmodus rotundus) inhibits inflammation and thrombosis in vivo. Blood 2013, 122, 4094–4106. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, S.; McManus, D.P. Structure and function of invertebrate Kunitz serine protease inhibitors. Dev. Comp. Immunol. 2013, 39, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Hao, J.; Luo, X.; Zhu, W.; Wu, Z.; Qian, Y.; Hu, F.; Liu, T.; Ruan, X.; Li, S.; et al. The Kv1.3 channel-inhibitory toxin BF9 also displays anticoagulant activity via inhibition of factor XIa. Toxicon Off. J. Int. Soc. Toxinol. 2018, 152, 9–15. [Google Scholar] [CrossRef]
- Chen, W.; Carvalho, L.P.; Chan, M.Y.; Kini, R.M.; Kang, T.S. Fasxiator, a novel factor XIa inhibitor from snake venom, and its site-specific mutagenesis to improve potency and selectivity. J. Thromb. Haemost. JTH 2015, 13, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Feng, J.; Wang, B.; Cao, Z.; Li, W.; Wu, Y.; Chen, Z. BF9, the first functionally characterized snake toxin peptide with Kunitz-type protease and potassium channel inhibiting properties. J. Biochem. Mol. Toxicol. 2014, 28, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Navaneetham, D.; Jin, L.; Pandey, P.; Strickler, J.E.; Babine, R.E.; Abdel-Meguid, S.S.; Walsh, P.N. Structural and mutational analyses of the molecular interactions between the catalytic domain of factor XIa and the Kunitz protease inhibitor domain of protease nexin 2. J. Biol. Chem. 2005, 280, 36165–36175. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, H.; Navaneetham, D.; Reichenbach, Z.W.; Tuma, R.F.; Walsh, P.N. The kunitz protease inhibitor domain of protease nexin-2 inhibits factor XIa and murine carotid artery and middle cerebral artery thrombosis. Blood 2012, 120, 671–677. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Ji, X.R.; Liu, Y.Y.; Jiang, S.; Yu, X.Y.; Jia, Z.P.; Zhao, Y.; Zhang, J.Q.; Zhang, J.L.; Kong, Y. WPK5, a Novel Kunitz-Type Peptide from the Leech Whitmania pigra Inhibiting Factor XIa, and Its Loop-Replaced Mutant to Improve Potency. Biomedicines 2021, 9, 1745. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Liu, Y.; Ji, X.; Zheng, Y.; Li, Z.; Jiang, S.; Li, H.; Kong, Y. DAKS1, a Kunitz Scaffold Peptide from the Venom Gland of Deinagkistrodon acutus Prevents Carotid-Artery and Middle-Cerebral-Artery Thrombosis via Targeting Factor XIa. Pharmaceuticals 2021, 14, 966. [Google Scholar] [CrossRef] [PubMed]
- Eltringham-Smith, L.J.; Bhakta, V.; Sheffield, W.P. Selection and in vitro and in vivo characterization of a Kunitz protease inhibitor domain of protease nexin 2 variant that inhibits factor XIa without inhibiting plasmin. J. Biotechnol. 2021, 330, 61–69. [Google Scholar] [CrossRef]
- Ding, L.; Hao, J.; Luo, X.; Chen, Z. Engineering varied serine protease inhibitors by converting P1 site of BF9, a weakly active Kunitz-type animal toxin. Int. J. Biol. Macromol. 2018, 120 Pt A, 1190–1197. [Google Scholar] [CrossRef]
- Sun, F.; Wang, W.; Li, Z.; Li, Y.; Guo, W.; Kong, Y. Design, expression and biological evaluation of DX-88mut as a novel selective factor XIa inhibitor for antithrombosis. Bioorg. Chem. 2024, 142, 106951. [Google Scholar] [CrossRef] [PubMed]
- Navaneetham, D.; Wu, W.; Li, H.; Sinha, D.; Tuma, R.F.; Walsh, P.N. P1 and P2′ site mutations convert protease nexin-2 from a factor XIa inhibitor to a plasmin inhibitor. J. Biochem. 2013, 153, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Miller, T.N.; Navaneetham, D.; Schoonmaker, R.T.; Sinha, D.; Walsh, P.N. The role of factor XIa (FXIa) catalytic domain exosite residues in substrate catalysis and inhibition by the Kunitz protease inhibitor domain of protease nexin 2. J. Biol. Chem. 2011, 286, 31904–31914. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Vadivel, K.; Schmidt, A.E.; Ogueli, G.I.; Ponnuraj, S.M.; Rannulu, N.; Loo, J.A.; Bajaj, M.S.; Bajaj, S.P. Decoy plasminogen receptor containing a selective Kunitz-inhibitory domain. Biochemistry 2014, 53, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, M.S.; Ogueli, G.I.; Kumar, Y.; Vadivel, K.; Lawson, G.; Shanker, S.; Schmidt, A.E.; Bajaj, S.P. Engineering kunitz domain 1 (KD1) of human tissue factor pathway inhibitor-2 to selectively inhibit fibrinolysis: Properties of KD1-L17R variant. J. Biol. Chem. 2011, 286, 4329–4340. [Google Scholar] [CrossRef] [PubMed]
