First Report of Safe Italian Peanut Production Regarding Aflatoxin
Abstract
1. Introduction
2. Results
2.1. Meteorological Data and Irrigation
2.2. Peanut Varieties
2.3. Production and Contamination Data for Lotos
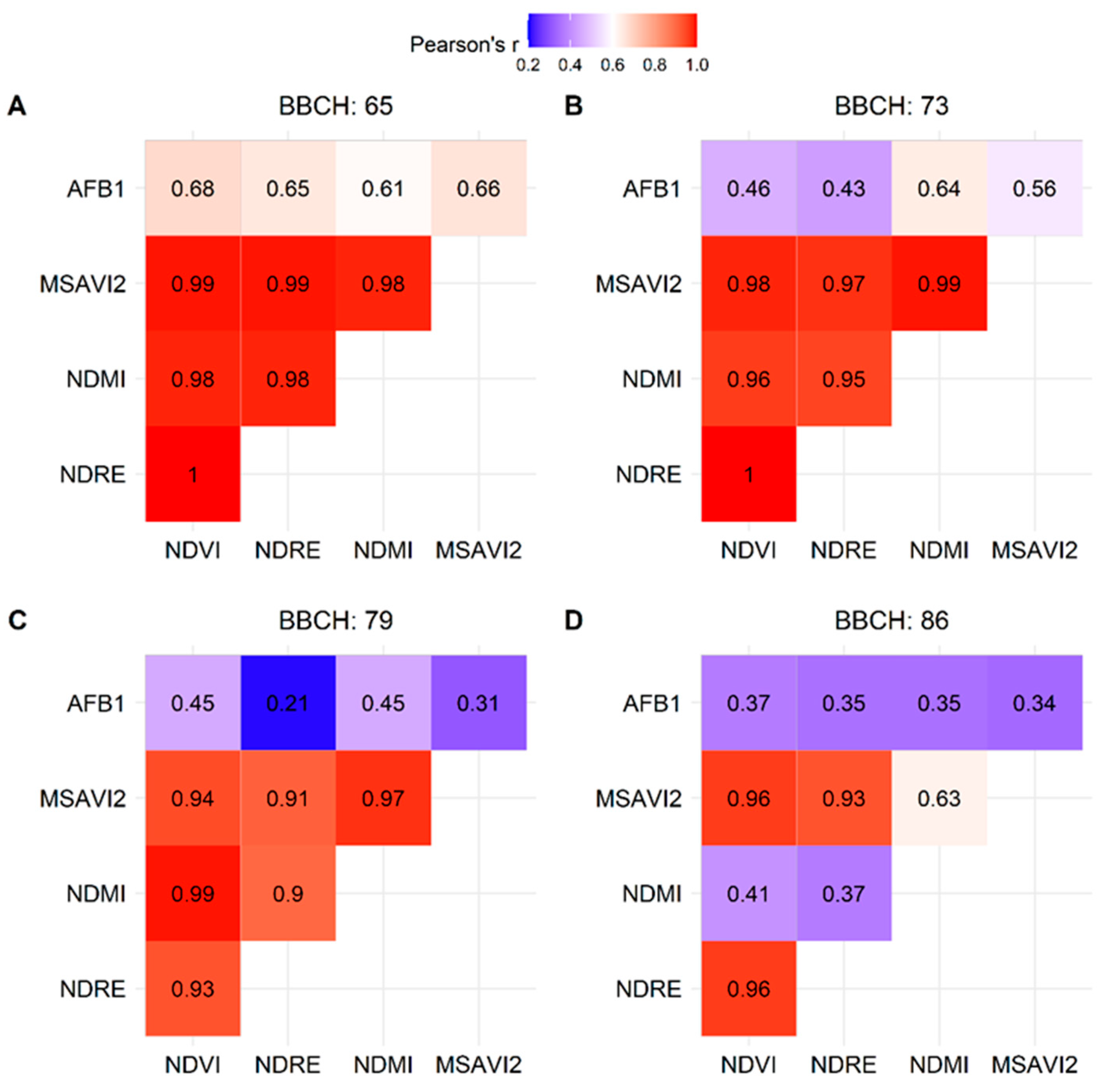
2.4. Vegetation Indices (VIs) and AFB1
3. Discussion and Conclusions
4. Materials and Methods
4.1. Selection of Location and Sampling
4.2. Meteorological Data and Irrigation
4.3. Fungal Population Analysis
4.3.1. Sample Preparation
4.3.2. Fungal Identification
4.4. Aflatoxin Analysis
4.4.1. Reagents and Chemicals
4.4.2. Aflatoxins Extraction
4.4.3. LC-MS/MS Analysis
4.5. Satellite Imagery
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 2 October 2024).
