The Elias University Hospital Approach: A Visual Guide to Ultrasound-Guided Botulinum Toxin Injection in Spasticity: Part I—Distal Upper Limb Muscles
Abstract
:1. Introduction
2. Distal Upper Limb Muscles Implicated in Post-Stroke Spasticity
2.1. Pronator Teres (PT)
2.1.1. Overview
2.1.2. Ultrasound Identification
- The PT, which appears oval-shaped on the lateral side;
- The flexor carpi radialis (FCR), which appears triangular-shaped on the medial side [8].
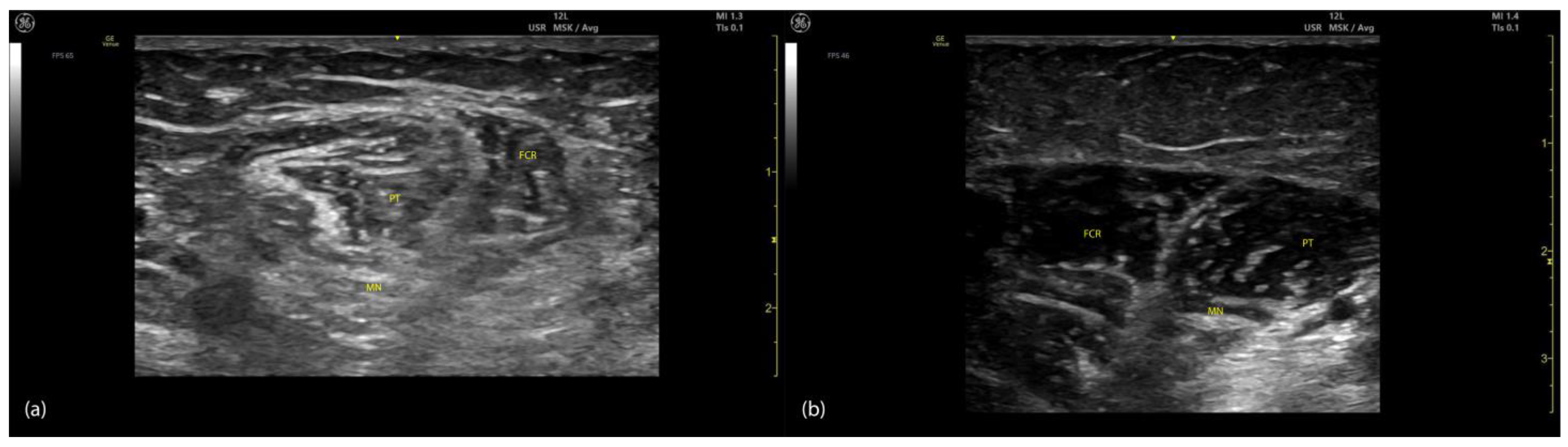
2.1.3. Key Ultrasound Landmarks (Figure 1)
- Muscle position: The PT is the first muscle mass from radial to ulnar on the volar aspect of the forearm [9].
- Intramuscular tendon: In the lateral portion of the muscle, it is positioned longitudinally along the muscle at this level.
- Internal fascia: The fascia separates the humeral (PTHH) and ulnar heads (PTUH) of the PT, while the external fascia distinctly demarcates the PT from adjacent muscle masses, facilitating precise BoNT-A injection.
- Median nerve: The nerve is located deep to the PT, approximately 2 cm distal to the elbow crease. Dynamic evaluation cranially towards the arm reveals the nerve transitioning to the lateral aspect of the muscle alongside the brachial artery [10].
- Dynamic evaluation (Video S1): Scanning proximally toward the medial epicondyle shows an increase in the PT muscle belly size and a concurrent decrease in the size of the FCR. At this level, the intramuscular fascia specific to the PT becomes evident, separating the two muscle heads—the humeral head and the ulnar head—allowing for individual targeting [11].

2.1.4. Clinical Implications and Injection Strategy
- The humeral head of the PT should receive a higher dose of BoNT-A because it contributes to elbow flexion [13];
- The motor point is located 20% distal to the intercondylar line along the muscle’s length, as described by Sung-Yoon Won et al. [17];
- In our clinical practice, to accurately target this motor point, we employ ultrasound to identify the point of maximum muscle thickness, which aligns closely with the motor point due to its innervation density. BoNT-A injections are then administered with the transducer placed transversely on the volar forearm, approximately 2–3 cm distal to the elbow crease in the radial region.
2.2. Flexor Carpi Radialis (FCR)
2.2.1. Overview
2.2.2. Ultrasound Identification
2.2.3. Key Ultrasound Landmarks (Figure 2)
- Muscle position: The FCR is the second muscle mass from radial to ulnar on the volar aspect of the forearm [20].
- Intramuscular tendon: The bipennate structure of the FCR is evident on ultrasound due to the presence of longitudinal intramuscular tendon [21].
- Median nerve: The median nerve is initially located deep to the PT, approximately 2 cm distal to the elbow crease. With distal scanning, the nerve transitions into the deeper plane of the FCR and maintains this relationship until the mid-forearm. Beyond this level, the median nerve passes deep to the flexor digitorum superficialis (FDS) [10].
- External fascia: The external fascia distinctly demarcates the FCR from adjacent muscle masses, facilitating precise BoNT-A injection.
- Dynamic evaluation: Muscle contraction is clearly visible during wrist flexion and abduction maneuvers. Scanning distally reveals an increase in the FCR muscle belly size while the PT decreases in size [11].

2.2.4. Clinical Implications and Injection Strategy
- A 2014 study investigating the localization of motor points and intramuscular nerve branches of wrist flexors relative to bony landmarks found that the highest concentration of motor points in the FCR is situated approximately 27% along the line connecting the most prominent point of the medial epicondyle to the base of the second metacarpal bone, equivalent to roughly one-fourth of the muscle’s length [22].
- In our clinical practice, ultrasound is used to precisely target the motor point by identifying the point of maximum muscle thickness. The transducer is placed transversely on the volar aspect of the forearm, approximately 4–5 cm distal to the elbow crease in the radial region, ensuring accurate and effective delivery.
2.3. Flexor Carpi Ulnaris (FCU)
2.3.1. Overview
2.3.2. Ultrasound Identification
2.3.3. Key Ultrasound Landmarks (Figure 3)
- Muscle position: The FCU is the most medial muscle mass in the flexor compartment of the wrist, located on the volar aspect of the forearm [24].
- Internal fascia: The intramuscular fascia, often referred to as “cloud fascia” due to its cloud-like appearance, separates the humeral and ulnar heads of the FCU. This fascia can be visualized from the muscle’s origin at the medial epicondyle, extending distally until the FCU transitions into its tendon [23].
- Ulnar nerve (Video S2): The ulnar nerve is located deep to the FCU approximately 2–3 cm distal to the elbow crease. When scanning cranially toward the medial epicondyle, the ulnar nerve can be seen passing between the humeral and ulnar heads of the FCU as it enters the volar compartment of the forearm [25,26]. Scanning distally reveals a gradual decrease in FCU muscle size and an increase in FDP muscle size. At the mid-forearm level, the ulnar nerve joins the ulnar artery, and both structures descend together into the distal forearm toward Guyon’s canal [26].
- External fascia: The external fascia distinctly demarcates the FCU from adjacent muscle masses, facilitating precise BoNT-A injection.
- Dynamic evaluation: Contraction of the FCU is observed during wrist flexion and adduction maneuvers.

2.3.4. Clinical Implications and Injection Strategy
- Electromyographic studies indicate that the region with the highest concentration of motor plates in the FCU is located approximately 32% along the line connecting the most prominent point of the medial epicondyle to the pisiform bone, corresponding to about one-third of the muscle’s length [22].
- In our clinical practice, ultrasound is used to precisely target the motor point by identifying the point of maximum muscle thickness. The transducer is positioned transversely on the volar aspect of the forearm, approximately 4 cm distal to the elbow crease in the ulnar portion, ensuring accurate and effective delivery of BoNT-A.
2.4. Flexor Digitorum Superficialis (FDS)
2.4.1. Overview
2.4.2. Ultrasound Identification
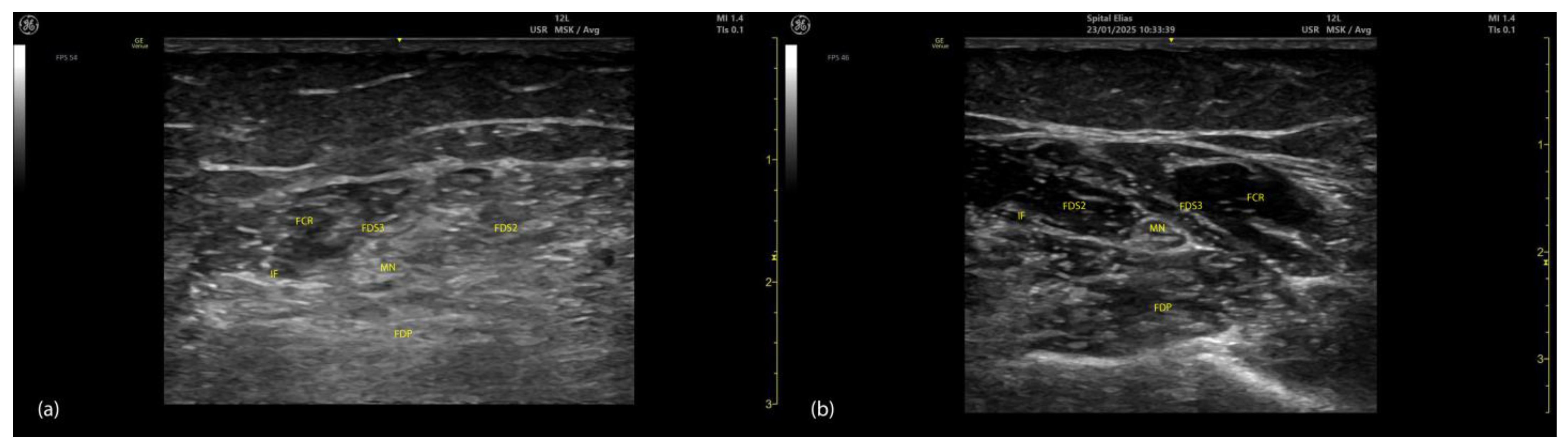
2.4.3. Key Ultrasound Landmarks (Figure 4 and Figure 5)
- Muscle position: At the mid-forearm, the FDS represents the second muscle mass from the cortical surfaces of the radius and ulna, moving from deep to superficial. Superficial to the FDS are the lateral FCR and medial FCU [29]. In the absence of US guidance, these adjacent muscles may be mistaken for the FDS, increasing the risk of injection errors.
