Pulsed Electric Field Induces Significant Changes in the Metabolome of Fusarium Species and Decreases Their Viability and Toxigenicity
Abstract
1. Introduction
2. Results
2.1. UHPLC–HRMS/MS Method Optimization
2.2. Chemometric Analysis
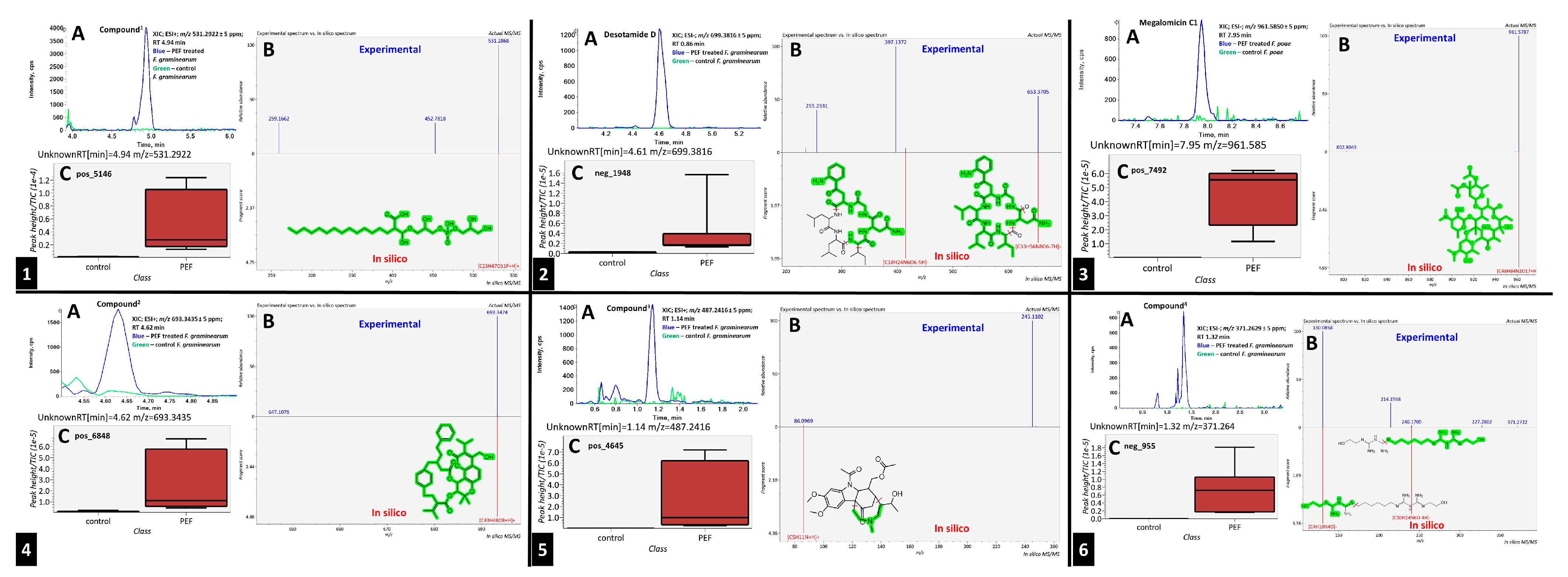
2.3. PEF Biomarkers
2.4. Influence of PEF on Fungal Viability and Mycotoxin Production
3. Discussion
4. Conclusions
- The PEF treatment significantly reduced the viability of the Fusarium species investigated in this study (i.e., F. culmorum, F. graminearum, F. sporotrichioides, and F. poae), where the extent of the reduction was the highest for F. poae (9.4% of surviving spores) and the lowest for F. culmorum (53.4% of surviving spores).
- The reduction in Fusarium species viability corresponded with a reduction in the mycotoxin content, specifically for DON in the F. culmorum isolates, ZEA in both the F. culmorum and F. graminearum isolates, and DAS and NEO in both the F. sporotrichioides and F. poae species.
- The overall metabolomes of the PEF-treated and untreated Fusarium species were significantly changed, and the statistically significant markers related to the PEF treatment were characterized using the tools of modern high-resolution mass spectrometry. Despite the fact that the biological interpretation of these markers was not successful at this stage, as the knowledge on complex metabolic pathways and the secondary metabolism of micromycetes, including Fusarium, is very poor, the well-characterized compounds directly associated with the PEF intervention will serve as a basis for follow-up multi-omics studies.