- Istat. Istat: Coltivazioni. Available online: https://www.istat.it/statistiche-per-temi/economia/agricoltura/#Microdati (accessed on 2 October 2024).
- Sannino, M.; Piscopo, R.; Assirelli, A.; Serrapica, F.; Caracciolo, G.; Ardito, L.; Antignano, A.; Ceriello, G.; Faugno, S. Evaluation of Arachis Hypogaea as New Multipurpose Crop for Central-Sud Italy. Eur. Biomass Conf. Exhib. Proc. 2020, 2020, 155–159. [Google Scholar]
- Sultan, Y.; Magan, N. Mycotoxigenic Fungi in Peanuts from Different Geographic Regions of Egypt. Mycotoxin Res. 2010, 26, 133–140. [Google Scholar] [CrossRef] [PubMed]
- RASFF. RASFF Window: Notifications. Available online: https://webgate.ec.europa.eu/rasff-window/screen/search (accessed on 12 November 2024).
- Aristil, J.; Venturini, G.; Maddalena, G.; Toffolatti, S.L.; Spada, A. Fungal Contamination and Aflatoxin Content of Maize, Moringa and Peanut Foods from Rural Subsistence Farms in South Haiti. J. Stored Prod. Res. 2020, 85, 101550. [Google Scholar] [CrossRef]
- Shephard, G.S. Impact of Mycotoxins on Human Health in Developing Countries. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Gupta, S.; Gupta, M. A Review Study on Biological Ill Effects and Health Hazards of Aflatoxins. Asian J. Adv. Med. 2021, 3, 1–8. [Google Scholar]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human Aflatoxicosis in Developing Countries: A Review of Toxicology, Exposure, Potential Health Consequences, and Interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef]
- Claeys, L.; Romano, C.; De Ruyck, K.; Wilson, H.; Fervers, B.; Korenjak, M.; Zavadil, J.; Gunter, M.J.; De Saeger, S.; De Boevre, M.; et al. Mycotoxin Exposure and Human Cancer Risk: A Systematic Review of Epidemiological Studies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1449–1464. [Google Scholar] [CrossRef]
- Meneely, J.P.; Kolawole, O.; Haughey, S.A.; Miller, S.J.; Krska, R.; Elliott, C.T. The Challenge of Global Aflatoxins Legislation with a Focus on Peanuts and Peanut Products: A Systematic Review. Expo. Heal. 2022, 15, 467–487. [Google Scholar] [CrossRef]
- Wu, F.; Guclu, H. Aflatoxin Regulations in a Network of Global Maize Trade. PLoS ONE 2012, 7, e45151. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2023/915 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- Amaike, S.; Keller, N.P. Aspergillus Flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Horn, B.W.; Greene, R.L.; Sobolev, V.S.; Dorner, J.W.; Powell, J.H.; Layton, R.C. Association of Morphology and Mycotoxin Production with Vegetative Compatibility Groups in Aspergillus Flavus, A. Parasiticus, and A. Tamarii. Mycologia 1996, 88, 574–587. [Google Scholar] [CrossRef]
- Payne, G.A. Process of Contamination by Aflatoxin-Producing Fungi and Their Impact on Crops. In Mycotoxins in Agriculture and Food Safety; CRC Press: Boca Raton, FL, USA, 1998; pp. 279–306. [Google Scholar]