- Median nerve: The median nerve is adhered to the deep surface of the FDS. At this level, the FDS and FDP are separated by the intermuscular fascia, which houses the median nerve, ulnar nerve, and ulnar artery [26].
- External fascia: FDS2 and FDS3 have a pronounced external fascia that distinctly demarcates them from adjacent muscle masses during BoNT-A injections, whereas FDS4 and FDS5 lack this feature.
- Dynamic evaluation (Video S3): The contraction of FDS muscle bellies corresponding to fingers II–V is observed during flexion of the metacarpophalangeal and proximal interphalangeal joints. A paradoxical arrangement of FDS2 and FDS3 bellies can be noted anatomically, as they appear inversely positioned medially and laterally relative to the median nerve [30]. Dynamic scanning proximal to the medial epicondyle reveals that the maximal thickness of FDS4 is located approximately 2–3 cm distal to the elbow crease, on the volar aspect of the forearm in the ulnar portion. At this level, the FDS4 is situated medial to the FCR and lateral to the FCU, following the line connecting the medial epicondyle to the pisiform bone [31]. A potential source of error in targeting FDS4 at this level is the palmaris longus (PL) muscle, which, if present, is located between the FCR and FDS4 muscle bellies. The PL is variably present in 74–97.5% of the population and is most commonly bilateral [32,33].

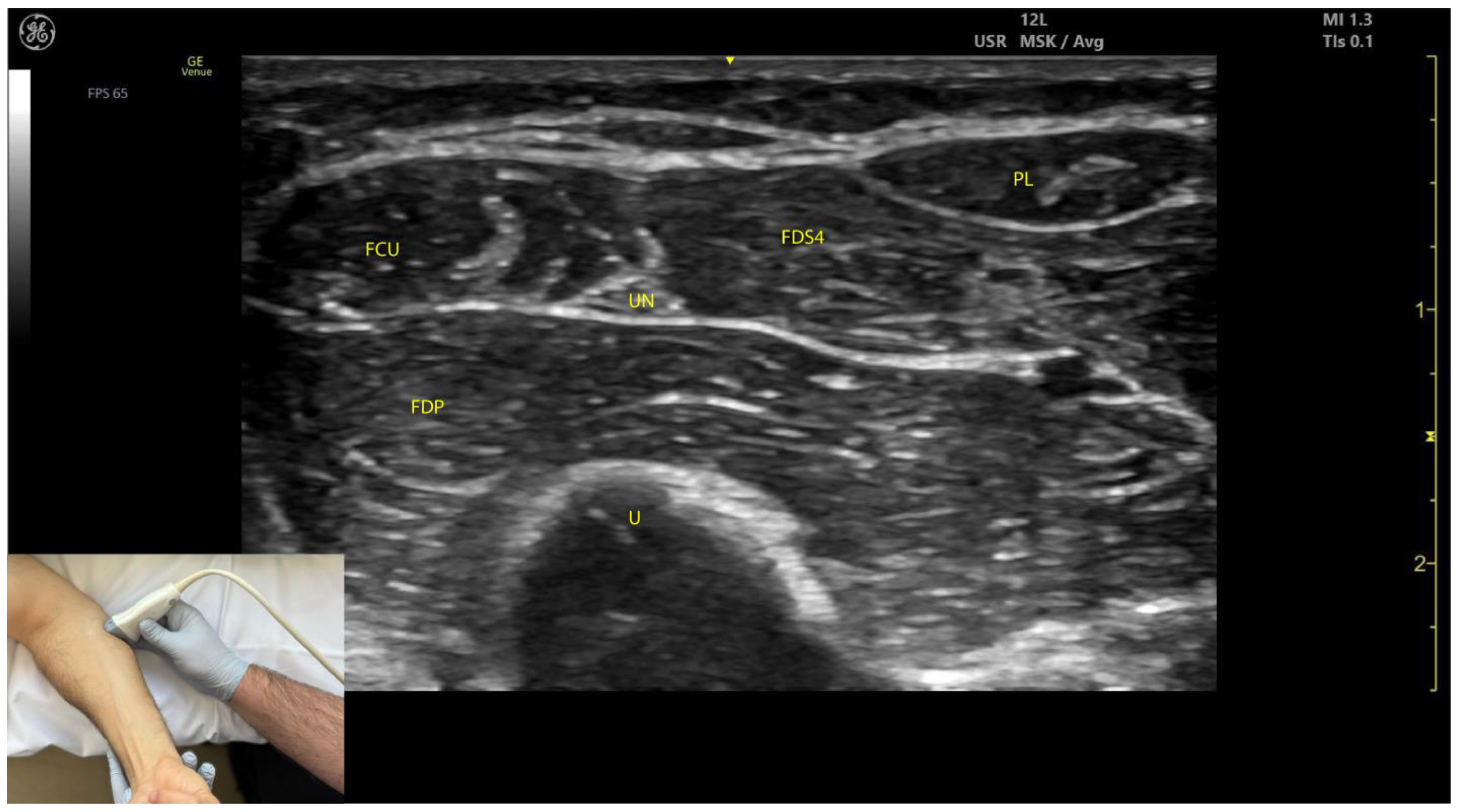
2.4.4. Clinical Implications and Injection Strategy
- The motor point of the FDS is located 30% along the interepicondylar-interstyloidar line in the humeroulnar portion and 50% along the interepicondylar-interstyloidar line in the radial portion [34].
- In our clinical practice, we target the FDS for BoNT-A injections at the point of maximum muscle thickness as determined by ultrasound:
- For FDS2, FDS3, and FDS5, this site is typically located at the volar aspect of the mid-forearm;
- For FDS4, this site is typically located 2–3 cm distal to the elbow crease in the ulnar portion of the forearm.
2.5. Flexor Digitorum Profundus (FDP)
2.5.1. Overview
2.5.2. Ultrasound Identification
2.5.3. Key Ultrasound Landmarks (Figure 4 and Figure 5)
- Muscle position: At the mid-forearm, the FDP is the first muscle mass located directly superficial to the cortical surfaces of the radius and ulna [29].
- Intermuscular fascia: The FDP is separated from the FDS by intermuscular fascia that houses the median nerve, ulnar nerve, and ulnar artery [31].
- Median nerve: The median nerve adheres to the deep surface of the FDS and is positioned superficially relative to the FDP at this level [31].
- Internal fascia: FDP 2–5 is a single continuous muscle with four distinct tendons, corresponding to digits 2–5. Unlike some other muscles, the FDP does not have a pronounced fascia that demarcates separate muscle masses for each individual finger, making it a continuous muscular structure without clear fascial boundaries for each digit during BoNT-A injection.
2.5.4. Clinical Implications and Injection Strategy
- The motor point of the FDP is located 40% along the interepicondylar-interstyloidar line along the muscle in the medial portion and 60% along the interepicondylar-interstyloidar line along the muscle in the lateral portion [34].
- In our practice, BoNT-A is administered into the point of maximum muscle thickness of the FDP as determined by ultrasound. For FDP 2–5, the optimal site is typically located on the volar aspect of the mid-forearm. The in-plane technique is often preferred, as it facilitates a single injection for FDP 2–5 at their points of maximum muscle thickness, reducing the number of injection sites and improving patient adherence. The out-of-plane technique can also be employed for targeted injections into individual muscle bellies when necessary.
2.6. Flexor Pollicis Longus (FPL)
2.6.1. Overview
2.6.2. Ultrasound Identification
2.6.3. Key Ultrasound Landmarks (Figure 6)
- Muscle position: The FPL is the first muscle mass medial to the radial cortex in the distal third of the forearm [38].
- Radial artery: The radial artery lies superficial to the FPL.
- Median nerve: The median nerve is positioned medial to the muscle [38].
- External fascia: The FPL muscle lacks a pronounced fascia to clearly separate it from adjacent muscle masses, such as the FDS, FDP, and FCR, which can make precise localization more challenging during BoNT-A injections.

2.6.4. Clinical Implications and Injection Strategy
- The motor points of the FLP are located at 50% and 65% along the intercondylar-interstyloidar line [34].
- In our clinical practice, BoNT-A is injected at the point of maximum muscle thickness of the FPL, typically located in the radial portion of the distal third of the forearm. The out-of-plane technique is typically employed due to the limited space between anatomical structures. The injection is performed through one of two ultrasound windows: between the radial artery and the radial cortex or between the radial artery and the median nerve. Both approaches require real-time ultrasound guidance to ensure accurate placement of the toxin within the muscle, prevent injury to the median nerve and radial artery, and minimize the risk of local hematoma formation.
2.7. Pronator Quadratus (PQ)
2.7.1. Overview
2.7.2. Ultrasound Identification
2.7.3. Key Ultrasound Landmarks (Figure 7)
- Muscle position: The PQ is the deepest muscle on the anterior forearm [31];
- Quadrilateral shape: It has a distinct, rectangular shape with parallel muscle fibers [41];
- Unique fiber orientation: The PQ is the only forearm muscle with fibers oriented perpendicularly to the limb, unlike all other forearm muscles, which run longitudinally;
- Two heads: The PQ has superficial and deep heads, separated by an intramuscular fascia that connects the convex surfaces of the radius and ulna [41];
- Deep to the PQ: There is the interosseous membrane and the extensor compartment of the distal forearm [42];
- Superficial to the PQ: There is the FDP, FDS, and FPL tendons, the median nerve, and, if present, the palmaris longus tendon [42];
- External fascia: The PQ muscle is characterized by a well-defined fascia that clearly separates it from adjacent muscle masses, facilitating precise localization during BoNT-A injections.
- Dynamic evaluation: When scanning distally toward the radioulnar joint, the PQ muscle enlarges, while the FDP, FPL, and FDS progressively decrease in size and transition into tendons [43].