5. Materials and Methods
5.1. Analytical Standards and Chemicals
5.2. PEF Treatment of Fusarium Spores
5.3. PDA Plate Preparation
5.4. Exraction of PDA Plates
5.5. UHPLC-HRMS/MS Metabolomic Fingeprinting
5.6. Metabolomic Data Processing and Statistical Analysis
5.7. Target Analysis of Mycotoxins by UHPLC-HRMS/MS and Statistical Evaluation
5.8. Viability Test and Statistical Evaluation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakker, M.G.; Brown, D.W.; Kelly, A.C.; Kim, H.S.; Kurtzman, C.P.; McCormick, S.P.; O’Donnell, K.L.; Proctor, R.H.; Vaughan, M.M.; Ward, T.J. Fusarium Mycotoxins: A Trans-Disciplinary Overview. Can. J. Plant Pathol. 2018, 40, 161–171. [Google Scholar] [CrossRef]
- Inbaia, S.; Farooqi, A.; Ray, R. V Aggressiveness and Mycotoxin Profile of Fusarium Avenaceum Isolates Causing Fusarium Seedling Blight and Fusarium Head Blight in UK Malting Barley. Front. Plant Sci. 2023, 14, 1121553. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Xie, Y.S.; Cui, Y.Y.; Xu, J.; He, W.; Chen, H.G.; Guo, J.H. Conjunctively Screening of Biocontrol Agents (BCAs) against Fusarium Root Rot and Fusarium Head Blight Caused by Fusarium Graminearum. Microbiol. Res. 2015, 177, 34–42. [Google Scholar] [CrossRef]
- Moonjely, S.; Ebert, M.; Paton-Glassbrook, D.; Noel, Z.A.; Roze, L.; Shay, R.; Watkins, T.; Trail, F. Update on the State of Research to Manage Fusarium Head Blight. Fungal Genet. Biol. 2023, 169, 103829. [Google Scholar] [CrossRef] [PubMed]
- Perincherry, L.; Lalak-Kanczugowska, J.; Stepien, L. Fusarium-Produced Mycotoxins in Plant-Pathogen Interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.M.; Palacios, S.A.; Yerkovich, N.; Palazzini, J.M.; Battilani, P.; Leslie, J.F.; Logrieco, A.F.; Chulze, S.N. Fusarium Head Blight and Mycotoxins in Wheat: Prevention and Control Strategies across the Food Chain. World Mycotoxin J. 2019, 12, 333–355. [Google Scholar] [CrossRef]
- Smaoui, S.; Agriopoulou, S.; D’Amore, T.; Tavares, L.; Mousavi Khaneghah, A. The Control of Fusarium Growth and Decontamination of Produced Mycotoxins by Lactic Acid Bacteria. Crit. Rev. Food Sci. Nutr. 2022, 63, 11125–11152. [Google Scholar] [CrossRef]
