Pain Reduction with Repeated Injections of Botulinum Toxin A in Upper Limb Spasticity: A Longitudinal Analysis from the ULIS-III Study
Abstract
1. Introduction
- (a)
- To determine the extent to which goals for pain reduction continued to be met through repeated cycles of injection over the 2-year period;
- (b)
- To examine serial changes in reported pain severity in order to identify any cumulative effect on pre- or post-injection pain scores;
- (c)
- To explore any patient-related factors that may determine which patients require more or less frequent injections;
- (d)
- To examine any patient-related predictors of pain reduction.
2. Results
2.1. Demographics
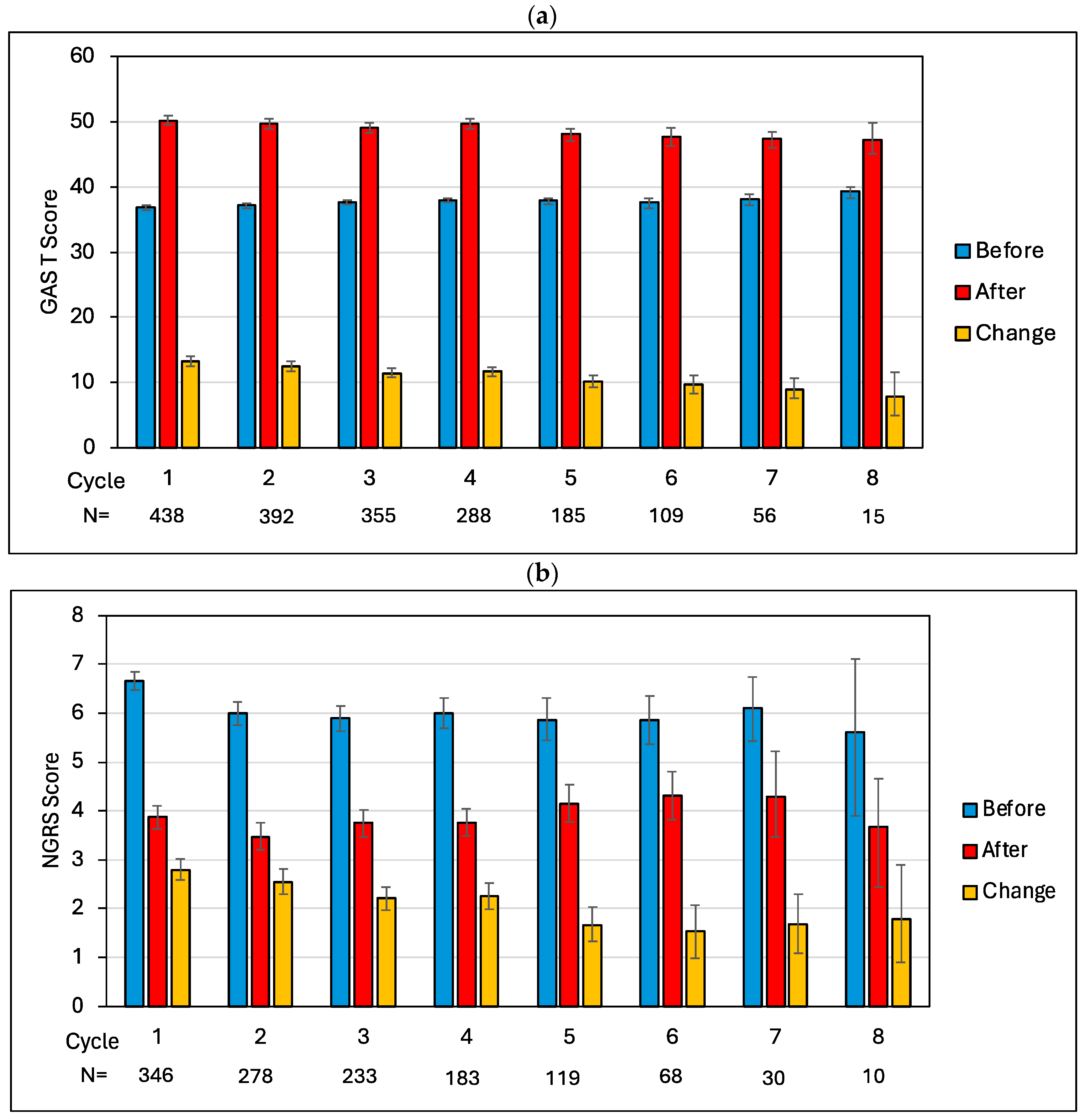
2.2. Cycle-by-Cycle Analysis of GAS T-Scores and Pain Scores
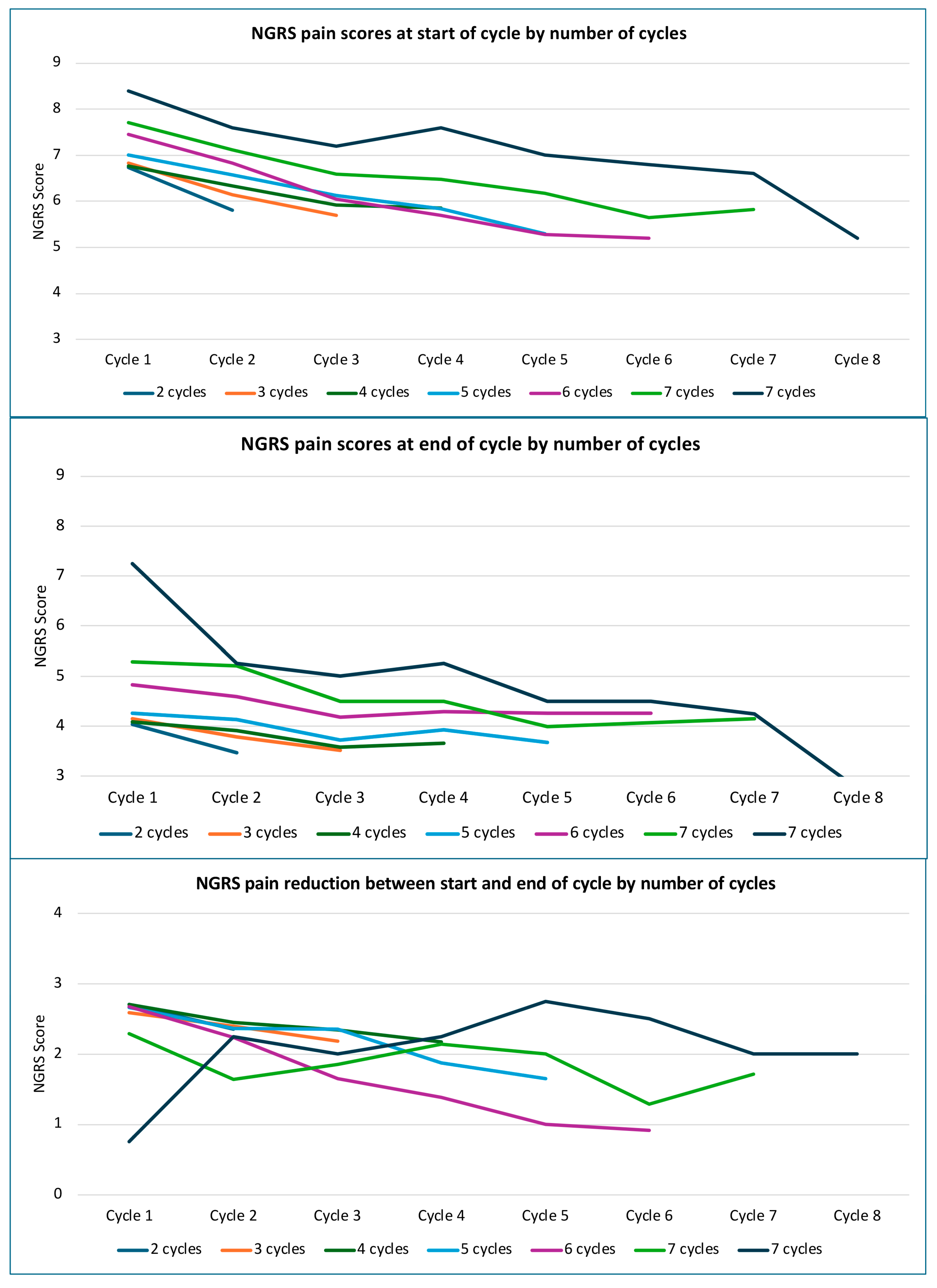
2.3. Serial Changes in Reported Pain Severity with Number of Cycles
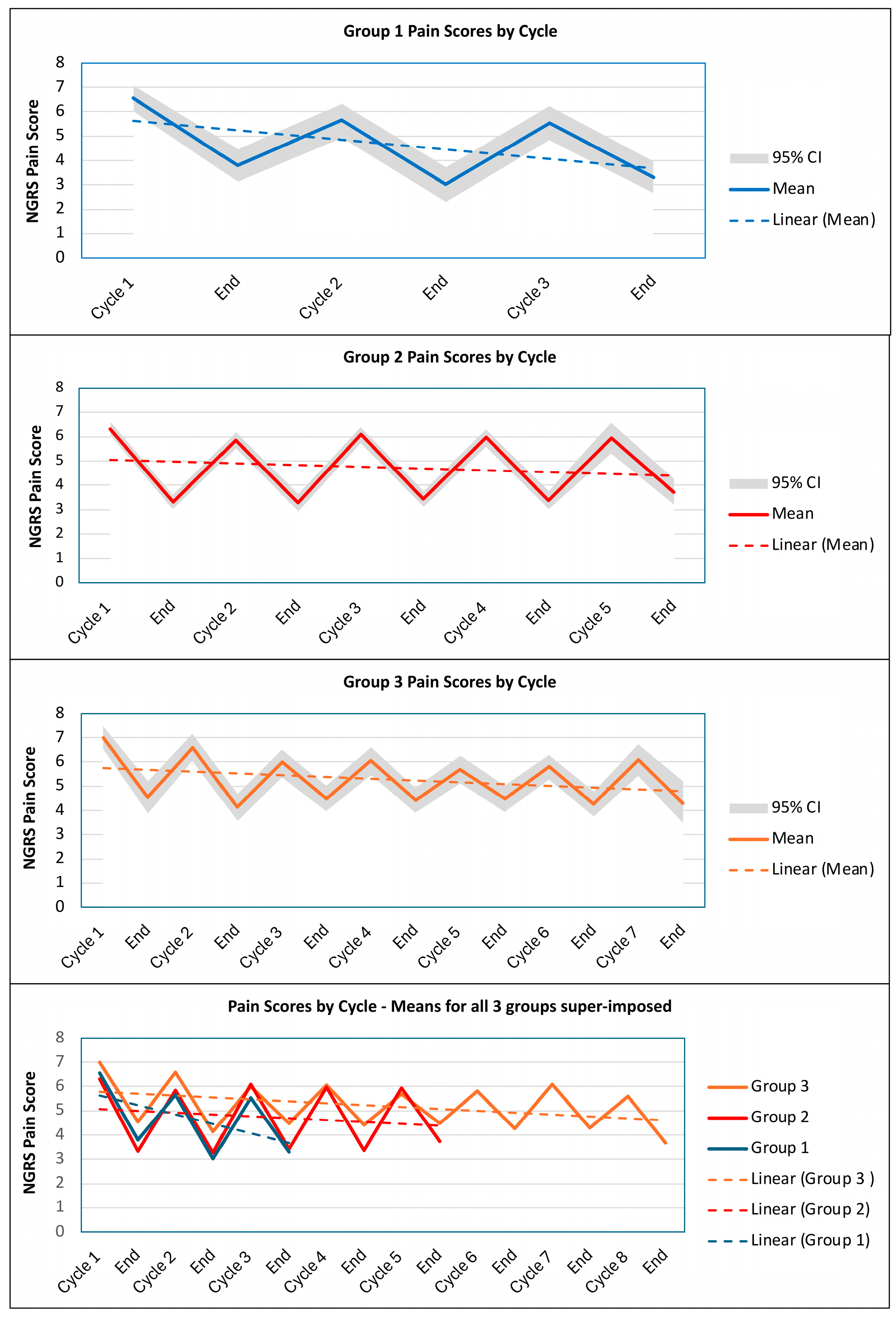
2.4. Cycle-Frequency Group Comparison
2.5. Predictors of Pain Scores
2.6. Predictors of Cycle Frequency
3. Discussion
3.1. Response to Repeated Injections
3.2. Serial Changes in Pain Severity
3.3. Patient-Related Predictors of Pain Reduction and the Frequency of Injection
3.4. Comparison of Findings with Other Studies
3.5. Important Messages for Clinicians Managing Upper Limb Spascticity
- Reduction in pain is a common goal for treatment in patients with upper limb spasticity and BoNT-A continues to provide relief from spasticity-related pain over repeated injections.
- There is evidence for both a short-term response to each injection and a longer-term cumulative effect of reduced pain over successive cycles, both of which are likely to be clinically significant.
- Some patients appear to require more frequent injections to manage their symptoms. Those who required more frequent injections had more severe and resistant pain over the course of the study and were more likely to have had previous treatment with BoNT-A, but as of yet, there is no clear algorithm for determining injection frequency based on patient characteristics or presentation. This can only be determined empirically over time.