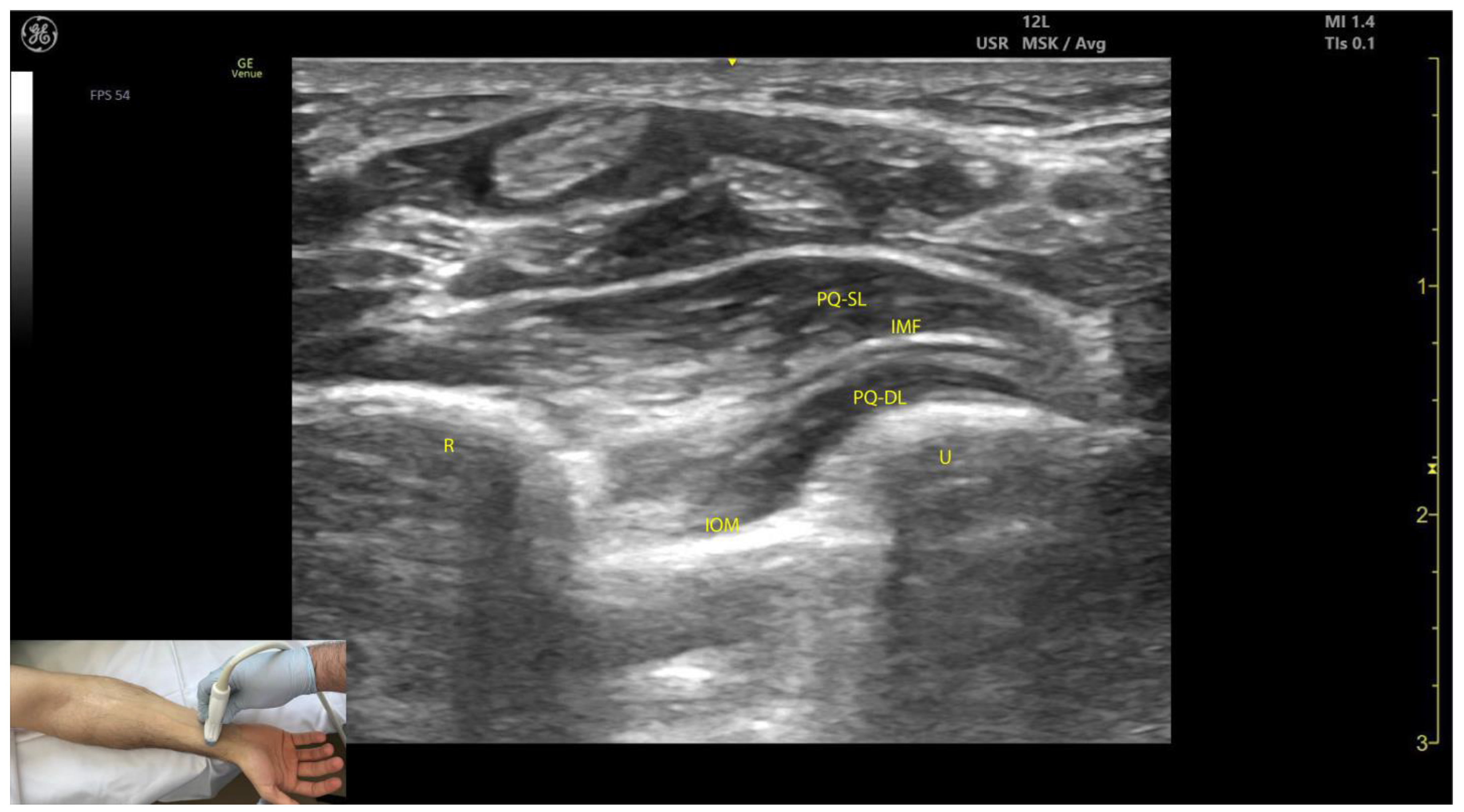
2.7.4. Clinical Implications and Injection Strategy
- According to Choung et al., the optimal injection site for the PQ, containing the highest concentration of motor points, is approximately 3 cm proximal to the ulnar styloid [46].
- In our clinical practice, the BoNT-A injections are performed at the point of maximum muscle thickness, as determined by ultrasound. The PQ can be injected either through the volar aspect of the forearm, avoiding the median nerve, or through the dorsal aspect via the extensor compartment and interosseous membrane. Both approaches require ultrasound guidance to ensure accurate delivery while avoiding neurovascular structures and minimizing the risk of complications.
2.8. Abductor Pollicis Brevis (APB)
2.8.1. Overview
2.8.2. Ultrasound Identification
2.8.3. Key Ultrasound Landmarks (Figure 8)
- Muscle position: The APB is the first muscle mass from lateral to medial within the thenar eminence, also being the most superficial muscle mass of the thenar eminence [49].
- External fascia: The APB muscle lacks a pronounced fascia to clearly separate it from adjacent muscle masses, such as the opponens pollicis and flexor pollicis brevis, which can complicate precise localization during BoNT-A injections.
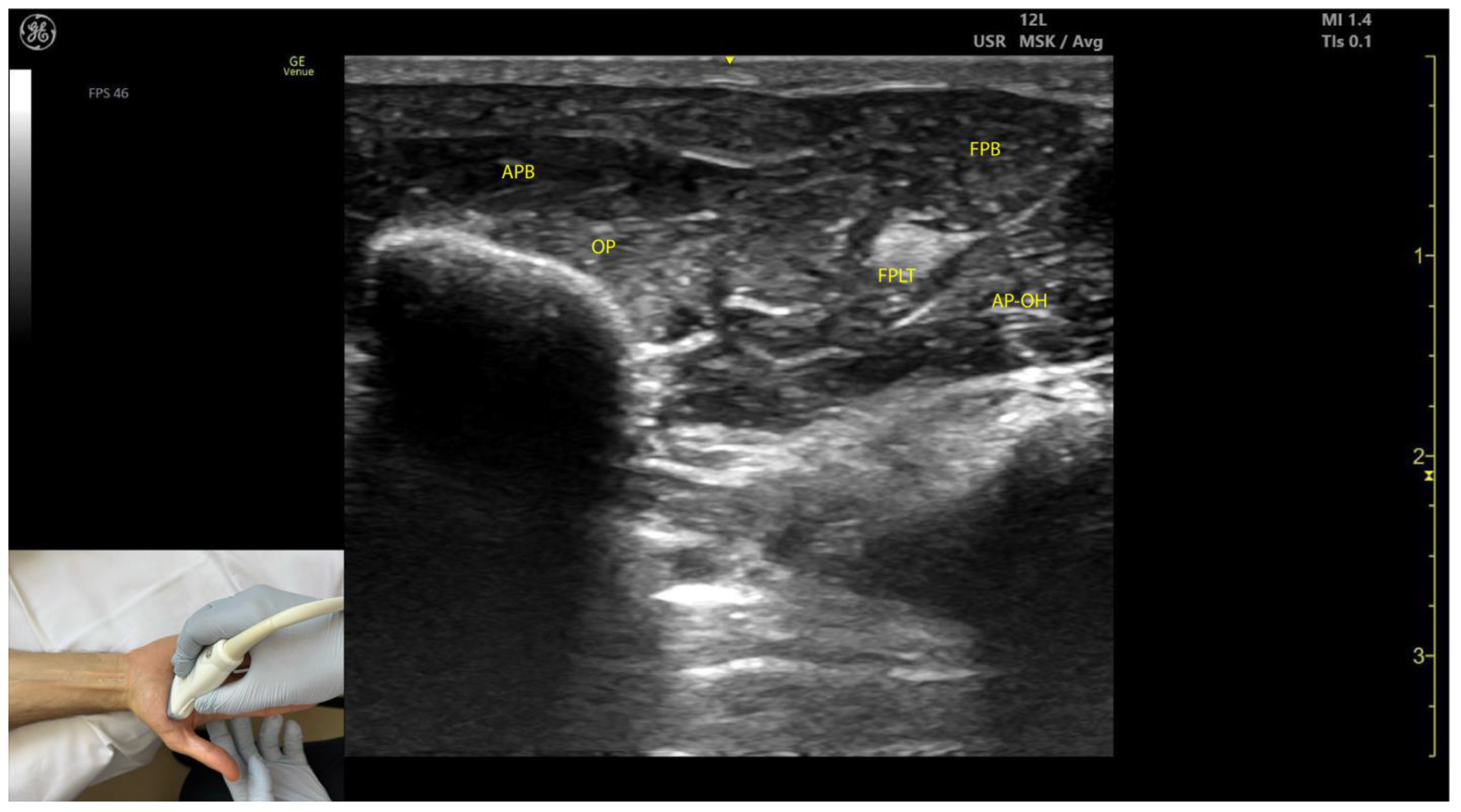
2.8.4. Clinical Implications and Injection Strategy
- The region with the highest density of intramuscular nerve arborizations of the APB is located 40% along the line connecting the hook of hamate to the head of the first metacarpal [51].
- In our clinical practice, the APB is targeted for BoNT-A injections at the point of maximum muscle thickness, determined via ultrasound, typically located at the base of the thenar eminence.
2.9. Opponens Pollicis (OP)
2.9.1. Overview
2.9.2. Ultrasound Identification
2.9.3. Key Ultrasound Landmarks (Figure 8)
- Muscle position: The OP is the second structure from superficial to deep within the thenar eminence [52].
- Muscle size: It is the largest muscle within the thenar eminence [53].
- Lateral position: The OP is located lateral to the flexor pollicis brevis (FPB) [53].
- External fascia: The OP muscle lacks a pronounced fascia to distinctly separate it from adjacent muscle masses, such as the abductor pollicis brevis and flexor pollicis brevis, which may make precise localization during BoNT-A injections more challenging.
- Dynamic evaluation: Scanning distally toward the MCP joint shows an increase in the size of the OP and a corresponding decrease in the size of the APB. At this level, the tendon of the FPL can be seen deep to the OP [54]. Muscle contraction of the OP is observed during adduction and medial rotation of the thumb at the CMC joint, flexion at the MCP joint, or during thumb opposition when the tip of the thumb contacts the fifth finger [55].
2.9.4. Clinical Implications and Injection Strategy
- The region with the highest density of intramuscular nerve arborizations of the opponens is located 60% along the line connecting the hook of hamate to the head of the first metacarpal [51].
- In our clinical practice, BoNT-A injections into the OP are performed at the point of maximum muscle thickness, as determined by ultrasound. The optimal injection site is typically located approximately 1 cm proximal to the MCP joint on the palmar aspect of the hand. Ultrasound guidance ensures accurate delivery while avoiding adjacent structures, such as the flexor pollicis longus tendon.