- Homa, K.; Barney, W.P.; Davis, W.P.; Guerrero, D.; Berger, M.J.; Lopez, J.L.; Wyenandt, C.A.; Simon, J.E. Cold Plasma Treatment Strategies for the Control of Fusarium oxysporum f. sp. basilici in Sweet Basil. HortScience 2021, 56, 42–51. [Google Scholar] [CrossRef]
- Filho, F.O.; de Oliveira Silva, E.; de Almeida Lopes, M.M.; Ribeiro, P.R.V.; Oster, A.H.; Guedes, J.A.C.; de Souza Zampieri, D.; do Nascimento Bordallo, P.; Zocolo, G.J. Effect of Pulsed Light on Postharvest Disease Control-Related Metabolomic Variation in Melon (Cucumis Melo) Artificially Inoculated with Fusarium Pallidoroseum. PLoS ONE 2020, 15, e0220097. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga-Calderón, B.; González, H.H.L.; Alzamora, S.M.; Coronel, M.B. Multi-Step Ozone Treatments of Malting Barley: Effect on the Incidence of Fusarium Graminearum and Grain Germination Parameters. Innov. Food Sci. Emerg. Technol. 2023, 83, 103219. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Sinigaglia, M.; Corbo, M.R. Ultrasound and Antimicrobial Compounds: A Suitable Way to Control Fusarium Oxysporum in Juices. Food Bioprocess Technol. 2013, 6, 1153–1163. [Google Scholar] [CrossRef]
- Evrendilek, G.A.; Karatas, B.; Uzuner, S.; Tanasov, I. Design and Effectiveness of Pulsed Electric Fields towards Seed Disinfection. J. Sci. Food Agric. 2019, 99, 3475–3480. [Google Scholar] [CrossRef]
- Han, Z.; Zeng, X.A.; Zhang, B.S.; Yu, S.J. Effects of Pulsed Electric Fields (PEF) Treatment on the Properties of Corn Starch. J. Food Eng. 2009, 93, 318–323. [Google Scholar] [CrossRef]
- García, D.; Gómez, N.; Mañas, P.; Raso, J.; Pagán, R. Pulsed Electric Fields Cause Bacterial Envelopes Permeabilization Depending on the Treatment Intensity, the Treatment Medium PH and the Microorganism Investigated. Int. J. Food Microbiol. 2007, 113, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.J.; Barbosa-Cánovas, G.V.; Swanson, B.G. Microbial Inactivation of Foods by Pulsed Electric Fields. J. Food Process. Preserv. 1993, 17, 47–73. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G.V.; Altunakar, B. Pulsed Electric Fields Processing of Foods: An Overview. In Pulsed Electric Fields Technology for the Food Industry; Raso, J., Heinz, V., Eds.; Springer: Boston, MA, USA, 2006; pp. 3–26. ISBN 978-0-387-31122-7. [Google Scholar]