- Klich, M.A. Biogeography of Aspergillus Species in Soil and Litter. Mycologia 2002, 94, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Klich, M.A. Aspergillus Flavus: The Major Producer of Aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; Van Der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B 1 Contamination in Maize in Europe Increases Due to Climate Change. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Piva, G.; Battilani, P.; Pietri, A. Emerging Issues in Southern Europe: Aflatoxins in Italy. In The Mycotoxin Factbook: Food & Feed Topics; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006; pp. 139–153. [Google Scholar]
- Cardwell, K.F.; Cotty, P.J. Distribution of Aspergillus Section Flavi among Field Soils from the Four Agroecological Zones of the Republic of Bénin, West Africa. Plant Dis. 2002, 86, 434–439. [Google Scholar] [CrossRef]
- Geiser, D.M.; Dorner, J.W.; Horn, B.W.; Taylor, J.W. The Phylogenetics of Mycotoxin and Sclerotium Production in Aspergillus Flavus and Aspergillus Oryzae. Fungal Genet. Biol. 2000, 31, 169–179. [Google Scholar] [CrossRef]
- Giorni, P.; Magan, N.; Pietri, A.; Bertuzzi, T.; Battilani, P. Studies on Aspergillus Section Flavi Isolated from Maize in Northern Italy. Int. J. Food Microbiol. 2007, 113, 330–338. [Google Scholar] [CrossRef]
- Sanchis, V.; Magan, N. Environmental Conditions Affecting Mycotoxins. Mycotoxins Food 2004, 174–189. [Google Scholar] [CrossRef]
- Giorni, P.; Bertuzzi, T.; Battilani, P. Aflatoxin in Maize, a Multifaceted Answer of Aspergillus Flavus Governed by Weather, Host-Plant and Competitor Fungi. J. Cereal Sci. 2016, 70, 256–262. [Google Scholar] [CrossRef]
- Jarvis, B.B. Mycotoxins–an Overview. Nat. Toxins 1989, 17–29. [Google Scholar]
- Pitt, J.I.; Hocking, A.D. Mycotoxins in Australia: Biocontrol of Aflatoxin in Peanuts. Mycopathologia 2006, 162, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Dorner, J.W.; Cole, R.J.; Sanders, T.H.; Blankenship, P.D. Interrelationship of Kernel Water Activity, Soil Temperature, Maturity, and Phytoalexin Production in Preharvest Aflatoxin Contamination of Drought-Stressed Peanuts. Mycopathologia 1989, 105, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.H.; Cole, R.J.; Blankenship, P.D.; Hill, R.A. Relation of Environmental Stress Duration to Aspergillus Flavus Invasion and Aflatoxin Production in Preharvest Peanuts. Peanut Sci. 1985, 12, 90–93. [Google Scholar] [CrossRef]
- Hill, R.A.; Blankenship, P.D.; Cole, R.J.; Sanders, T.H. Effects of Soil Moisture and Temperature on Preharvest Invasion of Peanuts by the Aspergillus Flavus Group and Subsequent Aflatoxin Development. Appl. Environ. Microbiol. 1983, 45, 628–633. [Google Scholar] [CrossRef]
- Jeyaramraja, P.R.; Meenakshi, S.N.; Woldesenbet, F. Relationship between Drought and Preharvest Aflatoxin Contamination in Groundnut (Arachis Hypogaea L.). World Mycotoxin J. 2018, 11, 187–199. [Google Scholar] [CrossRef]