- Patients who required only 1–3 injections tended to have a more rapid pain relief over successive cycles, and it is possible that some of these made such significant improvement that no further injections were required, although we cannot be certain about this from the data.
- The study was limited to 2 years for pragmatic reasons, but this does not mean that treatment should be limited to that period. Patients who require repeated injections to manage spasticity-related pain may require life-long treatment.
3.6. Strengths and Weaknesses
- Strengths include the large size of the study, conducted in real-life clinical practice with wide international representation, including all aetiologies and all BoNT-A products, which helps to ensure the generalisability of the findings.
- Limitations in the study’s design include the lack of a control group and the fact that some countries only had a few active sites, while others had several, so the findings may not be truly representative. Additional biases may include factors such as clinician expertise on treatment decisions, individual injector beliefs or habits or external prescribing restrictions that limit the number of permitted injections.
- The ULIS-III study was conducted in real-life clinical practice and spasticity-related pain may be multifactorial. Within the dataset, there is no specific information defining the specific cause(s) of pain—merely the clinical observation that pain management was a personal goal for treatment within that cycle.
- The ULIS-III study has provided a large and rich dataset for post hoc analyses. The wider the net is cast in a post hoc analysis, the more chance there is of introducing statistical error through multiple tests. In this study, we have explored in some detail the impact of patient characteristics (such as gender, age, severity distribution and chronicity of spasticity) on pain outcomes from repeated injection. However, we have not yet explored other possible variables, for example, related to treatment approach—such as the dose, agent, injection technique (e.g., number and distribution of muscles, use of targeting techniques, etc.) or concomitant therapies—all of which would need to be addressed in future analyses.
4. Conclusions
5. Materials and Methods
5.1. Study Design and Participants
5.2. Outcome Assessment and Measures
5.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Additional Tables and Figures

| Cycle | Total N in Sample | N (%) with a Pain Goal | N (%) with Pain Goal Achieved |
|---|---|---|---|
| 1 | 438 | 295 (67.4%) | 191 (64.7%) |
| 2 | 392 | 285 (72.7%) | 215 (75.4%) |
| 3 | 355 | 227 (63.9%) | 154 (67.8%) |
| 4 | 288 | 186 (64.6%) | 133 (71.5%) |
| 5 | 185 | 122 (65.9%) | 84 (68.9%) |
| 6 | 109 | 69 (63.3%) | 43 (62.3%) |
| 7 | 56 | 33 (58.9%) | 17 (51.5%) |
| 8 | 15 | 10 (66.7%) | 2 (20.0%) |
| Total | 1838 | 1189 (64.7%) | 839 (70.6%) |
| Start of Cycle | End of Cycle | Change | Paired T-Tests | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cycle | N | Mean | 95% CI | N | Mean | 95% CI | N | Mean | 95% CI | t | df | p | Adjusted p |
| GAS T-score | |||||||||||||
| 1 | 438 | 36.8 | 36.5, 37.1 | 434 | 50.2 | 49.5, 50.9 | 434 | 13.3 | 12.6, 14.1 | 35.0 | 433 | <0.001 | <0.001 |
| 2 | 392 | 37.2 | 36.8, 37.5 | 379 | 49.6 | 48.9, 50.4 | 379 | 12.5 | 11.7, 13.2 | 30.0 | 378 | <0.001 | <0.001 |
| 3 | 355 | 37.6 | 37.3, 38.0 | 338 | 49.1 | 48.3, 49.8 | 338 | 11.4 | 10.8, 12.2 | 29.7 | 337 | <0.001 | <0.001 |
| 4 | 288 | 38.0 | 37.7, 38.3 | 274 | 49.7 | 48.9, 50.4 | 274 | 11.7 | 10.9, 12.4 | 29.2 | 273 | <0.001 | <0.001 |
| 5 | 185 | 37.9 | 37.4, 38.4 | 177 | 48.1 | 47.1, 49.0 | 177 | 10.2 | 9.3, 11.2 | 20.8 | 176 | <0.001 | <0.001 |
| 6 | 109 | 37.6 | 36.8, 34.8 | 96 | 47.6 | 46.2, 49.1 | 96 | 9.8 | 8.4, 11.2 | 12.9 | 95 | <0.001 | <0.001 |
| 7 | 56 | 38.1 | 37.1, 39.0 | 47 | 47.3 | 46.0, 48.5 | 47 | 9.0 | 7.5, 10.7 | 10.8 | 46 | <0.001 | <0.001 |
| 8 | 15 | 39.4 | 38.3, 40.0 | 12 | 47.2 | 45.0, 49.8 | 12 | 7.9 | 5.0, 11.6 | 4.5 | 11 | 0.001 | 0.002 |
| NGRS pain score (pain goal sample) | |||||||||||||
| 1 | 346 | 6.7 | 6.5, 6.9 | 344 | 3.9 | 3.6, 4.1 | 344 | −2.8 | −3.0, −2.6 | −26.2 | 343 | <0.001 | <0.001 |
| 2 | 278 | 6.0 | 5.8, 6.2 | 269 | 3.5 | 3.2, 3.8 | 269 | −2.6 | −2.8, −2.3 | −18.4 | 266 | <0.001 | <0.001 |
| 3 | 233 | 5.9 | 5.6, 6.2 | 217 | 3.7 | 3.5, 4.0 | 215 | −2.2 | −2.5, −2.5 | −17.4 | 214 | <0.001 | <0.001 |
| 4 | 183 | 6.0 | 5.7, 6.3 | 174 | 3.8 | 3.5, 4.1 | 174 | −2.3 | −2.5, −2.0 | −16.1 | 173 | <0.001 | <0.001 |
| 5 | 119 | 5.8 | 5.5, 6.3 | 113 | 4.2 | 3.8, 4.5 | 113 | −1.7 | −2.0, −1.3 | −9.4 | 112 | <0.001 | <0.001 |
| 6 | 68 | 5.9 | 5.4, 6.4 | 60 | 4.3 | 3.8, 4.8 | 60 | −1.5 | −2.1, −1.0 | −5.6 | 59 | <0.001 | <0.001 |
| 7 | 30 | 6.1 | 5.4, 6.7 | 24 | 4.3 | 3.5, 5.2 | 24 | −1.7 | −2.3, −1.1 | −5.1 | 23 | <0.0001 | <0.0001 |