- Toepfl, S.; Heinz, V.; Knorr, D. High Intensity Pulsed Electric Fields Applied for Food Preservation. Chem. Eng. Process. Process Intensif. 2007, 46, 537–546. [Google Scholar] [CrossRef]
- Zhong, C.; Guan, X.; Fan, Z.; Song, W.; Chen, R.; Wang, Y.; Sun, X.; He, S. Pulsed Electric Field Disinfection Treatment of Fusarium Oxysporum in Nutrient Solution. Water Sci. Technol. Water Supply 2019, 19, 2116–2122. [Google Scholar] [CrossRef]
- Palicova, J.; Chrpova, J.; Tobolkova, A.; Ovesna, J.; Stranska, M. Effect of Pulsed Electric Field on Viability of Fusarium Micromycetes. Cereal Res. Commun. 2024. [Google Scholar] [CrossRef]
- Stranska, M.; Prusova, N.; Behner, A.; Dzuman, Z.; Lazarek, M.; Tobolkova, A.; Chrpova, J.; Hajslova, J. Influence of Pulsed Electric Field Treatment on the Fate of Fusarium and Alternaria Mycotoxins Present in Malting Barley. Food Control 2023, 145, 109440. [Google Scholar] [CrossRef]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS Models in Metabolomics: The Impact of Permutation of Dataset Rows on the K-Fold Cross-Validation Quality Parameters. Mol. Biosyst. 2015, 11, 13–19. [Google Scholar] [CrossRef]
- Schmid, R.; Heuckeroth, S.; Korf, A.; Smirnov, A.; Myers, O.; Dyrlund, T.S.; Bushuiev, R.; Murray, K.J.; Hoffmann, N.; Lu, M.; et al. Integrative Analysis of Multimodal Mass Spectrometry Data in MZmine 3. Nat. Biotechnol. 2023, 41, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Ikeda, K.; Takahashi, M.; Satoh, A.; Mori, Y.; Uchino, H.; Okahashi, N.; Yamada, Y.; Tada, I.; Bonini, P.; et al. A Lipidome Atlas in MS-DIAL 4. Nat. Biotechnol. 2020, 38, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Kvasnička, A.; Najdekr, L.; Dobešová, D.; Piskláková, B.; Ivanovová, E.; Friedecký, D. Clinical Lipidomics in the Era of the Big Data. Clin. Chem. Lab. Med. 2023, 61, 587–598. [Google Scholar] [CrossRef]
- Castillo, S.; Gopalacharyulu, P.; Yetukuri, L.; Orešič, M. Algorithms and Tools for the Preprocessing of LC-MS Metabolomics Data. Chemom. Intell. Lab. Syst. 2011, 108, 23–32. [Google Scholar] [CrossRef]