- Dorner, J.W. Management and Prevention of Mycotoxins in Peanuts. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 203–208. [Google Scholar] [CrossRef]
- Torres, A.M.; Barros, G.G.; Palacios, S.A.; Chulze, S.N.; Battilani, P. Review on Pre- and Post-Harvest Management of Peanuts to Minimize Aflatoxin Contamination. Food Res. Int. 2014, 62, 11–19. [Google Scholar] [CrossRef]
- Waliyar, F.; Traoré, A.; Fatondji, D.; Ntare, B.R. Effect of Irrigation Interval, Planting Date, and Cultivar on Aspergillus Flavus and Aflatoxin Contamination of Peanut in a Sandy Soil of Niger. Peanut Sci. 2003, 30, 79–84. [Google Scholar] [CrossRef]
- Voitik, A.; Kravchenko, V.; Pushka, O.; Kutkovetska, T.; Shchur, T.; Kocira, S. Comparison of NDVI, NDRE, MSAVI and NDSI Indices for Early Diagnosis of Crop Problems. Agric. Eng. 2023, 27, 47–57. [Google Scholar] [CrossRef]
- Kancheva, R.; Borisova, D. Vegetation Stress Indicators Derived from Multispectral and Multitemporal Data. Space Technol 2007, 26, 1–8. [Google Scholar] [CrossRef]
- Mimić, G.; Živaljević, B.; Blagojević, D.; Pejak, B.; Brdar, S. Quantifying the Effects of Drought Using the Crop Moisture Stress as an Indicator of Maize and Sunflower Yield Reduction in Serbia. Atmosphere 2022, 13, 1880. [Google Scholar] [CrossRef]
- Kerry, R.; Ingram, B.; Ortiz, B.V.; Salvacion, A. Using Soil, Plant, Topographic and Remotely Sensed Data to Determine the Best Method for Defining Aflatoxin Contamination Risk Zones within Fields for Precision Management. Agronomy 2022, 12, 2524. [Google Scholar] [CrossRef]
- Jędrejek, A.; Jadczyszyn, J.; Pudełko, R. Increasing Accuracy of the Soil-Agricultural Map by Sentinel-2 Images Analysis—Case Study of Maize Cultivation under Drought Conditions. Remote Sens. 2023, 15, 1281. [Google Scholar] [CrossRef]
- Ahmad, U.; Alvino, A.; Marino, S. A Review of CropWater Stress Assessment Using Remote Sensing. Remote Sens. 2021, 13, 4155. [Google Scholar] [CrossRef]
- Sannino, M.; Assirelli, A.; Piscopo, R.; Esposito, F.; Faugno, S. Harvesting of Arachis Hypogaea L. in an Italian Area: Synergy between Cultural Techniques and Mechanization. Agronomy 2024, 14, 27. [Google Scholar] [CrossRef]
- Tallarita, A.; Sannino, M.; Cozzolino, E.; Albanese, D.; Fratianni, F.; Faugno, S.; Piscopo, R.; Nazzaro, F.; Cuciniello, A.; Maiello, R.; et al. Yield, Quality, Antioxidants and Elemental Composition of Peanut as Affected by Plant Density and Harvest Time. Italus Hortus 2021, 28, 25–35. [Google Scholar] [CrossRef]
- Asare Bediako, K.; Ofori, K.; Offei, S.K.; Dzidzienyo, D.; Asibuo, J.Y.; Adu Amoah, R. Aflatoxin Contamination of Groundnut (Arachis Hypogaea L.): Predisposing Factors and Management Interventions. Food Control 2019, 98, 61–67. [Google Scholar] [CrossRef]
- Abou Kheira, A.A. Macromanagement of Deficit-Irrigated Peanut with Sprinkler Irrigation. Agric. Water Manag. 2009, 96, 1409–1420. [Google Scholar] [CrossRef]