2.10. Flexor Pollicis Brevis (FPB)
2.10.1. Overview
2.10.2. Ultrasound Identification
2.10.3. Key Ultrasound Landmarks (Figure 9)
- Muscle position: The FPB is the most medial muscle mass within the thenar eminence. It is situated medial to the APB and opponens pollicis and lateral to the adductor pollicis [56].
- Internal fascia: The FPB contains intramuscular fascia that separates its two heads—the superficial and deep heads—which can be targeted individually during injections [53];
- FPL tendon: Scanning toward the MCP joint reveals the FPL tendon located lateral to the superficial head of the FPB and deep to its deep head.
- External fascia: The FPB muscle lacks a pronounced fascia to clearly separate it from adjacent muscle masses, such as the abductor pollicis brevis and opponens pollicis, which may complicate precise localization during BoNT-A injections.
- Dynamic evaluation: Contraction of the FPB is observed during thumb flexion at the CMC and MCP joints [48].
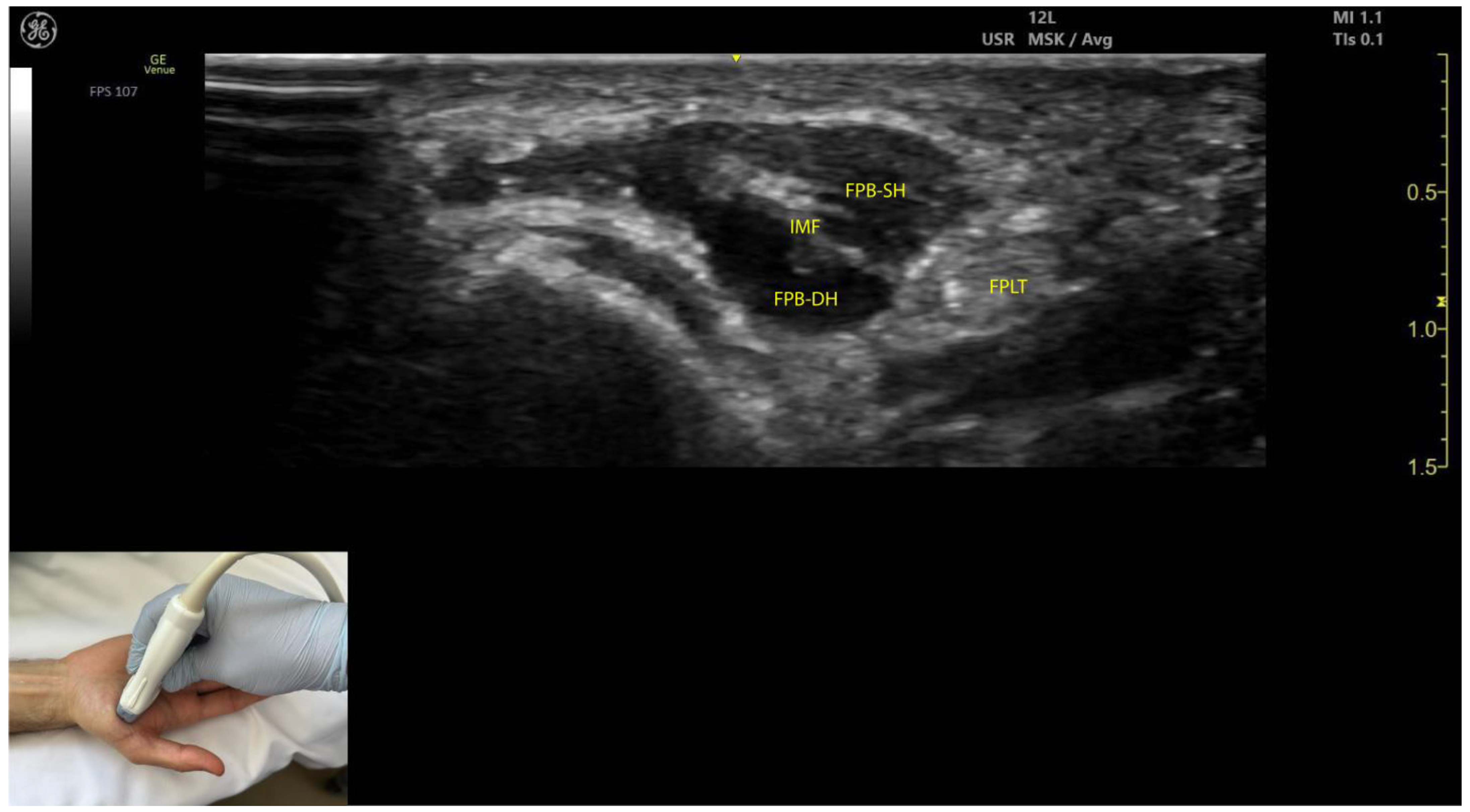
2.10.4. Clinical Implications and Injection Strategy
- The region with the highest density of intramuscular nerve arborizations of the FPB is located 50–70% along the line connecting the hook of hamate to the head of the first metacarpal [51].
- In our clinical practice, the FPB is targeted for BoNT-A injections at the point of maximum muscle thickness, as determined by ultrasound. The optimal injection site is typically located at the midpoint of the thenar eminence. Ultrasound guidance ensures precise toxin delivery and allows for individual targeting of the superficial and deep heads, minimizing the risk of complications.
2.11. Adductor Pollicis (AP)
2.11.1. Overview
2.11.2. Ultrasound Identification
2.11.3. Key Ultrasound Landmarks (Figure 10 and Figure 11)
- Muscle size: The AP is larger than the muscles of the thenar eminence [58].
- Unique anatomical compartment: The AP is the only muscle in the adductor compartment of the hand [59].
- Two heads: The AP consists of a transverse head and an oblique head: (i) The transverse head is identified with the transducer placed transversely on the palmar aspect at the midpoint of the second and third metacarpals. Scanning proximally (approximately 1 cm) reveals the muscle’s maximal thickness; (ii) the oblique head is visualized by angling the transducer approximately 45 degrees laterally toward the thumb. This head is located lateral to the FPL tendon and the FPB, with the first dorsal interosseous muscle in its depth [59,60];
- External fascia: The AP muscle is characterized by a pronounced fascia that clearly separates it from adjacent muscle masses, facilitating precise localization during BoNT-A injections.
- Dynamic evaluation: Contraction of the AP is observed during thumb adduction at the CMC and MCP joints or during pinch movements, such as bringing the thumb tip into contact with the index finger tip.
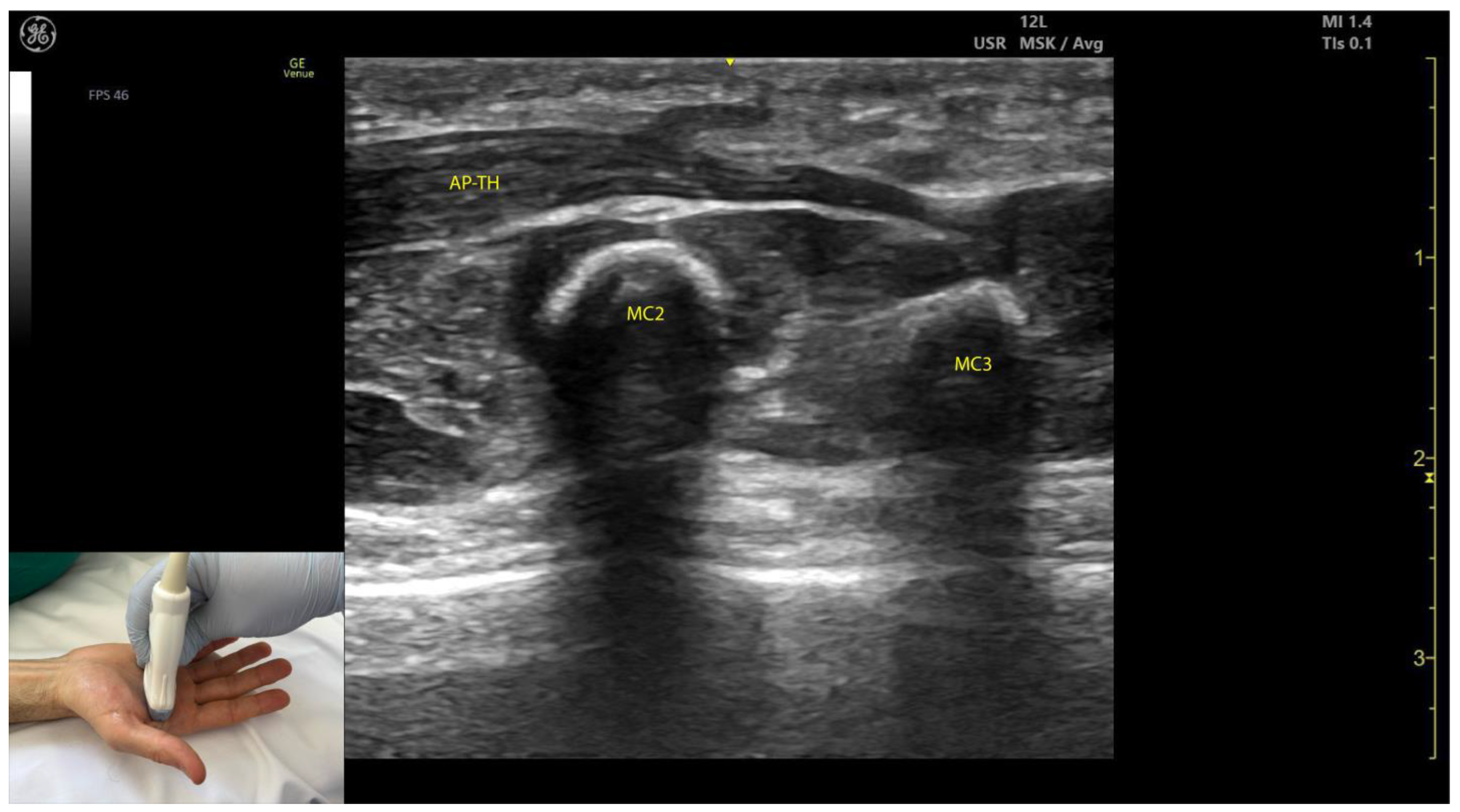
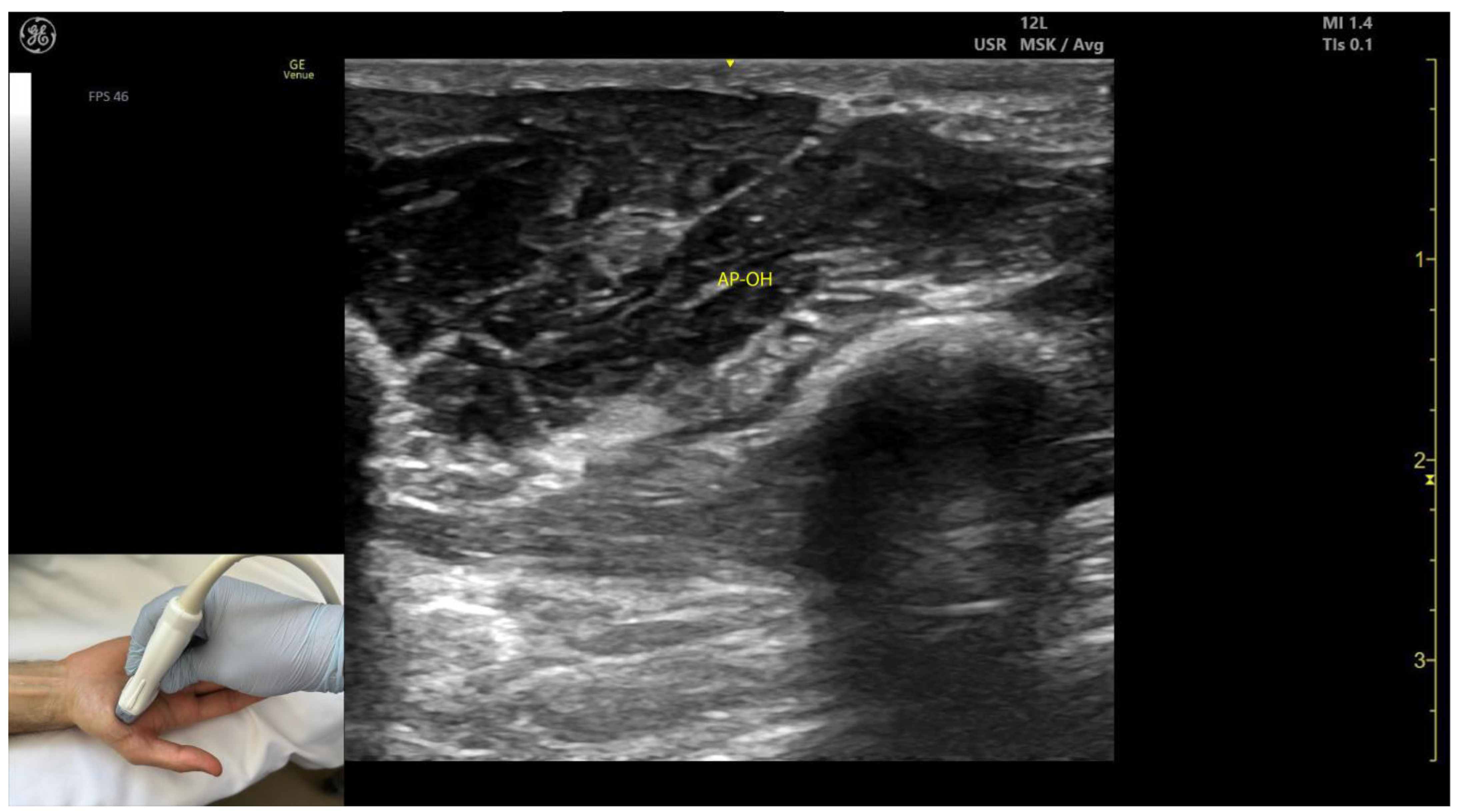
2.11.4. Clinical Implications and Injection Strategy