- Tsugawa, H.; Kind, T.; Nakabayashi, R.; Yukihira, D.; Tanaka, W.; Cajka, T.; Saito, K.; Fiehn, O.; Arita, M. Hydrogen Rearrangement Rules: Computational MS/MS Fragmentation and Structure Elucidation Using MS-FINDER Software. Anal. Chem. 2016, 88, 7946–7958. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A Rapid Tool for Turning Tandem Mass Spectra into Metabolite Structure Information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Fraisier-Vannier, O.; Chervin, J.; Cabanac, G.; Puech, V.; Fournier, S.; Durand, V.; Amiel, A.; André, O.; Benamar, O.A.; Dumas, B.; et al. MS-CleanR: A Feature-Filtering Workflow for Untargeted LC-MS Based Metabolomics. Anal. Chem. 2020, 92, 9971–9981. [Google Scholar] [CrossRef]
- Klåvus, A.; Kokla, M.; Noerman, S.; Koistinen, V.M.; Tuomainen, M.; Zarei, I.; Meuronen, T.; Häkkinen, M.R.; Rummukainen, S.; Babu, A.F.; et al. “Notame”: Workflow for Non-Targeted LC-MS Metabolic Profiling. Metabolites 2020, 10, 135. [Google Scholar] [CrossRef]
- Yonemoto, Y.; Yamashita, T.; Muraji, M.; Tatebe, W.; Ooshima, H.; Kato, J.; Kimura, A.; Murata, K. Resistance of Yeast and Bacterial Spores to High Voltage Electric Pulses. J. Ferment. Bioeng. 1993, 75, 99–102. [Google Scholar] [CrossRef]
- Qiu, X.; Chang, J.; Jin, Y.; Wu, W.J. Pulsed Electric Field Treatments with Nonlethal Field Strength Alter the Properties of Bacterial Spores. J. Food Prot. 2022, 85, 1053–1060. [Google Scholar] [CrossRef]
- Palmero Llamas, D.; De Cara Gonzalez, M.; Iglesias Gonzalez, C.; Ruíz Lopez, G.; Tello Marquina, J.C. Effects of Water Potential on Spore Germination and Viability of Fusarium Species. J. Ind. Microbiol. Biotechnol. 2008, 35, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Q.; Liu, X.; Chen, Y.; Zhang, Y.; Sun, A.; Zhang, W.; Zhang, J.; Ju, J. Cyclic Hexapeptides from the Deep South China Sea-Derived Streptomyces Scopuliridis SCSIO ZJ46 Active against Pathogenic Gram-Positive Bacteria. J. Nat. Prod. 2014, 77, 1937–1941. [Google Scholar] [CrossRef] [PubMed]