| 8 | 10 | 5.6 | 3.9, 7.1 | 9 | 3.7 | 2.4, 4.7 | 9 | −1.8 | −2.9, −0.9 | −3.1 | 8 | 0.014 | 0.014 |
| Group 1 (N = 164) | Group 2 N = 178) | Group 3 (N = 96) | Adjusted p-Value | ||||
|---|---|---|---|---|---|---|---|
| Demographic | N | % | N | % | N | % | χ2 |
| Sex—n, % | 1.0 | ||||||
| Male | 85 | 52% | 91 | 51% | 50 | 52% | |
| Female | 79 | 48% | 87 | 49% | 46 | 48% | |
| Age—n, % * | 0.026 | ||||||
| <51 | 47 | 29% | 82 | 46% | 29 | 30% | |
| 51-60 | 46 | 28% | 51 | 29% | 27 | 28% | |
| >60 | 71 | 43% | 45 | 25% | 40 | 42% | |
| Type of injury—n, % | 1.0 | ||||||
| Acquired brain injury | 148 | 90% | 158 | 89% | 85 | 89% | |
| Spinal cord injury | 2 | 1% | 1 | 1% | 3 | 3% | |
| Congenital | 6 | 3% | 13 | 7% | 5 | 5% | |
| Progressive neurological | 8 | 2% | 3 | 2% | 2 | 2% | |
| Other | 0 | 3 | 2% | 1 | 1% | ||
| Aetiology of acquired brain injury—n, % | 1.0 | ||||||
| Vascular (stroke) | 141 | 86% | 143 | 80% | 80 | 83% | |
| Trauma | 4 | 2% | 13 | 7% | 8 | 8% | |
| Hypoxic | 2 | 1% | 7 | 4% | 2 | 2% | |
| Tumour | 3 | 2% | 4 | 2% | 1 | 1% | |
| Inflammatory/infective/other | 14 | 1% | 11 | 3% | 2 | 2% | |
| Time since onset of injury | 1.0 | ||||||
| <1 year—n, % | 31 | 19% | 32 | 19% | 11 | 12% | |
| >1 year—n, % | 123 | 80% | 139 | 83% | 82 | 88% | |
| Years—mean (95% CI) | 6.6 | (5.2, 8.2) | 7.4 | (6.2, 8.8) | 9.8 | (7.7, 12.0) | |
| Missing—n, % | 10 | 7 | 3 | ||||
| Previous injections with BoNT-A for upper limb spasticity—n, % ** | <0.001 | ||||||
| No (Naïve) | 78 | 48% | 61 | 34% | 12 | 13% | |
| Yes (Non-Naïve) | 86 | 52% | 117 | 66% | 84 | 87% | |
| Distribution of spasticity—n, % | 0.759 | ||||||
| Focal | 30 | 18% | 17 | 10% | 13 | 13% | |
| Regional | 134 | 82% | 161 | 90% | 83 | 87% | |
| Baseline pain and spasticity scores | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ANOVA |
| Baseline DAS pain score * | 2.0 | (1.8, 2.1) | 1.6 | (1.5, 1.8) | 1.8 | (1.6, 2.0) | 0.051 |
| Proximal composite MAS score | 4.1 | (3.9, 4.3) | 3.7 | (3.5, 3.9) | 3.8 | (3.4, 4.2) | 0.968 |
| Distal composite MAS score | 6.2 | (5.8, 6.6) | 6.1 | (5.7, 6.4) | 6.0 | (5.4, 6.5) | 1.0 |
| Group 1 (n = 164) | Group 2 (n = 178) | Group 3 (n = 96) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |||||||
| Cycle | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI |
| GAS T-scores | ||||||||||||
| 1 | 36.6 | 36.0, 37.2 | 50.4 | 49.1, 51.6 | 37.0 | 36.8, 37.4 | 50.7 | 49.8, 51.6 | 36.8 | 36.0, 37.5 | 48.9 | 47.3, 50.5 |
| 2 | 37.8 | 37.4, 38.4 | 49.2 | 47.7, 50.8 | 37.3 | 36.9, 37.8 | 50.3 | 49.3, 51.4 | 36.0 | 35.0, 36.8 | 48.8 | 47.5, 50.2 |
| 3 | 37.8 | 37.1, 38.5 | 48.7 | 46.9, 50.7 | 37.8 | 37.3, 38.2 | 50.5 | 49.7, 51.3 | 37.2 | 36.4, 37.9 | 46.6 | 45.3, 48.0 |
| 4 | 38.3 | 37.9, 38.6 | 50.7 | 49.7, 51.5 | 37.5 | 36.6, 38.2 | 47.9 | 46.4, 49.3 | ||||
| 5 | 37.8 | 37.2, 38.4 | 49.6 | 48.5, 50.6 | 37.9 | 37.1, 38.7 | 46.8 | 45.2, 48.3 | ||||
| 6 | 37.9 | 37.0, 38.6 | 47.6 | 46.1, 48.9 | ||||||||
| 7 | 38.0 | 37.1, 38.9 | 47.3 | 46.1, 48.5 | ||||||||
| 8 | 39.4 | 37.9, 40.0 | 47.2 | 45.0, 49.6 | ||||||||
| NGRS scores | ||||||||||||
| 1 | 6.9 | 6.6, 7.2 | 4.1 | 3.7, 4.5 | 6.3 | 6.0, 6.6 | 3.3 | 3.0, 3.6 | 7.0 | 6.6, 7.5 | 4.6 | 3.9, 5.2 |
| 2 | 5.7 | 5.3, 6.1 | 3.2 | 2.7, 3.6 | 5.9 | 5.5, 6.2 | 3.3 | 2.9, 3.7 | 6.6 | 6.0, 7.1 | 4.1 | 3.6, 4.8 |
| 3 | 5.4 | 4.8, 6.0 | 3.3 | 2.7, 4.0 | 6.1 | 5.8, 6.5 | 3.5 | 3.1, 3.9 | 6.0 | 5.4, 6.6 | 4.5 | 4.0, 5.0 |
| 4 | 6.0 | 5.6, 6.4 | 3.4 | 3.1, 3.8 | 6.0 | 5.4, 6.6 | 4.4 | 3.9, 4.9 | ||||
| 5 | 6.0 | 5.3, 6.6 | 3.8 | 3.2, 4.4 | 5.7 | 5.1, 6.3 | 4.5 | 4.0, 5.0 | ||||
| 6 | 5.8 | 5.3, 6.3 | 4.3 | 3.9, 4.8 | ||||||||
| 7 | 6.1 | 5.5, 6.7 | 4.3 | 3.5, 5.3 | ||||||||
| 8 | 5.6 | 4.0, 7.2 | 3.7 | 2.4, 4.8 | ||||||||

References
- Trompetto, C.; Marinelli, L.; Mori, L.; Pelosin, E.; Curra, A.; Molfetta, L.; Abbruzzese, G. Pathophysiology of spasticity: Implications for neurorehabilitation. Biomed. Res. Int. 2014, 2014, 354906. [Google Scholar] [CrossRef]
- Gracies, J.M.; Brashear, A.; Jech, R.; McAllister, P.; Banach, M.; Valkovic, P.; Walker, H.; Marciniak, C.; Deltombe, T.; Skoromets, A.; et al. Safety and efficacy of abobotulinumtoxinA for hemiparesis in adults with upper limb spasticity after stroke or traumatic brain injury: A double-blind randomised controlled trial. Lancet Neurol. 2015, 14, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Elovic, E.P.; Munin, M.C.; Kanovsky, P.; Hanschmann, A.; Hiersemenzel, R.; Marciniak, C. Randomized, placebo-controlled trial of incobotulinumtoxina for upper-limb post-stroke spasticity. Muscle Nerve 2016, 53, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.M.; Gracies, J.M.; Yablon, S.A.; Barbano, R.; Brashear, A.; Bo, N.T.T.Z.D.S.T. Botulinum neurotoxin versus tizanidine in upper limb spasticity: A placebo-controlled study. J. Neurol. Neurosurg. Psychiatry 2009, 80, 380–385. [Google Scholar] [CrossRef]