- The AP is commonly injected with BoNT-A due to its critical role in thumb adduction, a movement it performs alongside the FPB and OP [61]. According to a 2023 study by Yi et al., the highest concentration of motor endplates is located on 1/5–3/5 of the muscle from the midline of the third metacarpal bone to the base of the first proximal phalanx [61].
- In our clinical practice, BoNT-A is administered into the point of maximum muscle thickness, as determined by ultrasound. The injection is typically performed approximately 1 cm proximal to the midpoint of the second and third metacarpals, with the transducer angled 45 degrees toward the thumb to target both the transverse and oblique heads.
2.12. Lumbricals (L)
2.12.1. Overview
2.12.2. Ultrasound Identification
2.12.3. Key Ultrasound Landmarks [63,64,65,66] (Figure 12)
- Muscle morphology: Lumbricals L1 and L2 are unipennate, while L3 and L4 are bipennate. Intramuscular fascia corresponding to L1 and L2 can be visualized.
- Innervation and vascular supply: Superficial to L1 and L2 are branches of the median nerve, while superficial to L3 and L4 are branches of the ulnar nerve, each accompanied by arterial branches from the dorsal carpal arch.
- External fascia: The lumbrical muscles lack a pronounced fascia to distinctly separate them from the FDS tendons (adjacent structures), which may pose challenges for precise localization during BoNT-A injections.
- Dynamic evaluation: Scanning distally toward the MCP joints reveals an increase in the size of L1–L4 and a corresponding decrease in the size of the AP. Placing the transducer at the midpoint of the metacarpals II–V and performing flexion and extension maneuvers of the fingers highlights the contraction of L1–L4 and the associated action on the tendons of FDS and FDP. The lumbricals appear as hypoechoic structures with hyperechoic speckles, described as a “starry sky” pattern, while the FDS and FDP tendons are hyperechoic with parallel fibrillar lines.
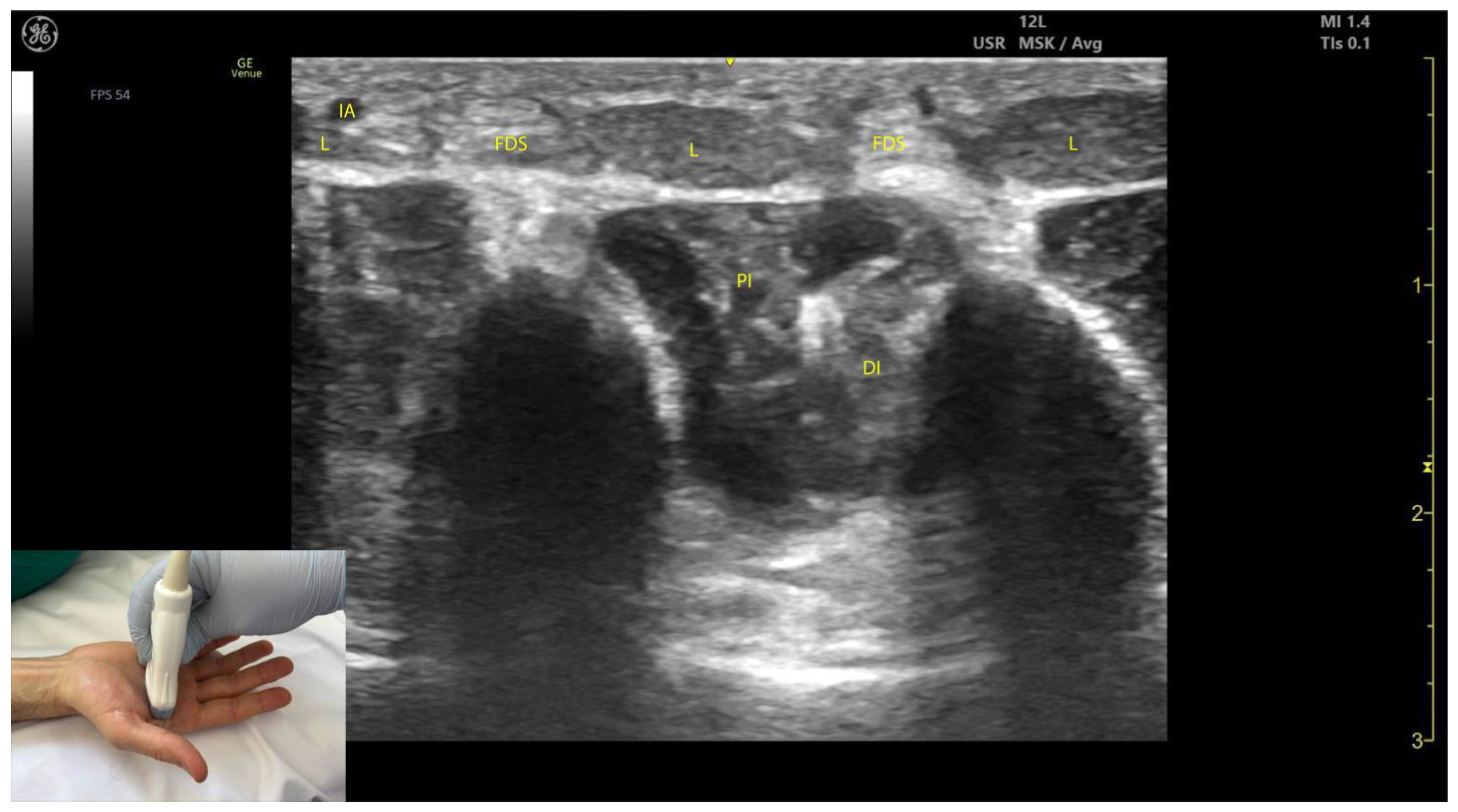
2.12.4. Clinical Implications and Injection Strategy
2.13. Interossei Muscles
2.13.1. Overview
2.13.2. Ultrasound Identification
2.13.3. Key Ultrasound Landmarks [60,67,70] (Figure 13)
- Muscle position: The dorsal interossei are the most superficial muscle structures on the dorsal aspect of the hand.
- Muscle morphology: The dorsal interossei are bipennate muscles, whereas the palmar interossei are unipennate. Musculoskeletal ultrasound also allows visualization of the intramuscular fascia within these structures.
- External fascia: The interossei muscles lack a pronounced fascia to clearly demarcate the dorsal and palmar interossei (adjacent muscle masses), which can make precise localization during BoNT-A injections more challenging.
- Muscle size: The first dorsal interosseous muscle is larger compared to the other dorsal interossei. Deep to it lies the AP, which decreases in size as the first dorsal interosseous muscle increases during dynamic scanning cranially.