- Sanders, T.H.; Hill, R.A.; Cole, R.J.; Blankenship, P.D. Effect of Drought on Occurrence of Aspergillus Flavus in Maturing Peanuts. J. Am. Oil Chem. Soc. 1981, 58, A966–A970. [Google Scholar] [CrossRef]
- Sannino, M.; Faugno, S.; Piscopo, R.; Tallarita, A.V.; Serrapica, F.; Assirelli, A.; Caruso, G. Effetts of Irrigation, Fertilization and Mechanical Harvesting on Yield Performances of Peanut (Arachis Hypogaea L.) Grown in Southern Italy. In Proceedings of the European Biomass Conference and Exhibition, Marseille, France and Online, 26–29 April 2021; pp. 345–351. [Google Scholar]
- Sezen, S.M.; Yucel, S.; Tekin, S.; Yildiz, M. Determination of Optimum Irrigation and Effect of Deficit Irrigation Strategies on Yield and Disease Rate of Peanut Irrigated with Drip System in Eastern Mediterranean. Agric. Water Manag. 2019, 221, 211–219. [Google Scholar] [CrossRef]
- Chalwe, H.; Mweetwa, A.M.; Lungu, O.I.; Phiri, E.; Njoroge, S.; Brandenburg, R.L. Reducing Pre-Harvest Aflatoxin Content in Groundnuts through Soil Water Management. In Proceedings of the Fifth African Higher Education Week and RUFORUM Biennial Conference, Cape Town, South Africa, 17–21 October 2016; pp. 921–926. [Google Scholar]
- Pitt, J.I.; Dyer, S.K.; McCammon, S. Systemic Invasion of Developing Peanut Plants by Aspergillus Flavus. Lett. Appl. Microbiol. 1991, 13, 16–20. [Google Scholar] [CrossRef]
- Cole, R.J.; Hill, R.A.; Blankenship, P.D.; Sanders, T.H. Color Mutants of Aspergillus Flavus and Aspergillus Parasiticus in a Study of Preharvest Invasion of Peanuts. Appl. Environ. Microbiol. 1986, 52, 1128–1131. [Google Scholar] [CrossRef] [PubMed]
- Virnodkar, S.S.; Pachghare, V.K.; Patil, V.C.; Jha, S.K. Remote Sensing and Machine Learning for Crop Water Stress Determination in Various Crops: A Critical Review; Springer: New York, NY, USA, 2020; Volume 21. [Google Scholar]
- Boken, V.K.; Hoogenboom, G.; Williams, J.H.; Diarra, B.; Dione, S.; Easson, G.L. Monitoring Peanut Contamination in Mali (Africa) Using AVHRR Satellite Data and a Crop Simulation Model. Int. J. Remote Sens. 2008, 29, 117–129. [Google Scholar] [CrossRef]
- Crosta, M.; Leggieri, M.C.; Battilani, P. “AFLA-Peanut”, a Mechanistic Prototype Model to Predict Aflatoxin B1 Contamination. Phytopathol. Mediterr. 2024, 2024, 481–488. [Google Scholar] [CrossRef]
- European Commission. Common Catalogue of Varieties of Agricultural Plant Species. Supplement A 2024/7. Off. J. 2024, C/2024/4620. [Google Scholar]
- Njoki, L.; Okoth, S.; Wachira, P.; Ouko, A.; Mwololo, J.; Rizzu, M.; Oufensou, S.; Amakhobe, T. Evaluation of Agronomic Characteristics, Disease Incidence, Yield Performance, and Aflatoxin Accumulation among Six Peanut Varieties (Arachis Hypogea L.) Grown in Kenya. Toxins 2023, 15, 26–28. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spolage; Springer Nature: London, UK, 2013; Volume 53, ISBN 9788578110796. [Google Scholar]