- Andringa, A.; van de Port, I.; van Wegen, E.; Ket, J.; Meskers, C.; Kwakkel, G. Effectiveness of Botulinum Toxin Treatment for Upper Limb Spasticity Poststroke Over Different ICF Domains: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2019, 100, 1703–1725. [Google Scholar] [CrossRef]
- Wissel, J.; Ward, A.B.; Erztgaard, P.; Bensmail, D.; Hecht, M.J.; Lejeune, T.M.; Schnider, P.; Altavista, M.C.; Cavazza, S.; Deltombe, T.; et al. European consensus table on the use of botulinum toxin type A in adult spasticity. J. Rehabil. Med. 2009, 41, 13–25. [Google Scholar] [CrossRef]
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef]
- Ashford, S.A.; Turner-Stokes, L.; Allison, R.; Duke, L.; Bavikatte, G.; Kirker, S.; Moore, P.; Ward, A.B.; Bilton, D. Spasticity in adults: Management using Botulinum Toxin. In National Clinical Guidelines, 2nd ed.; Royal College of Physicians: London, UK, 2018; Available online: https://www.rcp.ac.uk/improving-care/resources/spasticity-in-adults-management-using-botulinum-toxin/ (accessed on 23 February 2025).
- Truini, A.; Barbanti, P.; Pozzilli, C.; Cruccu, G. A mechanism-based classification of pain in multiple sclerosis. J. Neurol. 2013, 260, 351–367. [Google Scholar] [CrossRef]
- Chang, Y.J.; Liang, J.N.; Hsu, M.J.; Lien, H.Y.; Fang, C.Y.; Lin, C.H. Effects of continuous passive motion on reversing the adapted spinal circuit in humans with chronic spinal cord injury. Arch. Phys. Med. Rehabil. 2013, 94, 822–828. [Google Scholar] [CrossRef]
- Sheean, G.; McGuire, J.R. Spastic hypertonia and movement disorders: Pathophysiology, clinical presentation, and quantification. PM R 2009, 1, 827–833. [Google Scholar] [CrossRef]
- Ward, A.B.; Kadies, M. The management of pain in spasticity. Disabil. Rehabil. 2002, 24, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Dietz, V.; Berger, W. Normal and impaired regulation of muscle stiffness in gait: A new hypothesis about muscle hypertonia. Exp. Neurol. 1983, 79, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Galiana, L.; Fung, J.; Kearney, R. Identification of intrinsic and reflex ankle stiffness components in stroke patients. Exp. Brain Res. 2005, 165, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.M. Pathophysiology of spastic paresis. I: Paresis and soft tissue changes. Muscle Nerve 2005, 31, 535–551. [Google Scholar] [CrossRef]
- Kalichman, L.; Ratmansky, M. Underlying pathology and associated factors of hemiplegic shoulder pain. Am. J. Phys. Med. Rehabil. 2011, 90, 768–780. [Google Scholar] [CrossRef]
- O’Donnell, M.J.; Diener, H.C.; Sacco, R.L.; Panju, A.A.; Vinisko, R.; Yusuf, S.; Investigators, P.R. Chronic pain syndromes after ischemic stroke: PRoFESS trial. Stroke 2013, 44, 1238–1243. [Google Scholar] [CrossRef]
- Klit, H.; Finnerup, N.B.; Jensen, T.S. Central post-stroke pain: Clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009, 8, 857–868. [Google Scholar] [CrossRef]
- Chae, J. Poststroke complex regional pain syndrome. Top. Stroke Rehabil. 2010, 17, 151–162. [Google Scholar] [CrossRef]
- Kocabas, H.; Levendoglu, F.; Ozerbil, O.M.; Yuruten, B. Complex regional pain syndrome in stroke patients. Int. J. Rehabil. Res. 2007, 30, 33–38. [Google Scholar] [CrossRef]
- Yelnik, A.P.; Colle, F.M.; Bonan, I.V.; Vicaut, E. Treatment of shoulder pain in spastic hemiplegia by reducing spasticity of the subscapular muscle: A randomised, double blind, placebo controlled study of botulinum toxin A. J. Neurol. Neurosurg. Psychiatr. 2007, 78, 845–848. [Google Scholar] [CrossRef]
- Arezzo, J.C. Possible mechanisms for the effects of botulinum toxin on pain. Clin. J. Pain. 2002, 18, S125–S132. [Google Scholar] [CrossRef]
- Kumar, R. Therapeutic use of botulinum toxin in pain treatment. Neuronal Signal 2018, 2, NS20180058. [Google Scholar] [CrossRef] [PubMed]
- Matak, I.; Bolcskei, K.; Bach-Rojecky, L.; Helyes, Z. Mechanisms of Botulinum Toxin Type A Action on Pain. Toxins 2019, 11, 459. [Google Scholar] [CrossRef]
- Shaikh, A.; Phadke, C.P.; Ismail, F.; Boulias, C. Relationship Between Botulinum Toxin, Spasticity, and Pain: A Survey of Patient Perception. Can. J. Neurol. Sci. 2016, 43, 311–315. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Fheodoroff, K.; Jacinto, J.; Maisonobe, P.; Ashford, S. ULIS (Upper Limb International Spasticity), a 10-year Odyssey: An international, multicentric, longitudinal cohort of person-centered spasticity management in real-life practice. J. Int. Soc. Phys. Rehabil. Med. 2019, 2, 138–150. [Google Scholar] [CrossRef]