- Volchegursky, Y.; Hu, Z.; Katz, L.; McDaniel, R. Biosynthesis of the Antiparasitic Agent Megalomicin: Transformation of Erythromycin to Megalomicin in Saccharopolyspora Erythraea. Mol. Microbiol. 2000, 37, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Smedsgaard, J.; Nielsen, J. Metabolite Profiling of Fungi and Yeast: From Phenotype to Metabolome by MS and Informatics. J. Exp. Bot. 2005, 56, 273–286. [Google Scholar] [CrossRef]
- Šíp, V.; Stuchlíková, E. Evaluation of the Response of Selected Winter Wheat Cultivars to Artificial Infection with Fusarium Culmorum in Field Conditions. Czech J. Genet. Plant Breed. 2000, 36, 49–58. [Google Scholar]
- Prusova, N.; Karabin, M.; Jelinek, L.; Chrpova, J.; Ovesna, J.; Svoboda, P.; Dolezalova, T.; Behner, A.; Hajslova, J.; Stranska, M. Application of Pulsed Electric Field During Malting: Impact on Fusarium Species Growth and Mycotoxin Production. Toxins 2024, 16, 537. [Google Scholar] [CrossRef] [PubMed]
- Stranska, M.; Uttl, L.; Bechynska, K.; Hurkova, K.; Behner, A.; Hajslova, J. Metabolomic Fingerprinting as a Tool for Authentication of Grapevine (Vitis vinifera L.) Biomass Used in Food Production. Food Chem. 2021, 361, 130166. [Google Scholar] [CrossRef]
- Dzuman, Z.; Zachariasova, M.; Lacina, O.; Veprikova, Z.; Slavikova, P.; Hajslova, J. A Rugged High-Throughput Analytical Approach for the Determination and Quantification of Multiple Mycotoxins in Complex Feed Matrices. Talanta 2014, 121, 263–272. [Google Scholar] [CrossRef] [PubMed]
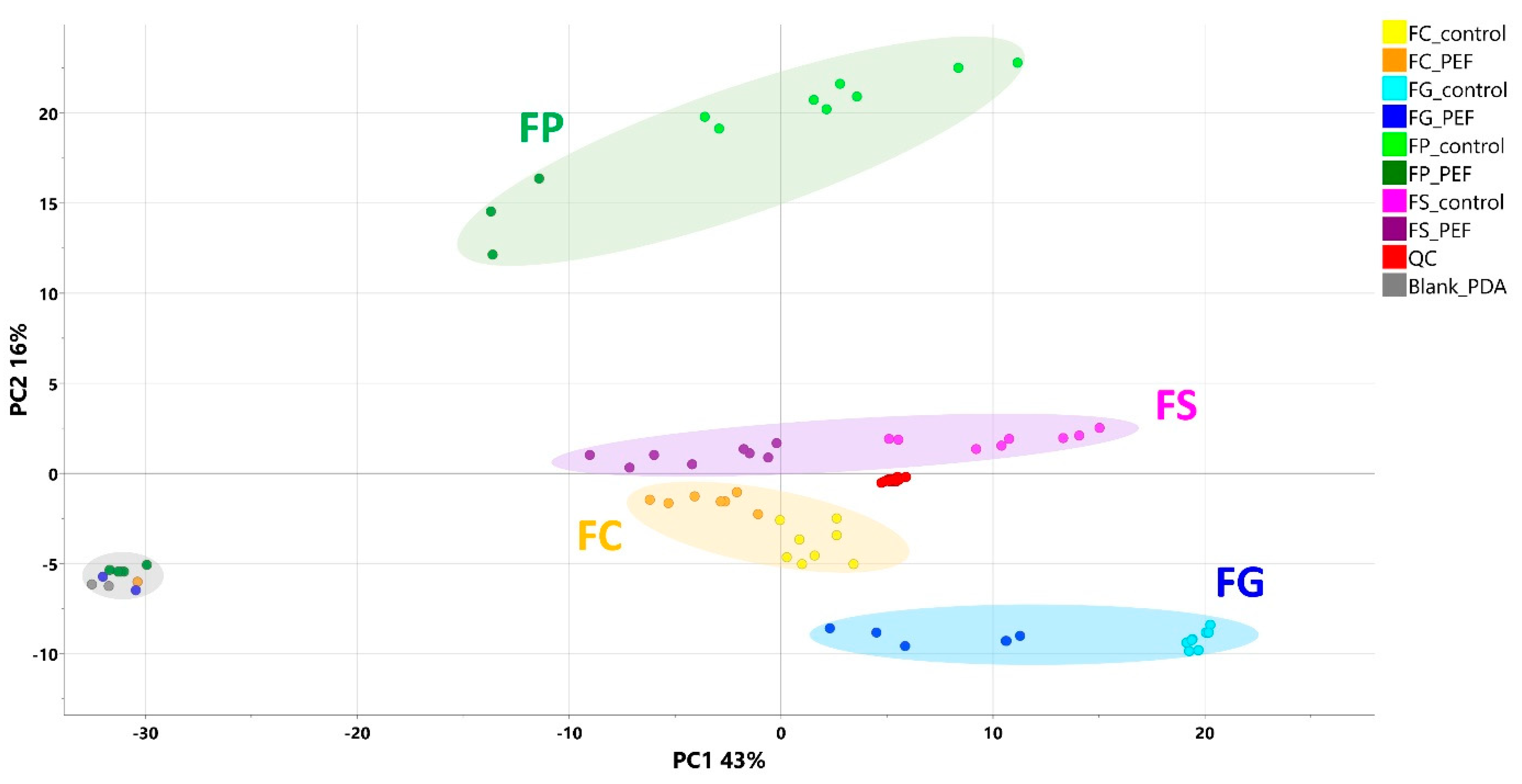

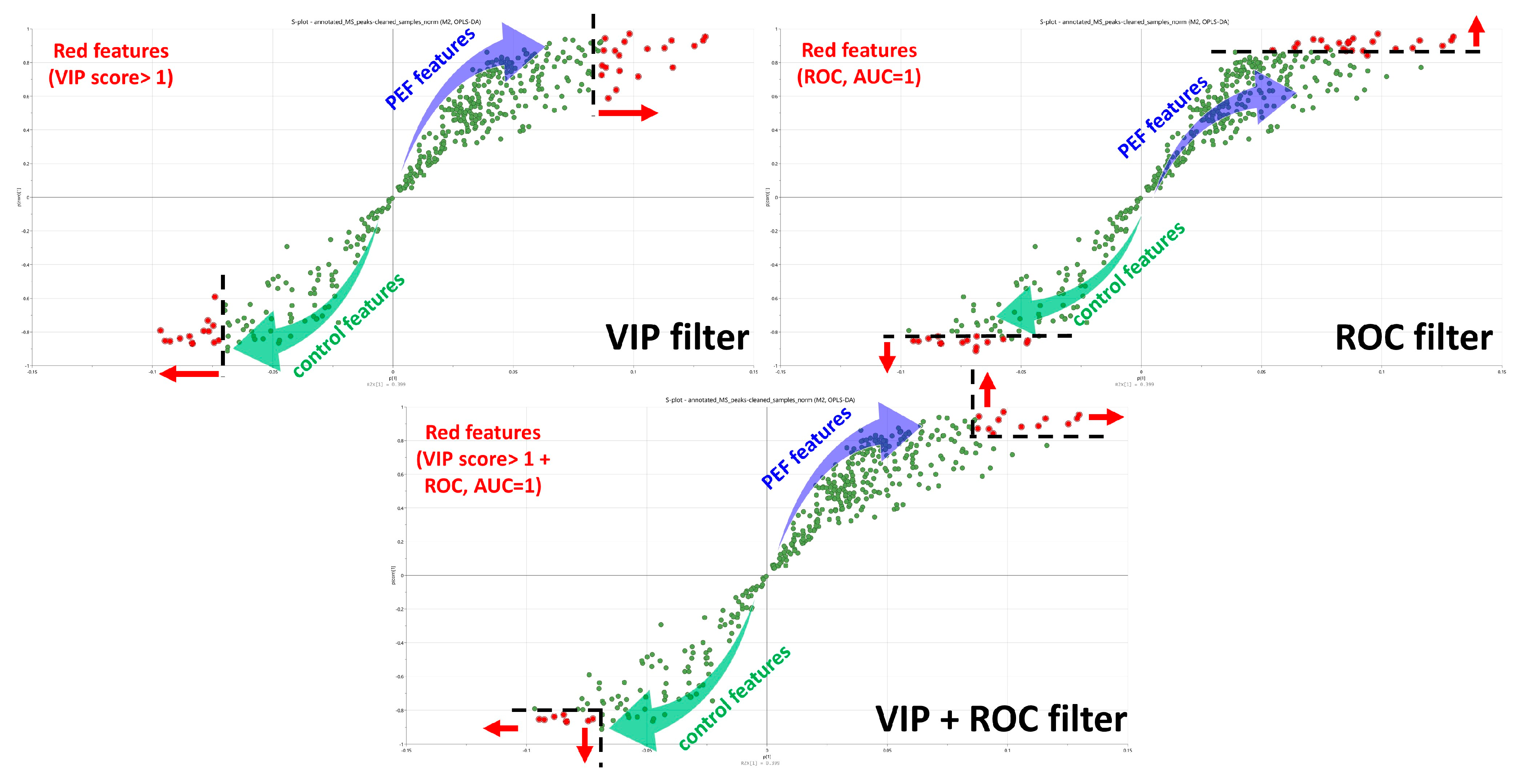

| Polarity of Features | Extraction Solvent/Solvent Mixtures | Number of Features | ||
|---|---|---|---|---|
| ESI+ | ESI- | SUM. | ||
| Polar (0–6 min Rt) | water | 4179 | 2962 | 7141 |
| methanol/water (50:50, v/v) | 5822 | 4165 | 9987 | |
| methanol | 6208 | 4179 | 10,387 | |
| methanol/propan-2-ol (50:50, v/v) | 5790 | 3798 | 9588 | |
| Middle-polar (6–12 min Rt) | water | 1517 | 197 | 1714 |
| methanol/water (50:50, v/v) | 1191 | 178 | 1369 | |
| methanol | 4466 | 1285 | 5751 | |
| methanol/propan-2-ol (50:50, v/v) | 4738 | 1272 | 6010 | |
| Nonpolar (12–19 min Rt) | water | 1416 | 15 | 1431 |