- Sartori, A.V.; De Mattos, J.S.; Souza, Y.P.; Dos Santos, R.P.; Paulino de Moraes, M.H.; Da Nóbrega, A.W. Determination of Aflatoxins M1, M2, B1, B2, G1 and G2 in Peanut by Modified QuEChERS Method and Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. Vigilância Sanitária Em Debate 2014, 3, 115–121. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-Scale Geospatial Analysis for Everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Wilson, E.H.; Sader, S.A. Detection of Forest Harvest Type Using Multiple Dates of Landsat TM Imagery. Remote Sens. Environ. 2002, 80, 385–396. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Quantitative Estimation of Chlorophyll-a Using Reflectance Spectra: Experiments with Autumn Chestnut and Maple Leaves. J. Photochem. Photobiol. B Biol. 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Rouse, J.W.J.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. NASA Spec. Publ. 1974, 351, 309. [Google Scholar]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A Modified Soil Adjusted Vegetation Index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono-and Dicotyledonous Plants: BBCH Monograph, 2nd Ed. Fed. Biol. Res. Cent. Agric. For. 2001, 49, 66–70. [Google Scholar] [CrossRef]
- Pearson, K.L.I.I.I. On Lines and Planes of Closest Fit to Systems of Points in Space. Lond. Edinb. Dublin Philos. Mag. J. Sci. 2010, 2, 559–572. [Google Scholar] [CrossRef]
| Area | Province | Long | Lat | Year | Tmax (°C) | Tmin (°C) | Tmean (°C) | Precipitations (mm) | Irrigation (n) | Irrigation (mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ferrara | 11.32894 | 44.75973 | 2022 | 32.12 | 16.53 | 24.51 | 123.90 | 5 | 189 |
| Modena | 11.28777 | 44.85639 | 2022 | 32.66 | 16.94 | 25.01 | 115.70 | 6 | 214 | |
| Ferrara | 11.48193 | 44.88769 | 2023 | 31.22 | 17.45 | 24.54 | 143.50 | 1 | 40 | |
| 2 | Ferrara | 12.18881 | 44.78862 | 2022 | 29.75 | 18.64 | 24.29 | 63.60 | 1 | 48 |
| Ferrara | 12.07147 | 44.91150 | 2022 | 31.69 | 18.37 | 24.89 | 67.00 | 4 | 155 | |
| Ferrara | 12.06913 | 44.91158 | 2022 | 31.25 | 17.99 | 24.50 | 93.70 | 4 | 204 | |
| Ferrara | 12.05803 | 44.88688 | 2023 | 28.77 | 17.98 | 23.33 | 165.71 | NA * | NA | |
| 3 | Ferrara | 12.04619 | 44.69533 | 2022 | 31.90 | 18.31 | 25.02 | 60.10 | 5 | 228 |
| Ferrara | 12.11603 | 44.72886 | 2022 | 31.02 | 18.66 | 24.80 | 58.10 | 5 | 234 | |
| Ferrara | 11.92636 | 44.62356 | 2022 | 31.84 | 17.72 | 24.75 | 63.50 | 5 | 175 | |
| Ferrara | 11.89168 | 44.74335 | 2023 | 31.18 | 17.89 | 24.59 | 98.90 | 5 | 190 | |
| 4 | Verona | 11.10991 | 45.27143 | 2023 | 29.81 | 18.25 | 23.89 | 55.40 | 5 | 125 |
| Verona | 11.10933 | 45.27024 | 2023 | 29.77 | 18.43 | 23.97 | 55.40 | 5 | 125 | |
| Verona | 11.11122 | 45.26857 | 2023 | 29.77 | 18.43 | 23.97 | 55.40 | 5 | 125 | |