- Wissel, J.; Camoes-Barbosa, A.; Comes, G.; Althaus, M.; Scheschonka, A.; Simpson, D.M. Pain Reduction in Adults with Limb Spasticity Following Treatment with IncobotulinumtoxinA: A Pooled Analysis. Toxins 2021, 13, 887. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Muller, J.; Dressnandt, J.; Heinen, F.; Naumann, M.; Topka, H.; Poewe, W. Management of spasticity associated pain with botulinum toxin A. J. Pain. Symptom Manag. 2000, 20, 44–49. [Google Scholar] [CrossRef]
- Shaw, L.; Rodgers, H.; Price, C.; van Wijck, F.; Shackley, P.; Steen, M.; Barnes, M.; Ford, G.; Graham, L. BoTULS: A multicentre randomised controlled trial to evaluate the clinical effectiveness and cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type A. Health Technol. Assess. 2010, 14, 1–113. [Google Scholar] [CrossRef]
- Baker, J.A.; Pereira, G. The efficacy of Botulinum Toxin A for spasticity and pain in adults: A systematic review and meta-analysis using the Grades of Recommendation, Assessment, Development and Evaluation approach. Clin. Rehabil. 2013, 27, 1084–1096. [Google Scholar] [CrossRef]
- Riberto, M.; Frances, J.A.; Chueire, R.; Amorim, A.; Xerez, D.; Chung, T.M.; Mercuri, L.H.C.; Lianza, S.; Rocha, E.C.M.; Maisonobe, P.; et al. Post Hoc Subgroup Analysis of the BCause Study Assessing the Effect of AbobotulinumtoxinA on Post-Stroke Shoulder Pain in Adults. Toxins 2022, 14, 809. [Google Scholar] [CrossRef]
- Trompetto, C.; Marinelli, L.; Mori, L.; Puce, L.; Avanti, C.; Saretti, E.; Biasotti, G.; Amella, R.; Cotellessa, F.; Restivo, D.A.; et al. Effectiveness of Botulinum Toxin on Pain in Stroke Patients Suffering from Upper Limb Spastic Dystonia. Toxins 2022, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.M.; Guo, T.T.; Sun, X.; Ge, H.X.; Chen, X.D.; Zhao, K.J.; Zhang, L.N. Effectiveness of Botulinum Toxin A in Treatment of Hemiplegic Shoulder Pain: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2021, 102, 1775–1787. [Google Scholar] [CrossRef]
- Kiresuk, T.; Sherman, R. Goal attainment scaling: A general method of evaluating comprehensive mental health programmes. Community Ment. Health J. 1968, 4, 443–453. [Google Scholar] [CrossRef]
- Malec, J.F. Goal Attainment Scaling in Rehabilitation. Neuropsychol. Rehabil. 1999, 9, 253–275. [Google Scholar] [CrossRef]
- Bovend’Eerdt, T.J.; Botell, R.E.; Wade, D.T. Writing SMART rehabilitation goals and achieving goal attainment scaling: A practical guide. Clin. Rehabil. 2009, 23, 352–361. [Google Scholar] [CrossRef]
- Ashford, S.A.; Turner-Stokes, L. Goal Attainment Scaling (GAS) in adult neurorehabilitation. In Rehabilitation Goal Setting: Theory. Practice and Evidence; Siegert, R.J., Levack, W., Eds.; CRC Press, Taylor and Francis: Boca Raton, FL, USA, 2014; pp. 119–137. [Google Scholar]
- Grant, M.; Ponsford, J. Goal attainment scaling in brain injury rehabilitation: Strengths, limitations and recommendations for future applications. Neuropsychol. Rehabil. 2014, 24, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Bard-Pondarre, R.; Villepinte, C.; Roumenoff, F.; Lebrault, H.; Bonnyaud, C.; Pradeau, C.; Bensmail, D.; Isner-Horobeti, M.E.; Krasny-Pacini, A. Goal Attainment Scaling in rehabilitation: An educational review providing a comprehensive didactical tool box for implementing Goal Attainment Scaling. J. Rehabil. Med. 2023, 55, jrm6498. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Ashford, S.; Jacinto, J.; Maisonobe, P.; Balcaitiene, J.; Fheodoroff, K. Impact of integrated upper limb spasticity management including botulinum toxin A on patient-centred goal attainment: Rationale and protocol for an international prospective, longitudinal cohort study (ULIS-III). BMJ Open 2016, 6, e011157. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Fheodoroff, K.; Jacinto, J.; Maisonobe, P. Results from the Upper Limb International Spasticity Study-II (ULIS-II): A large, international, prospective cohort study investigating practice and goal attainment following treatment with botulinum toxin A in real-life clinical management. BMJ Open 2013, 3, e002771. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Jacinto, J.; Fheodoroff, K.; Brashear, A.; Maisonobe, P.; Lysandropoulos, A.; Ashford, S.; The ULIS-III Study Group. Longitudinal goal attainment with integrated upper limb spasticity management including repeat injections of botulinum toxin A: Findings from the prospective, observational Upper Limb International Spasticity (ULIS-III) cohort study. J. Rehabil. Med. 2021, 53, jrm00157. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Jacinto, J.; Fheodoroff, K.; Brashear, A.; Maisonobe, P.; Lysandropoulos, A.; Ashford, S.; Upper Limb International Spasticity-III (ULIS-III) Study Group. Assessing the effectiveness of upper-limb spasticity management using a structured approach to goal-setting and outcome measurement: First cycle results from the ULIS-III Study. J. Rehabil. Med. 2021, 53, jrm00133. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, B.; Balouch, S.A.; Dengler, R.; Kollewe, K. Long-term treatment of spasticity with botulinum toxin type A: An analysis of 1221 treatments in 137 patients. Neurol. Res. 2010, 32, 309–313. [Google Scholar] [CrossRef]