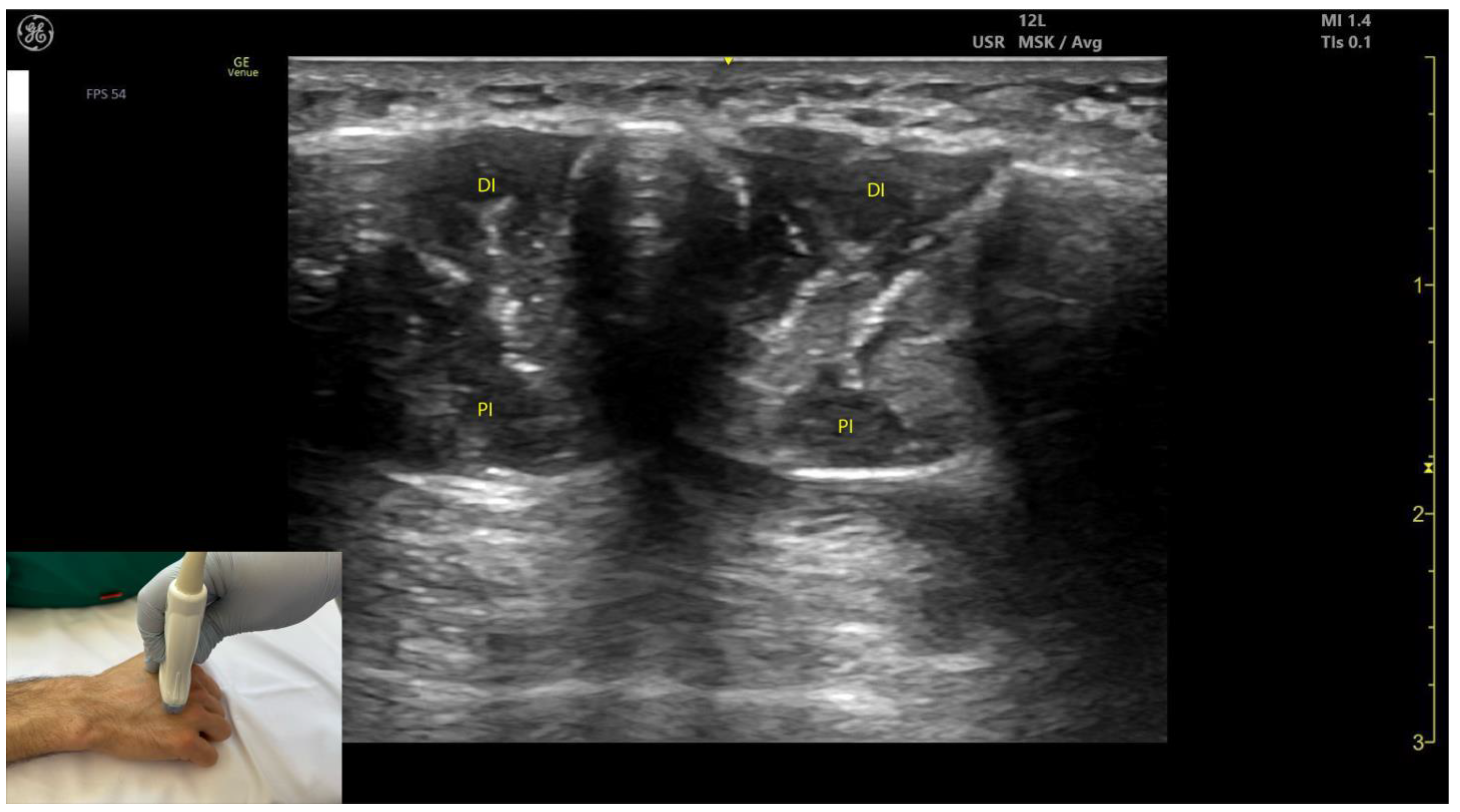
2.13.4. Clinical Implications and Injection Strategy
3. Ultrasound Characteristics of Spastic Muscles and Their Clinical Relevance
- Atrophy: On ultrasound, spastic muscles often appear smaller, with a reduced cross-sectional area compared to normal muscles. The reduction in muscle volume correlates with decreased contractile strength and functionality, necessitating precise targeting during BoNT-A injections to maximize therapeutic outcomes [69];
- Increased echogenicity: Spastic muscles commonly exhibit increased echogenicity due to intramuscular connective tissue accumulation (fibrosis) and fatty infiltration. This “whiter” appearance on ultrasound indicates pathological changes in the muscle tissue, which can complicate the identification of motor points or regions of maximum muscle activity [74];
- Loss of homogeneous texture: Normal muscles display a striated echotexture due to their uniform fibrous and contractile elements. In contrast, spastic muscles appear heterogeneous, with disrupted striations caused by fibrosis and fatty infiltration. This heterogeneity can make it challenging to differentiate between functional muscle tissue and pathological regions [75];
- Altered dynamic response: Dynamic ultrasound evaluation of spastic muscles often reveals reduced or paradoxical contraction patterns during voluntary or passive movements. This is due to sarcomere loss and shortening, which impair the muscle’s mechanical efficiency and responsiveness to stretch [76,77];
- Increased stiffness: On elastography, spastic muscles show increased stiffness compared to normal muscles. This stiffness reflects both increased connective tissue deposition and muscle hypertonicity, influencing the choice of injection technique and dose distribution [78].
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BoNT-A | Botulinum Toxin-A |
| EMG | Electromyography |
| EUH | Elias University Hospital |
| PT | Pronator Teres |
| PTT | Pronator Teres (tendon) |
| FCR | Flexor Carpi Radialis |
| PTHH | Pronator Teres Humeral Head |
| PTUH | Pronator Teres Ulnar Head |
| FCU | Flexor Carpi Ulnaris |
| CF | Cloud Fascia |
| FDS | Flexor Digitorum Superficialis |
| FDP | Flexor Digitorum Profundus |
| FPL | Flexor Pollicis Longus |
| PL | Palmaris Longus |
| PQ | Pronator Quadratus |
| PQ-SL | Pronator Quadratus Superficial Layer |
| PQ-DL | Pronator Quadratus Deep Layer |
| APB | Abductor Pollicis Brevis |
| OP | Opponens Pollicis |
| FPB | Flexor Pollicis Brevis |
| FPB-SH | Flexor Pollicis Brevis Superficial Head |
| FPB-DH | Flexor Pollicis Brevis Deep Head |
| AP | Adductor Pollicis |
| AP-TH | Adductor Pollicis Transverse Head |
| AP-OH | Adductor Pollicis Oblique Head |
| MCP | Metacarpophalangeal (joint) |
| IP | Interphalangeal (joint) |
| DIP | Distal Interphalangeal (joint) |
| CMC | Carpometacarpal (joint) |
| IMF | Intramuscular Fascia |
| IF | Intermuscular Fascia |
| RA | Radial Artery |
| IA | Interdigital Artery |
| MN | Median Nerve |
| UN | Ulnar Nerve |
| IOM | Interosseous Membrane |
| PI | Palmar Interossei |
| DI | Dorsal Interossei |
| FPLT | Flexor Pollicis Longus (tendon) |
| R | Radius (bone) |
| U | Ulna (bone) |
| MC | Metacarpal (bone) |
| L | Lumbrical (muscle) |
References
- Suputtitada, A.; Chatromyen, S.; Chen, C.P.C.; Simpson, D.M. Best Practice Guidelines for the Management of Patients with Post-Stroke Spasticity: A Modified Scoping Review. Toxins 2024, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.N.; Petca, R.C.; Beiu, C.; Dumitrascu, M.C.; Petca, A.; Mehedintu, C.; Farcasanu, P.D.; Sandru, F. Efficiency of Different Preparations of Botulinum Toxin Type A, Xeomin and Dysport, in the Management of Spastic Upper Limb After Stroke. Rev. Chim. 2019, 70, 3490–3494. [Google Scholar] [CrossRef]
- Santamato, A.; Micello, M.F.; Panza, F.; Fortunato, F.; Baricich, A.; Cisari, C.; Pilotto, A.; Logroscino, G.; Fiore, P.; Ranieri, M. Can Botulinum Toxin Type A Injection Technique Influence the Clinical Outcome of Patients with Post-Stroke Upper Limb Spasticity? A Randomized Controlled Trial Comparing Manual Needle Placement and Ultrasound-Guided Injection Techniques. J. Neurol. Sci. 2014, 347, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Buyukavci, R.; Akturk, S.; Ersoy, Y. Evaluating the Functional Outcomes of Ultrasound-Guided Botulinum Toxin Type A Injections Using the Euro-Musculus Approach for Upper Limb Spasticity Treatment in Post-Stroke Patients: An Observational Study. Eur. J. Phys. Rehabil. Med. 2018, 54, 738–744. [Google Scholar] [CrossRef]
- Francisco, G.E.; Balbert, A.; Bavikatte, G.; Bensmail, D.; Carda, S.; Deltombe, T.; Draulans, N.; Escaldi, S.; Gross, R.; Jacinto, J.; et al. A Practical Guide to Optimizing the Benefits of Post-Stroke Spasticity Interventions With Botulinum Toxin A: An International Group Consensus. J. Rehabil. Med. 2020, 53, 2715. [Google Scholar] [CrossRef]
- Créteur, V.; Madani, A.; Sattari, A.; Bianchi, S. Sonography of the Pronator Teres: Normal and Pathologic Appearances. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2017, 36, 2585–2597. [Google Scholar] [CrossRef]
- Lagnau, P.; Lo, A.; Sandarage, R.; Alter, K.; Picelli, A.; Wissel, J.; Verduzco-Gutierrez, M.; Suputtitada, A.; Munin, M.C.; Carda, S.; et al. Ergonomic Recommendations in Ultrasound-Guided Botulinum Neurotoxin Chemodenervation for Spasticity: An International Expert Group Opinion. Toxins 2021, 13, 249. [Google Scholar] [CrossRef]
- Roberts, C.; Crystal, R.; Eastwood, D.M. Optimal Injection Points for the Neuromuscular Blockade of Forearm Flexor Muscles: A Cadaveric Study. J. Pediatr. Orthop. Part B 2006, 15, 351–355. [Google Scholar] [CrossRef]
- Moore, K.L.; Agur, A.M.R.; Dalley, A.F. Clinically Oriented Anatomy, 7th ed.; Lippincott Williams: Philadelphia, PA, USA, 2013; ISBN 978-81-8473-912-1. [Google Scholar]
- Shojaie, P.; Botchu, R.; Iyengar, K.; Drakonaki, E.; Sharma, G.K. Ultrasound-Guided Median Nerve Hydrodissection of Pronator Teres Syndrome: A Case Report and a Literature Review. J. Ultrason. 2023, 23, e165–e169. [Google Scholar] [CrossRef]
- Chang, K.-V.; Wu, W.-T.; Hsu, P.-C.; Yang, Y.-C.; Özçakar, L. Ultrasonography in Pronator Teres Syndrome: Dynamic Examination and Guided Hydrodissection. Pain Med. Malden Mass. 2022, 23, 219–220. [Google Scholar] [CrossRef]
- Gharbaoui, I.; Kania, K.; Cole, P. Spastic Paralysis of the Elbow and Forearm. Semin. Plast. Surg. 2016, 30, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Ghroubi, S.; Alila, S.; Elleuch, W.; Ayed, H.B.; Mhiri, C.; Elleuch, M.H. Efficacy of Botulinum Toxin A for the Treatment of Hemiparesis in Adults with Chronic Upper Limb Spasticity. Pan Afr. Med. J. 2020, 35, 55. [Google Scholar] [CrossRef]
- Luxenburg, D.; Rizzo, M.G. Anatomy, Shoulder and Upper Limb, Pronator Teres. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Şengül, İ.; Aşkin, A.; Bayram, K.; Tosun, A. Assessment of Post-Stroke Elbow Flexor Spasticity in Different Forearm Positions. Somatosens. Mot. Res. 2018, 35, 218–222. [Google Scholar] [CrossRef] [PubMed]
- MD, E.E. Spasticity: Diagnosis and Management, 1st ed.; MD, A.B., Ed.; Demos Medical: New York, NY, USA, 2010; ISBN 978-1-933864-51-8. [Google Scholar]
- Won, S.-Y.; Hur, M.-S.; Rha, D.-W.; Park, H.-D.; Hu, K.-S.; Fontaine, C.; Kim, H.-J. Extra- and Intramuscular Nerve Distribution Patterns of the Muscles of the Ventral Compartment of the Forearm. Am. J. Phys. Med. Rehabil. 2010, 89, 644–652. [Google Scholar] [CrossRef]
- Nigel Palastanga, D.F. Anatomy and Human Movement; Butterworth-Heinemann: Oxford, UK, 2013; ISBN 978-1-4831-9274-1. [Google Scholar]
- Olchowy, C.; Soliński, D.; Łasecki, M.; Dąbrowski, P.; Urban, S.; Zaleska-Dorobisz, U. Wrist Ultrasound Examination—Scanning Technique and Ultrasound Anatomy. Part 2: Ventral Wrist. J. Ultrason. 2017, 17, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Atlas of Human Anatomy; 2014; ISBN 978-1-4557-0418-7. Available online: https://books.google.com.hk/books/about/Atlas_of_Human_Anatomy_Sixth_Edition.html?id=9-2ZBQAAQBAJ&redir_esc=y (accessed on 29 January 2025).