| 5 | Pordenone | 12.88952 | 45.86698 | 2023 | 30.31 | 17.35 | 23.84 | 149.80 | 0 | 0 |
| Pordenone | 12.91461 | 45.87008 | 2023 | 30.33 | 17.62 | 24.00 | 148.40 | 1 | 30 | |
| 6 | Cuneo | 7.68155 | 44.71550 | 2022 | 30.24 | 16.75 | 23.60 | 140.10 | 0 | 0 |
| 7 | Avellino | 14.77357 | 40.82467 | 2022 | NA | NA | NA | NA | NA | NA |
| Factor | Pods Weight (g) | Seeds Weight (g) | Number of Seeds (n) | Shelling Percentage % 1 | Mean Seeds per Pod (n) | 1000 Seeds Weight (g) 2 |
| Variety | ** | ** | ** | NS | ** | ** |
| Lotos | 143.72 a | 102.79 a | 109.43 b | 71.48 | 2.2 b | 937.18 a |
| IPG914 | 111.10 b | 78.61 b | 93.50 c | 70.99 | 1.9 c | 840.31 a |
| SIS_AR_01 | 136.61 a | 98.90 a | 158.30 a | 72.18 | 3.2 a | 625.23 b |
| Factor | Aspergillus sec. Flavi (CFU/g) | Aspergillus sec. Nigri (CFU/g) | Fusarium spp. (CFU/g) | Penicillium spp. (CFU/g) | Total fungi (CFU/g) | AFB1 (µg/kg) |
| Variety | ** | * | NS | ** | NS | NS |
| Lotos | 2.11 × 101 b | 1.10 × 104 | 3.73 × 103 | 4.54 × 104 ab | 1.01 × 105 | 0.18 |
| IPG914 | 4.00 × 101 b | 2.17 × 102 | 1.77 × 102 | 2.40 × 102 b | 1.56 × 103 | 0.00 |
| SIS_AR_01 | 6.44 × 102 a | 4.63 × 103 | 0.00 × 100 | 2.09 × 104 a | 2.73 × 104 | 0.39 |
| Factors | Pods Weight (g) | Seeds Weight (g) | Number of Seeds (n) | 2 Shelling Percentage % | Mean Seeds per Pod (n) | 3 1000 Seeds Weight (g) |
| Geographical area 1 | ** | ** | ** | ** | ** | ** |
| 1 | 162.25 a | 115.95 a | 112.73 a | 71.52 a | 2.25 a | 1028.30 a |
| 2 | 155.28 a | 113.27 a | 111.10 a | 72.91 a | 2.22 a | 1021.13 a |
| 3 | 150.40 ab | 110.08 a | 110.45 a | 73.27 a | 2.21 a | 998.09 a |
| 4 | 135.71 bc | 89.30 bc | 109.40 ab | 65.85 b | 2.19 ab | 816.33 b |
| 5 | 126.84 cd | 91.38 b | 111.40 a | 72.03 a | 2.23 a | 821.22 b |
| 6 | 106.70 e | 76.58 c | 100.00 b | 71.74 a | 2.00 b | 766.39 b |
| 7 | 108.20 de | 77.76 bc | 99.60 b | 71.85 a | 1.99 b | 780.72 b |
| Year | NS | ** | NS | ** | NS | ** |
| 2022 | 145.79 | 106.24 a | 109.0 | 72.87 a | 2.18 | 971.24 a |
| 2023 | 140.00 | 96.58 b | 110.3 | 68.99 b | 2.21 | 875.87 b |
| Factors | Aspergillus sec. Flavi (CFU/g) | Aspergillus sec. Nigri (CFU/g) | Fusarium spp. (CFU/g) | Penicillium spp. (CFU/g) | Total fungi (CFU/g) | AFB1 (µg/kg) |
| Geographical area | NS | ** | * | NS | * | NS |
| 1 | 9.52 × 100 | 3.15 × 102 bc | 1.45 × 104 | 7.63 × 104 | 2.07 × 105 a | 0.10 |
| 2 | 0.00 × 100 | 6.93 × 104 b | 7.00 × 101 | 3.29 × 104 | 1.58 × 105 a | 0.00 |
| 3 | 1.50 × 101 | 3.17 × 102 bc | 8.62 × 102 | 8.52 × 104 | 8.68 × 104 ab | 0.04 |
| 4 | 3.20 × 101 | 2.14 × 102 b | 1.10 × 101 | 9.28 × 101 | 6.79 × 102 b | 0.66 |
| 5 | 1.20 × 102 | 9.90 × 103 a | 3.53 × 102 | 4.71 × 103 | 1.54 × 104 ab | 0.76 |
| 6 | 2.00 × 101 | 0.00 × 100 c | 5.34 × 103 | 8.20 × 102 | 6.61 × 103 ab | 0.00 |
| 7 | 0.00 × 100 | 0.00 × 100 c | 1.73 × 103 | 7.23 × 102 | 1.30 × 105 ab | 0.00 |
| Year | * | ** | NS | NS | * | * |