| methanol/water (50:50, v/v) | 899 | 9 | 908 | |
| methanol | 1435 | 10 | 1445 | |
| methanol/propan-2-ol (50:50, v/v) | 2480 | 149 | 2629 | |
| Dataset Overlays | MS/MS spec. Match Score * | Trend | Structure | Ontology | InChIKey Chemical Structure |
|---|---|---|---|---|---|
| FG ∩ FP ∩ FS | 1 | PEF | Compound 1 | Oxidized long-chain fatty acid derivatives | IGLRXLRPYYHFQN-UHFFFAOYSA-N |
| FG ∩ FP | 3 | PEF | Desotamide D | Cyclic hexapeptides | LZYIIJVZWQGRJH-CBHSLMNUSA-N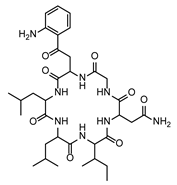 |
| FP ∩ FS | 1 | PEF | Megalomicin C1 | Macrolides | NGOSGEYHKQYUTN-XIBKBKGSSA-N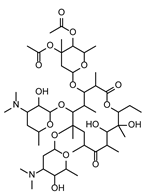 |
| FG ∩ FS | 1 | PEF | Compound 2 | Angular pyranocoumarins | PRZGTCWIDNLJEN-UHFFFAOYSA-N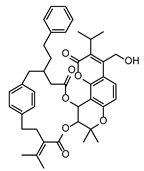 |
| FG ∩ FS | 2 | PEF | Compound 3 | Carbazoles | CBMRXUBLKLUUQW-CHRKOGDLNA-N |
| FG ∩ FS | 3 | PEF | Compound 4 | Guanidines | YMMKPCXKLMUKEC-UHFFFAOYSA-N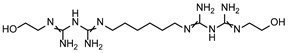 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behner, A.; Palicova, J.; Tobolkova, A.-H.; Prusova, N.; Stranska, M. Pulsed Electric Field Induces Significant Changes in the Metabolome of Fusarium Species and Decreases Their Viability and Toxigenicity. Toxins 2025, 17, 33. https://doi.org/10.3390/toxins17010033
Behner A, Palicova J, Tobolkova A-H, Prusova N, Stranska M. Pulsed Electric Field Induces Significant Changes in the Metabolome of Fusarium Species and Decreases Their Viability and Toxigenicity. Toxins. 2025; 17(1):33. https://doi.org/10.3390/toxins17010033
Chicago/Turabian StyleBehner, Adam, Jana Palicova, Anna-Hirt Tobolkova, Nela Prusova, and Milena Stranska. 2025. "Pulsed Electric Field Induces Significant Changes in the Metabolome of Fusarium Species and Decreases Their Viability and Toxigenicity" Toxins 17, no. 1: 33. https://doi.org/10.3390/toxins17010033
APA StyleBehner, A., Palicova, J., Tobolkova, A.-H., Prusova, N., & Stranska, M. (2025). Pulsed Electric Field Induces Significant Changes in the Metabolome of Fusarium Species and Decreases Their Viability and Toxigenicity. Toxins, 17(1), 33. https://doi.org/10.3390/toxins17010033