- Deshpande, S.; Gormley, M.E.; Carey, J.R. Muscle Fiber Orientation in Muscles Commonly Injected with Botulinum Toxin: An Anatomical Pilot Study. Neurotox. Res. 2006, 9, 115–120. [Google Scholar] [CrossRef]
- Song, D.H.; Chung, M.E.; Han, Z.-A.; Kim, S.Y.; Park, H.K.; Seo, Y.J. Anatomic Localization of Motor Points of Wrist Flexors. Am. J. Phys. Med. Rehabil. 2014, 93, 282–286. [Google Scholar] [CrossRef]
- Lung, B.E.; Siwiec, R.M. Anatomy, Shoulder and Upper Limb, Forearm Flexor Carpi Ulnaris Muscle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Sharpe, F.; Barry, P.; Lin, S.D.; Stevanovic, M. Anatomic Study of the Flexor Carpi Ulnaris Muscle and Its Application to Soft Tissue Coverage of the Elbow with Clinical Correlation. J. Shoulder Elbow Surg. 2014, 23, 82–90. [Google Scholar] [CrossRef]
- Becciolini, M.; Pivec, C.; Riegler, G. Ultrasonography of the Ulnar Nerve Loop in Relation to the Flexor Carpi Ulnaris Tendon. J. Ultrason. 2024, 24, 1–5. [Google Scholar] [CrossRef]
- Bargalló, X.; Carrera, A.; Sala-Blanch, X.; Santamaría, G.; Morro, R.; Llusá, M.; Gilabert, R. Ultrasound-Anatomic Correlation of the Peripheral Nerves of the Upper Limb. Surg. Radiol. Anat. SRA 2010, 32, 305–314. [Google Scholar] [CrossRef]
- Okafor, L.; Varacallo, M. Anatomy, Shoulder and Upper Limb, Hand Flexor Digitorum Superficialis Muscle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Drake, R.L.; Vogl, A.W.; Mitchell, A.W.M. Gray’s Anatomy for Students E-Book, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 978-0-323-93504-3. [Google Scholar]
- Bianchi, S.; Martinoli, C.; de Gautard, R.; Gaignot, C. Ultrasound of the Digital Flexor System: Normal and Pathological Findings. J. Ultrasound 2007, 10, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Walter, U.; Dressler, D. Ultrasound-Guided Botulinum Toxin Injections in Neurology: Technique, Indications and Future Perspectives. Expert Rev. Neurother. 2014, 14, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Henzel, M.K.; Munin, M.C.; Niyonkuru, C.; Skidmore, E.R.; Weber, D.J.; Zafonte, R.D. Comparison of Surface and Ultrasound Localization to Identify Forearm Flexor Muscles for Botulinum Toxin Injections. PM R 2010, 2, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Yong, M.W.; Yusof, N.; Rampal, L.; Arumugam, M. Prevalence of Absence of Palmaris Longus and Its Association with Gender, Hand Dominance and Absence of FDS Tendon to Little Finger Among Malay Population. J. Hand Surg. Asian-Pac. Vol. 2017, 22, 484–489. [Google Scholar] [CrossRef]
- Cetin, A.; Genc, M.; Sevil, S.; Coban, Y.K. Prevalence of the Palmaris Longus Muscle and Its Relationship with Grip and Pinch Strength: A Study in a Turkish Pediatric Population. Hand 2013, 8, 215–220. [Google Scholar] [CrossRef]
- Kara, M.; Kaymak, B.; Ulaşli, A.M.; Tok, F.; Öztürk, G.T.; Chang, K.-V.; Hsiao, M.-Y.; Hung, C.-Y.; Yağiz On, A.; Özçakar, L. Sonographic Guide for Botulinum Toxin Injections of the Upper Limb: EUROMUSCULUS/USPRM Spasticity Approach. Eur. J. Phys. Rehabil. Med. 2018, 54, 469–485. [Google Scholar] [CrossRef]
- Meiser, V.C.; Kreysa, H.; Guntinas-Lichius, O.; Volk, G.F. Comparison of In-Plane and out-of-Plane Needle Insertion with vs. without Needle Guidance. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 2697–2705. [Google Scholar] [CrossRef]
- Lung, B.E.; Burns, B. Anatomy, Shoulder and Upper Limb, Hand Flexor Digitorum Profundus Muscle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Park, H.J.; Woo, S.R.; Park, S.J.; Yoon, J.S. Ultrasonographic Examination of the Safe Zone for Medial Needle Approach towards the Median-Innervated Flexor Digitorum Profundus Muscle: Effect of Changes in Position of the Forearm. Medicine 2023, 102, e34720. [Google Scholar] [CrossRef]
- Benson, D.C.; Miao, K.H.; Varacallo, M. Anatomy, Shoulder and Upper Limb, Hand Flexor Pollicis Longus Muscle. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Schlickum, L.; Quadlbauer, S.; Pezzei, C.; Stöphasius, E.; Hausner, T.; Leixnering, M. Three-Dimensional Kinematics of the Flexor Pollicis Longus Tendon in Relation to the Position of the FPL Plate and Distal Radius Width. Arch. Orthop. Trauma Surg. 2019, 139, 269–279. [Google Scholar] [CrossRef]
- Gupta, A.D.; Addison, S. Healing Hand Ulcers Caused by Focal Spasticity. Int. Wound J. 2020, 17, 774–780. [Google Scholar] [CrossRef]
- Sato, J.; Ishii, Y.; Noguchi, H.; Takeda, M.; Toyabe, S. Sonographic Appearance of the Pronator Quadratus Muscle in Healthy Volunteers. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2014, 33, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Nasu, H.; Nimura, A.; Hamada, J.; Akita, K. An Anatomic Study of the Structure and Innervation of the Pronator Quadratus Muscle. Anat. Sci. Int. 2015, 90, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.-J.; Kim, J.-P.; Kim, J.-S.; Park, H.-J. Sonographic Quantification of Pronator Quadratus Activity During Gripping Effort. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2015, 34, 2269–2278. [Google Scholar] [CrossRef]
- McConkey, M.O.; Schwab, T.D.; Travlos, A.; Oxland, T.R.; Goetz, T. Quantification of Pronator Quadratus Contribution to Isometric Pronation Torque of the Forearm. J. Hand Surg. 2009, 34, 1612–1617. [Google Scholar] [CrossRef]
- Santamato, A. Safety and Efficacy of incobotulinumtoxinA as a Potential Treatment for Poststroke Spasticity. Neuropsychiatr. Dis. Treat. 2016, 12, 251–263. [Google Scholar] [CrossRef]
- Choung, P.W.; Kim, M.Y.; Im, H.S.; Kim, K.H.; Rhyu, I.J.; Park, B.K.; Kim, D.H. Anatomic Characteristics of Pronator Quadratus Muscle: A Cadaver Study. Ann. Rehabil. Med. 2016, 40, 496–501. [Google Scholar] [CrossRef]
- Draganich, L.F.; Greenspahn, S.; Mass, D.P. Effects of the Adductor Pollicis and Abductor Pollicis Brevis on Thumb Metacarpophalangeal Joint Laxity before and after Ulnar Collateral Ligament Reconstruction. J. Hand Surg. 2004, 29, 481–488. [Google Scholar] [CrossRef]
- Picasso, R.; Zaottini, F.; Pistoia, F.; Perez, M.M.; Macciò, M.; Bianco, D.; Rinaldi, S.; Pansecchi, M.; Rossi, G.; Tovt, L.; et al. Ultrasound of the Palmar Aspect of the Hand: Normal Anatomy and Clinical Applications of Intrinsic Muscles Imaging. J. Ultrason. 2023, 23, e122–e130. [Google Scholar] [CrossRef]
- Li, R.; Zheng, S.; Zhang, Y.; Zhang, H.; Du, L.; Cheng, L.; Li, H.; Zhang, W.; Du, K.; He, W.; et al. Quantitative Assessment of Thenar to Evaluate Hand Function after Stroke by Bayes Discriminant. BMC Musculoskelet. Disord. 2023, 24, 682. [Google Scholar] [CrossRef]
- Ohno, K.; Fujino, K.; Fujiwara, K.; Yokota, A.; Neo, M. Sonographic Evaluation of the Abductor Pollicis Brevis Muscle Reflects Muscle Strength Recovery after Carpal Tunnel Release. J. Med. Ultrason. 2001 2022, 49, 279–287. [Google Scholar] [CrossRef]