| 2022 | 4.92 × 100 b | 1.59 × 104 a | 5.80 × 103 | 7.01 × 104 | 1.56 × 105 a | 0.00 b |
| 2023 | 4.95 × 101 a | 2.32 × 103 b | 1.03 × 102 | 1.99 × 103 | 4.98 × 103 b | 0.50 a |
| Area | Province | Long | Lat | Year | Peanut Variety | Previous Crop | Sowing Time | Sampling Time |
|---|---|---|---|---|---|---|---|---|
| 1 | Ferrara | 11.328936 | 44.75973 | 2022 | Lotos | Maize | 10/05 | 04/10 |
| 1 | Modena | 11.287771 | 44.856394 | 2022 | Lotos | Maize | 19/05 | 29/09 |
| 1 | Ferrara | 11.481934 | 44.887692 | 2023 | Lotos | Wheat | 30/05 | 29/09 |
| 2 | Ferrara | 12.188809 | 44.788623 | 2022 | Lotos | Chard seed | 10/05 | 07/09 |
| 2 | Ferrara | 12.071466 | 44.911498 | 2022 | Lotos | Wheat | 09/05 | 16/09 |
| 2 | Ferrara | 12.069126 | 44.911584 | 2022 | SIS_AR_01 | Wheat | 09/05 | 22/09 |
| 2 | Ferrara | 12.058032 | 44.886884 | 2023 | SIS_AR_01 | NA * | 11/05 | 21/09 |
| 3 | Ferrara | 12.046193 | 44.695327 | 2022 | Lotos | Maize | 10/05 | 13/09 |
| 3 | Ferrara | 12.116027 | 44.728864 | 2022 | Lotos | Maize | 10/05 | 10/09 |
| 3 | Ferrara | 11.926363 | 44.623563 | 2022 | Lotos | Ryegrass | 11/05 | 22/09 |
| 3 | Ferrara | 11.891678 | 44.743345 | 2023 | Lotos | Soybean | 27/05 | 21/09 |
| 4 | Verona | 11.109907 | 45.271434 | 2023 | IPG914 | Mixed crops | 15/06 | 11/10 |
| 4 | Verona | 11.109327 | 45.270235 | 2023 | Lotos | Mixed crops | 14/06 | 03/10 |
| 4 | Verona | 11.111219 | 45.26857 | 2023 | Lotos | Mixed crops | 14/06 | 03/10 |
| 5 | Pordenone | 12.889521 | 45.866977 | 2023 | IPG914 | Soybean | 29/06 | 11/10 |
| 5 | Pordenone | 12.914613 | 45.870081 | 2023 | Lotos | Soybean | 27/06 | 03/10 |
| 6 | Cuneo | 7.681548 | 44.7155 | 2022 | Lotos | Fallow field | 07/05 | 28/09 |
| 7 | Avellino | 14.77357 | 40.824669 | 2022 | Lotos | NA | NA | NA |
| BBCH | Phase Description |
|---|---|
| 65 | Flowering |
| 73 | Pods development and pod filling |
| 79 | Seeds fill the inner space of the pods, which have reached their full size |
| 86 | 60% of fully developed pods are ripe |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crosta, M.; Croci, M.; Dall’Asta, C.; Pisante, M.; Battilani, P. First Report of Safe Italian Peanut Production Regarding Aflatoxin. Toxins 2025, 17, 90. https://doi.org/10.3390/toxins17020090
Crosta M, Croci M, Dall’Asta C, Pisante M, Battilani P. First Report of Safe Italian Peanut Production Regarding Aflatoxin. Toxins. 2025; 17(2):90. https://doi.org/10.3390/toxins17020090
Chicago/Turabian StyleCrosta, Matteo, Michele Croci, Chiara Dall’Asta, Michele Pisante, and Paola Battilani. 2025. "First Report of Safe Italian Peanut Production Regarding Aflatoxin" Toxins 17, no. 2: 90. https://doi.org/10.3390/toxins17020090
APA StyleCrosta, M., Croci, M., Dall’Asta, C., Pisante, M., & Battilani, P. (2025). First Report of Safe Italian Peanut Production Regarding Aflatoxin. Toxins, 17(2), 90. https://doi.org/10.3390/toxins17020090