- Salameh, M.A.; Soares, A.S.; Hockla, A.; Radisky, D.C.; Radisky, E.S. The P(2)′ residue is a key determinant of mesotrypsin specificity: Engineering a high-affinity inhibitor with anticancer activity. Biochem. J. 2011, 440, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, X.; Liu, H.; San, M.; Xu, Y.; Li, J.; Li, S.; Cao, Z.; Li, W.; Wu, Y.; et al. A new Kunitz-type plasmin inhibitor from scorpion venom. Toxicon Off. J. Int. Soc. Toxinol. 2015, 106, 7–13. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Hu, Y.T.; Yang, W.S.; He, Y.W.; Feng, J.; Wang, B.; Zhao, R.M.; Ding, J.P.; Cao, Z.J.; Li, W.X.; et al. Hg1, novel peptide inhibitor specific for Kv1.3 channels from first scorpion Kunitz-type potassium channel toxin family. J. Biol. Chem. 2012, 287, 13813–13821. [Google Scholar] [CrossRef] [PubMed]
- Mega, J.L.; Simon, T. Pharmacology of antithrombotic drugs: An assessment of oral antiplatelet and anticoagulant treatments. Lancet 2015, 386, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Straub, A.; Roehrig, S.; Hillisch, A. Oral, direct thrombin and factor Xa inhibitors: The replacement for warfarin, leeches, and pig intestines? Angewandte Chemie 2011, 50, 4574–4590. [Google Scholar] [CrossRef] [PubMed]
- Perzborn, E.; Roehrig, S.; Straub, A.; Kubitza, D.; Misselwitz, F. The discovery and development of rivaroxaban, an oral, direct factor Xa inhibitor. Nature reviews. Drug Discov. 2011, 10, 61–75. [Google Scholar] [CrossRef]
- Desai, U.R. New antithrombin-based anticoagulants. Med. Res. Rev. 2004, 24, 151–181. [Google Scholar] [CrossRef] [PubMed]
- Jmel, M.A.; Voet, H.; Araujo, R.N.; Tirloni, L.; Sa-Nunes, A.; Kotsyfakis, M. Tick Salivary Kunitz-Type Inhibitors: Targeting Host Hemostasis and Immunity to Mediate Successful Blood Feeding. Int. J. Mol. Sci. 2023, 24, 1556. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, S.; Li, Q.; Kong, Y. Advances of Kunitz-type serine protease inhibitors. Sheng Wu Gong Cheng Xue Bao = Chin. J. Biotechnol. 2021, 37, 3988–4000. [Google Scholar] [PubMed]
- Sun, F.; Deng, X.; Gao, H.; Ding, L.; Zhu, W.; Luo, H.; Ye, X.; Luo, X.; Chen, Z.; Qin, C. Characterization of Kunitz-Domain Anticoagulation Peptides Derived from Acinetobacter baumannii Exotoxin Protein F6W77. Toxins 2024, 16, 450. [Google Scholar] [CrossRef] [PubMed]
- Simeon, R.; Chen, Z. In vitro-engineered non-antibody protein therapeutics. Protein Cell 2018, 9, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Navaneetham, D.; Sinha, D.; Walsh, P.N. Mechanisms and specificity of factor XIa and trypsin inhibition by protease nexin 2 and basic pancreatic trypsin inhibitor. J. Biochem. 2010, 148, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Martian, P.C.; Tertis, M.; Leonte, D.; Hadade, N.; Cristea, C.; Crisan, O. Cyclic peptides: A powerful instrument for advancing biomedical nanotechnologies and drug development. J. Pharm. Biomed. Anal. 2025, 252, 116488. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Nielsen, A.L.; Heinis, C. Cyclic Peptides for Drug Development. Angew. Chem. 2024, 63, e202308251. [Google Scholar] [CrossRef]
- Imai, M.; Colas, K.; Suga, H. Protein Grafting Techniques: From Peptide Epitopes to Lasso-Grafted Neobiologics. ChemPlusChem 2024, 89, e202400152. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, A.A.; Yin, Y.; Suga, H. Macrocyclic Peptides as Drug Candidates: Recent Progress and Remaining Challenges. J. Am. Chem. Soc. 2019, 141, 4167–4181. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Shu, Z.; Hao, J.; Luo, X.; Ye, X.; Zhu, W.; Duan, W.; Chen, Z. Schixator, a new FXa inhibitor from Schistosoma japonicum with antithrombotic effect and low bleeding risk. Biochem. Biophys. Res. Commun. 2022, 603, 138–143. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Zhai, Z.; Qin, T.; Lin, Y.; Shuang, Z.; Sun, F.; Qin, C.; Luo, H.; Zhu, W.; Ye, X.; et al. Improvement in XIa Selectivity of Snake Venom Peptide Analogue BF9-N17K Using P2′ Amino Acid Replacements. Toxins 2025, 17, 23. https://doi.org/10.3390/toxins17010023
Ding L, Zhai Z, Qin T, Lin Y, Shuang Z, Sun F, Qin C, Luo H, Zhu W, Ye X, et al. Improvement in XIa Selectivity of Snake Venom Peptide Analogue BF9-N17K Using P2′ Amino Acid Replacements. Toxins. 2025; 17(1):23. https://doi.org/10.3390/toxins17010023
Chicago/Turabian StyleDing, Li, Zhiping Zhai, Tianxiang Qin, Yuexi Lin, Zhicheng Shuang, Fang Sun, Chenhu Qin, Hongyi Luo, Wen Zhu, Xiangdong Ye, and et al. 2025. "Improvement in XIa Selectivity of Snake Venom Peptide Analogue BF9-N17K Using P2′ Amino Acid Replacements" Toxins 17, no. 1: 23. https://doi.org/10.3390/toxins17010023
APA StyleDing, L., Zhai, Z., Qin, T., Lin, Y., Shuang, Z., Sun, F., Qin, C., Luo, H., Zhu, W., Ye, X., Chen, Z., & Luo, X. (2025). Improvement in XIa Selectivity of Snake Venom Peptide Analogue BF9-N17K Using P2′ Amino Acid Replacements. Toxins, 17(1), 23. https://doi.org/10.3390/toxins17010023