- Im, S.; Han, S.H.; Choi, J.H.; Lee, J.H.; Ko, Y.J.; Lee, J.I.; Kim, H.W. Anatomic Localization of Motor Points for the Neuromuscular Blockade of Hand Intrinsic Muscles Involved in Thumb-in-Palm. Am. J. Phys. Med. Rehabil. 2008, 87, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.D.; Duong, H. Anatomy, Shoulder and Upper Limb, Hand Opponens Pollicis Muscle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Luxenburg, D.; Rizzo, M.G. Anatomy, Shoulder and Upper Limb, Hand Thenar Eminence. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lee, S.A.; Kim, B.H.; Kim, S.-J.; Kim, J.N.; Park, S.-Y.; Choi, K. Current Status of Ultrasonography of the Finger. Ultrasonography 2016, 35, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Kumar, S.; Kathiria, A.V.; Harjai, R.; Jawed, A.; Gupta, V. Thumb Ultrasound: Technique and Pathologies. Indian J. Radiol. Imaging 2016, 26, 386–396. [Google Scholar] [CrossRef]
- Caetano, E.B.; Nakamichi, Y.d.C.; Alves de Andrade, R.; Sawada, M.M.; Nakasone, M.T.; Vieira, L.A.; Sabongi, R.G. Flexor Pollicis Brevis Muscle. Anatomical Study and Clinical Implications. Open Orthop. J. 2017, 11, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Rha, D.-W.; Kim, S.A.; Park, E.S. The Dynamic Thumb-in-Palm Pattern in Children with Spastic Cerebral Palsy and Its Effects on Upper Limb Function. Children 2020, 8, 17. [Google Scholar] [CrossRef]
- Acosta, J.R.; Graefe, S.B.; Varacallo, M. Anatomy, Shoulder and Upper Limb, Hand Adductor Pollicis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Russo, A.; Reginelli, A.; Lacasella, G.V.; Grassi, E.; Karaboue, M.A.A.; Quarto, T.; Busetto, G.M.; Aliprandi, A.; Grassi, R.; Berritto, D. Clinical Application of Ultra-High-Frequency Ultrasound. J. Pers. Med. 2022, 12, 1733. [Google Scholar] [CrossRef]
- Wu, W.-T.; Chang, K.-V.; Hsu, Y.-C.; Tsai, Y.-Y.; Mezian, K.; Ricci, V.; Özçakar, L. Ultrasound Imaging and Guidance for Distal Peripheral Nerve Pathologies at the Wrist/Hand. Diagnostics 2023, 13, 1928. [Google Scholar] [CrossRef]
- Yi, K.-H.; Lee, K.-W.; Hwang, Y.; An, M.H.; Ahn, H.-S.; Hu, H.; Lee, J.-H.; Lee, H.-J. Intramuscular Neural Distribution of Adductor Pollicis Muscle Spasticity in Cadaver Model Regarding Botulinum Neurotoxin Treatment. Yonsei Med. J. 2023, 64, 581–585. [Google Scholar] [CrossRef]
- Palti, R.; Vigler, M. Anatomy and Function of Lumbrical Muscles. Hand Clin. 2012, 28, 13–17. [Google Scholar] [CrossRef]
- Wang, K.; McGlinn, E.P.; Chung, K.C. A Biomechanical and Evolutionary Perspective on the Function of the Lumbrical Muscle. J. Hand Surg. 2014, 39, 149–155. [Google Scholar] [CrossRef]
- Nadar, M.S.; Amr, H.A.; Manee, F.S.; Ali, A.A. In Vivo Evidence of Lumbricals Incursion into the Carpal Tunnel in Healthy Hands: An Ultrasonographic Cross Sectional Study. J. Hand Ther. Off. J. Am. Soc. Hand Ther. 2022, 35, 261–266. [Google Scholar] [CrossRef] [PubMed]
- De Maeseneer, M.; Meng, J.; Marcelis, S.; Jager, T.; Provyn, S.; Shahabpour, M. Ultrasound Anatomy of the Fingers: Flexor and Extensor System with Emphasis on Variations and Anatomical Detail. J. Ultrason. 2020, 20, e122–e128. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.R.; Piagkou, M.; Monticelli, A.; Tiengo, C.; Bassetto, F.; Sonda, R.; Battiston, B.; Titolo, P.; Tos, P.; Fazio, A.; et al. Lumbrical Muscles Neural Branching Patterns: A Cadaveric Study With Potential Clinical Implications. Hand 2022, 17, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Toliopoulos, A. In-Plane Ultrasound-Guided Botulinum Toxin Injection to Lumbrical and Interosseus Upper Limb Muscles: Technical Report. Cureus 2023, 15, e45073. [Google Scholar] [CrossRef]
- Valenzuela, M.; Bordoni, B. Anatomy, Shoulder and Upper Limb, Hand Palmar Interosseous Muscle. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Williams, S.A.; Reid, S.; Elliott, C.; Shipman, P.; Valentine, J. Muscle Volume Alterations in Spastic Muscles Immediately Following Botulinum Toxin Type-A Treatment in Children with Cerebral Palsy. Dev. Med. Child Neurol. 2013, 55, 813–820. [Google Scholar] [CrossRef]
- Infantolino, B.W.; Challis, J.H. Estimating the Volume of the First Dorsal Interossoeus Using Ultrasound. Med. Eng. Phys. 2011, 33, 391–394. [Google Scholar] [CrossRef]
- Gatin, L.; Schnitzler, A.; Calé, F.; Genêt, G.; Denormandie, P.; Genêt, F. Soft Tissue Surgery for Adults With Nonfunctional, Spastic Hands Following Central Nervous System Lesions: A Retrospective Study. J. Hand Surg. 2017, 42, 1035.e1–1035.e7. [Google Scholar] [CrossRef]
- Valenzuela, M.; Bordoni, B. Anatomy, Shoulder and Upper Limb, Hand Dorsal Interossei Muscle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Picelli, A.; Roncari, L.; Baldessarelli, S.; Berto, G.; Lobba, D.; Santamato, A.; Fiore, P.; Smania, N. Accuracy of Botulinum Toxin Type A Injection into the Forearm Muscles of Chronic Stroke Patients with Spastic Flexed Wrist and Clenched Fist: Manual Needle Placement Evaluated Using Ultrasonography. J. Rehabil. Med. 2014, 46, 1042–1045. [Google Scholar] [CrossRef]
- Pillen, S.; Tak, R.O.; Zwarts, M.J.; Lammens, M.M.Y.; Verrijp, K.N.; Arts, I.M.P.; van der Laak, J.A.; Hoogerbrugge, P.M.; van Engelen, B.G.M.; Verrips, A. Skeletal Muscle Ultrasound: Correlation between Fibrous Tissue and Echo Intensity. Ultrasound Med. Biol. 2009, 35, 443–446. [Google Scholar] [CrossRef]
- Lieber, R.L.; Steinman, S.; Barash, I.A.; Chambers, H. Structural and Functional Changes in Spastic Skeletal Muscle. Muscle Nerve 2004, 29, 615–627. [Google Scholar] [CrossRef]
- Hodges, P.W.; Pengel, L.H.M.; Herbert, R.D.; Gandevia, S.C. Measurement of Muscle Contraction with Ultrasound Imaging. Muscle Nerve 2003, 27, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Lieber, R.L.; Fridén, J. Muscle Contracture and Passive Mechanics in Cerebral Palsy. J. Appl. Physiol. 2019, 126, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Ultrasound Elastography in the Assessment of the Stiffness of Spastic Muscles: A Systematic Review—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/33707090/ (accessed on 6 January 2025).
- Santamato, A. High Doses of Botulinum Toxin Type A for the Treatment of Post-Stroke Spasticity: Rationale for a Real Benefit for the Patients. Toxins 2022, 14, 332. [Google Scholar] [CrossRef] [PubMed]
- Boissonnault, È.; Jeon, A.; Munin, M.C.; Filippetti, M.; Picelli, A.; Haldane, C.; Reebye, R. Assessing Muscle Architecture with Ultrasound: Implications for Spasticity. Eur. J. Transl. Myol. 2024, 34, 12397. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, M.N.; Căpeț, C.; Beiu, C.; Berteanu, M. The Elias University Hospital Approach: A Visual Guide to Ultrasound-Guided Botulinum Toxin Injection in Spasticity: Part I—Distal Upper Limb Muscles. Toxins 2025, 17, 107. https://doi.org/10.3390/toxins17030107
Popescu MN, Căpeț C, Beiu C, Berteanu M. The Elias University Hospital Approach: A Visual Guide to Ultrasound-Guided Botulinum Toxin Injection in Spasticity: Part I—Distal Upper Limb Muscles. Toxins. 2025; 17(3):107. https://doi.org/10.3390/toxins17030107
Chicago/Turabian StylePopescu, Marius Nicolae, Claudiu Căpeț, Cristina Beiu, and Mihai Berteanu. 2025. "The Elias University Hospital Approach: A Visual Guide to Ultrasound-Guided Botulinum Toxin Injection in Spasticity: Part I—Distal Upper Limb Muscles" Toxins 17, no. 3: 107. https://doi.org/10.3390/toxins17030107
APA StylePopescu, M. N., Căpeț, C., Beiu, C., & Berteanu, M. (2025). The Elias University Hospital Approach: A Visual Guide to Ultrasound-Guided Botulinum Toxin Injection in Spasticity: Part I—Distal Upper Limb Muscles. Toxins, 17(3), 107. https://doi.org/10.3390/toxins17030107