- Gracies, J.M.; O’Dell, M.; Vecchio, M.; Hedera, P.; Kocer, S.; Rudzinska-Bar, M.; Rubin, B.; Timerbaeva, S.L.; Lusakowska, A.; Boyer, F.C.; et al. Effects of repeated abobotulinumtoxinA injections in upper limb spasticity. Muscle Nerve 2018, 57, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.M.; Jech, R.; Valkovic, P.; Marque, P.; Vecchio, M.; Denes, Z.; Vilain, C.; Delafont, B.; Picaut, P. When can maximal efficacy occur with repeat botulinum toxin injection in upper limb spastic paresis? Brain Commun. 2021, 3, fcaa201. [Google Scholar] [CrossRef] [PubMed]
- Elovic, E.P.; Brashear, A.; Kaelin, D.; Liu, J.; Millis, S.R.; Barron, R.; Turkel, C. Repeated treatments with botulinum toxin type a produce sustained decreases in the limitations associated with focal upper-limb poststroke spasticity for caregivers and patients. Arch. Phys. Med. Rehabil. 2008, 89, 799–806. [Google Scholar] [CrossRef]
- Francisco, G.E.; Jost, W.H.; Bavikatte, G.; Bandari, D.S.; Tang, S.F.T.; Munin, M.C.; Largent, J.; Adams, A.M.; Zuzek, A.; Esquenazi, A. Individualized OnabotulinumtoxinA Treatment for Upper Limb Spasticity Resulted in High Clinician- and Patient-Reported Satisfaction: Long-Term Observational Results from the ASPIRE Study. PM R. 2020, 12, 1120–1133. [Google Scholar] [CrossRef]
- Fheodoroff, K.; Jacinto, J.; Ashford, S.; Danchenko, N.; Grandoulier, A.-S.; Pietri, G.; Bourhis, Y.; Spurden, D.; Turner-Stokes, L. Predictors of real-world response in adults treated with botulinumtoxin-A for upper limb spasticity. J. Int. Soc. Phys. Rehabil. Med. 2024, 7, 24–32. [Google Scholar] [CrossRef]
- Turner-Stokes, L. Goal attainment scaling in rehabilitation; a practical guide. Clin. Rehabil. 2009, 23, 362–370. [Google Scholar] [CrossRef]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Geisser, S.; Greenhouse, S.W. An Extension of Box’s Results on the Use of the $F$ Distribution in Multivariate Analysis. Ann. Math. Stat. 1958, 29, 885–891. [Google Scholar] [CrossRef]
| Effective Population (N = 953) | Pain Subgroup (N = 438) | Adjusted p-Value * | |||
|---|---|---|---|---|---|
| Demographic | N | % | N | % | χ2 |
| Sex—n, % | 0.109 | ||||
| Male | 537 | 56.3% | 226 | 51.6% | |
| Female | 416 | 43.7% | 212 | 48.4% | |
| Age—n, % | 1.0 | ||||
| <51 years | 364 | 38.2% | 158 | 36.1% | |
| 51–60 years | 254 | 26.7% | 124 | 28.3% | |
| >60 years | 335 | 35.2% | 156 | 35.6% | |
| Type of injury—n, % | 0.704 | ||||
| Acquired brain injury | 870 | 91.3% | 391 | 89.3% | |
| Spinal cord injury | 15 | 1.6% | 6 | 1.4% | |
| Congenital | 44 | 4.6% | 24 | 5.5% | |
| Progressive neurological | 20 | 2.1% | 13 | 3.0% | |
| Other | 4 | 0.4% | 4 | 0.9% | |
| Aetiology of acquired brain injury—n, % | 1.0 | ||||
| Vascular (stroke) | 786 | 90.3% | 364 | 93.1% | |
| Trauma | 71 | 8.2% | 25 | 6.4% | |
| Hypoxic | 25 | 2.9% | 11 | 2.8% | |
| Tumour | 19 | 2.2% | 8 | 2.0% | |
| Inflammatory/infective/other | 52 | 6.0% | 30 | 7.7% | |
| Time since onset of injury | 1.0 | ||||
| <1 year—n, % | 145 | 15.2% | 74 | 16.9% | |
| >1 year—n, % | 769 | 80.7% | 344 | 78.5% | |
| Years—mean (SD) | 7.6 | (9.4) | 7.6 | (9.6) | |
| Missing—n, % | 39 | 4.1% | 20 | 4.6% | |
| Previous injections with BoNT-A for upper limb spasticity—n, % | 1.0 | ||||
| No (Naïve) | 318 | 33.4% | 151 | 34.5% | |
| Yes (Non-Naïve) | 635 | 66.6% | 287 | 65.5% | |
| Distribution of spasticity—n, % | <0.001 | ||||
| Focal | 190 | 19.9% | 60 | 13.7% | |
| Regional | 763 | 80.1% | 378 | 86.3% | |
| Baseline pain and spasticity | Mean | 95% CI | Mean | 95% CI | t-test |
| Baseline DAS pain score | 1.1 | 1.0, 1.1 | 1.8 | 1.7, 1.9 | <0.001 ** |
| Proximal composite MAS score | 3.7 | 3.6, 3.8 | 3.9 | 3.7, 4.0 | 0.014 |
| Distal composite MAS score | 6.1 | 5.9, 6.2 | 6.1 | 5.9, 6.3 | 1.0 |
| (a) NGRS Scores at the Start of Cycle | ||||||
|---|---|---|---|---|---|---|
| Cycles | Effect Size (η2) | df1 | df2 | F | p-Value | Adjusted p * |
| 2 | 0.17 | 1 | 230 | 46.6 | <0.001 | <0.001 |
| 3 | 0.14 | 2.0 | 320.6 | 25.4 | <0.001 | <0.001 |
| 4 | 0.08 | 2.6 | 299.5 | 9.6 | <0.001 | <0.001 |
| 5 | 0.16 | 3.1 | 227.6 | 13.9 | <0.0001 | <0.001 |
| 6 | 0.27 | 3.7 | 142.9 | 14.3 | <0.001 | <0.001 |
| 7 | 0.18 | 3.7 | 59.3 | 3.4 | 0.016 | 0.160 |
| 8 | 0.31 | 7 | 28 | 1.8 | 0.129 | 0.742 |
| (b) NGRS Scores at the End of Cycle | ||||||
| Cycles | Effect Size (η2) | df1 | df2 | F | p-Value | Adjusted p |
| 2 | 0.08 | 1 | 222 | 19.5 | <0.001 | <0.001 |
| 3 | 0.06 | 2.0 | 298.5 | 9.4 | <0.001 | 0.002 |
| 4 | 0.03 | 2.7 | 293.0 | 3.8 | 0.014 | 0.154 |
| 5 | 0.04 | 3.3 | 227.7 | 2.8 | 0.036 | 0.324 |
| 6 | 0.04 | 4.0 | 132.1 | 1.2 | 0.297 | 0.891 |
| 7 | 0.11 | 3.1 | 40.8 | 1.6 | 0.192 | 0.768 |
| 8 | 0.65 | 7 | 21 | 5.6 | 0.001 | 0.013 |
| (c) Change in NGRS Scores in Each Cycle | ||||||
| Cycles | Effect Size (η2) | df1 | df2 | F | p-Value | Adjusted p |
| 2 | 0.01 | 1 | 221 | 4.0 | 0.047 | 0.376 |
| 3 | 0.01 | 2 | 294.8 | 2.2 | 0.114 | 0.742 |
| 4 | 0.02 | 2.7 | 288.7 | 2.1 | 0.106 | 0.742 |
| 5 | 0.06 | 3.2 | 226.3 | 4.5 | 0.004 | 0.048 |
| 6 | 0.14 | 3.5 | 116.9 | 5.5 | 0.001 | 0.011 |
| 7 | 0.03 | 3.0 | 39.5 | 0.4 | 0.732 | 1 |
| 8 | 0.13 | 7 | 21 | 0.4 | 0.862 | 1 |
| (a) Start of Cycle | ||||||
|---|---|---|---|---|---|---|
| Variable | B | SE | df | Z | p-Value | Adjusted p * |
| Intercept | 6.45 | 0.33 | 521.6 | 19.75 | <0.001 | <0.001 |
| Group Group 2 | −0.55 | 0.23 | 770.0 | −2.41 | 0.016 | 0.312 |
| Group 3 | −0.09 | 0.27 | 668.9 | −0.34 | 0.738 | 1 |
| Age | ||||||
| 51–60 years | 0.12 | 0.21 | 418.2 | 0.58 | 0.560 | 1 |
| 61+ years | −0.08 | 0.20 | 431.5 | −0.41 | 0.679 | 1 |
| Sex | ||||||
| Female | 0.32 | 0.17 | 424.9 | 1.94 | 0.053 | 0.953 |
| Time since injury | <0.01 | <0.01 | 439.5 | −0.15 | 0.879 | 1 |
| Previous BoNT-A for ULS | ||||||
| Yes | 0.09 | 0.19 | 456.3 | 0.50 | 0.618 | 1 |
| Distribution of spasticity | ||||||
| Regional | 0.09 | 0.24 | 442.6 | 0.38 | 0.704 | 1 |
| Severity of spasticity MAS proximal | <0.01 | <0.01 | 433.4 | 1.30 | 0.193 | 1 |
| MAS distal | <0.01 | <0.01 | 409.4 | 0.62 | 0.538 | 1 |
| Cycle number | −0.65 | 0.12 | 1158 | −5.62 | <0.001 | <0.001 |
| Group 2*cycle number | 0.50 | 0.13 | 1140 | 3.98 | <0.001 | 0.002 |
| Group 3*cycle number | 0.33 | 0.12 | 1142 | 2.73 | 0.006 | 0.140 |
| (b) End of cycle | ||||||
| Variable | B | SE | df | Z | p-Value | Adjusted p * |
| Intercept | 3.40 | 0.36 | 467.6 | 9.34 | <0.001 | <0.001 |
| Group | ||||||
| Group 2 | −0.63 | 0.25 | 641.3 | −2.57 | 0.010 | 0.206 |
| Group 3 | −0.31 | 0.29 | 558.4 | −1.06 | 0.290 | 1 |
| Age | ||||||
| 51–60 years | 0.11 | 0.24 | 394.1 | 0.48 | 0.632 | 1 |
| 61+ years | 0.08 | 0.23 | 405.3 | 0.35 | 0.729 | 1 |
| Sex | ||||||
| Female | 0.34 | 0.19 | 398.8 | 1.79 | 0.074 | 1 |
| Time since injury | <0.01 | <0.01 | 407.4 | −0.23 | 0.821 | 1 |
| Previous BoNT-A for ULS | ||||||
| Yes | −0.09 | 0.21 | 420.3 | −0.46 | 0.647 | 1 |
| Distribution of spasticity | ||||||
| Regional | 0.46 | 0.27 | 411.4 | 1.69 | 0.092 | 1 |
| Severity of spasticity MAS proximal | <0.01 | <0.01 | 403.8 | 0.72 | 0.472 | 1 |
| MAS distal | <0.01 | <0.01 | 388.1 | 0.59 | 0.553 | 1 |
| Cycle number | −0.49 | 0.14 | 1067 | −3.57 | <0.001 | 0.009 |
| Group 2*cycle number | 0.45 | 0.15 | 1049 | 3.08 | 0.002 | 0.049 |
| Group 3*cycle number | 0.38 | 0.14 | 1053 | 2.66 | 0.008 | 0.165 |
| Variable | B | SE | Z | p-Value | Adjusted p * |
|---|---|---|---|---|---|
| Intercept | −1.43 | 0.79 | −1.80 | 0.071 | 0.713 |
| Age | |||||
| 51–60 years | −0.02 | 0.42 | −0.05 | 0.965 | 1 |
| 61+ years | −0.15 | 0.39 | −0.39 | 0.697 | 1 |
| Sex | |||||
| Female | −0.24 | 0.32 | −0.76 | 0.447 | 1 |
| DAS pain score at baseline | |||||
| 1 | 0.46 | 0.66 | 0.70 | 0.487 | 1 |
| 2 | 0.24 | 0.58 | 0.41 | 0.679 | 1 |
| 3 | 0.05 | 0.62 | 0.09 | 0.931 | 1 |
| Previous BoNT-A for ULS | |||||
| Yes | 1.74 | 0.40 | 4.34 | <0.001 | <0.001 |
| Severity of spasticity MAS proximal | −0.02 | 0.11 | −0.23 | 0.817 | 1 |
| MAS distal | −0.07 | 0.07 | −1.01 | 0.312 | 1 |
| Years since stroke | −0.01 | 0.02 | −0.34 | 0.736 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner-Stokes, L.; Buchwald, K.; Ashford, S.A.; Fheodoroff, K.; Jacinto, J.; Narayanan, A.; Siegert, R.J. Pain Reduction with Repeated Injections of Botulinum Toxin A in Upper Limb Spasticity: A Longitudinal Analysis from the ULIS-III Study. Toxins 2025, 17, 117. https://doi.org/10.3390/toxins17030117
Turner-Stokes L, Buchwald K, Ashford SA, Fheodoroff K, Jacinto J, Narayanan A, Siegert RJ. Pain Reduction with Repeated Injections of Botulinum Toxin A in Upper Limb Spasticity: A Longitudinal Analysis from the ULIS-III Study. Toxins. 2025; 17(3):117. https://doi.org/10.3390/toxins17030117
Chicago/Turabian StyleTurner-Stokes, Lynne, Khan Buchwald, Stephen A. Ashford, Klemens Fheodoroff, Jorge Jacinto, Ajit Narayanan, and Richard J. Siegert. 2025. "Pain Reduction with Repeated Injections of Botulinum Toxin A in Upper Limb Spasticity: A Longitudinal Analysis from the ULIS-III Study" Toxins 17, no. 3: 117. https://doi.org/10.3390/toxins17030117
APA StyleTurner-Stokes, L., Buchwald, K., Ashford, S. A., Fheodoroff, K., Jacinto, J., Narayanan, A., & Siegert, R. J. (2025). Pain Reduction with Repeated Injections of Botulinum Toxin A in Upper Limb Spasticity: A Longitudinal Analysis from the ULIS-III Study. Toxins, 17(3), 117. https://doi.org/10.3390/toxins17030117






