Mechanism of Mycotoxin Contamination of Medicinal Herbs
Abstract
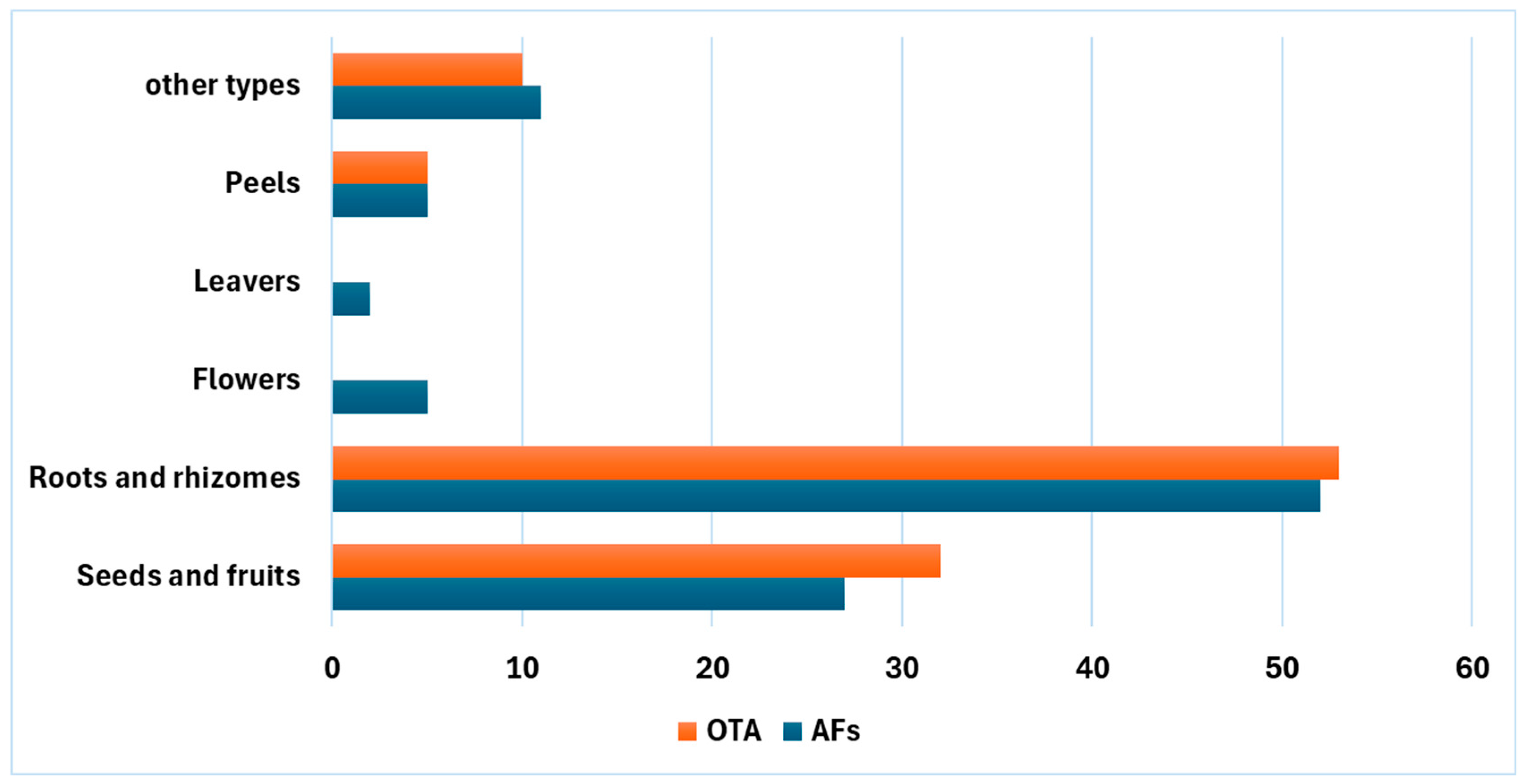
:1. Mycotoxin Contamination of Medicinal Herbs
1.1. Aflatoxins (AFs)
1.2. Ochratoxins (OTs)
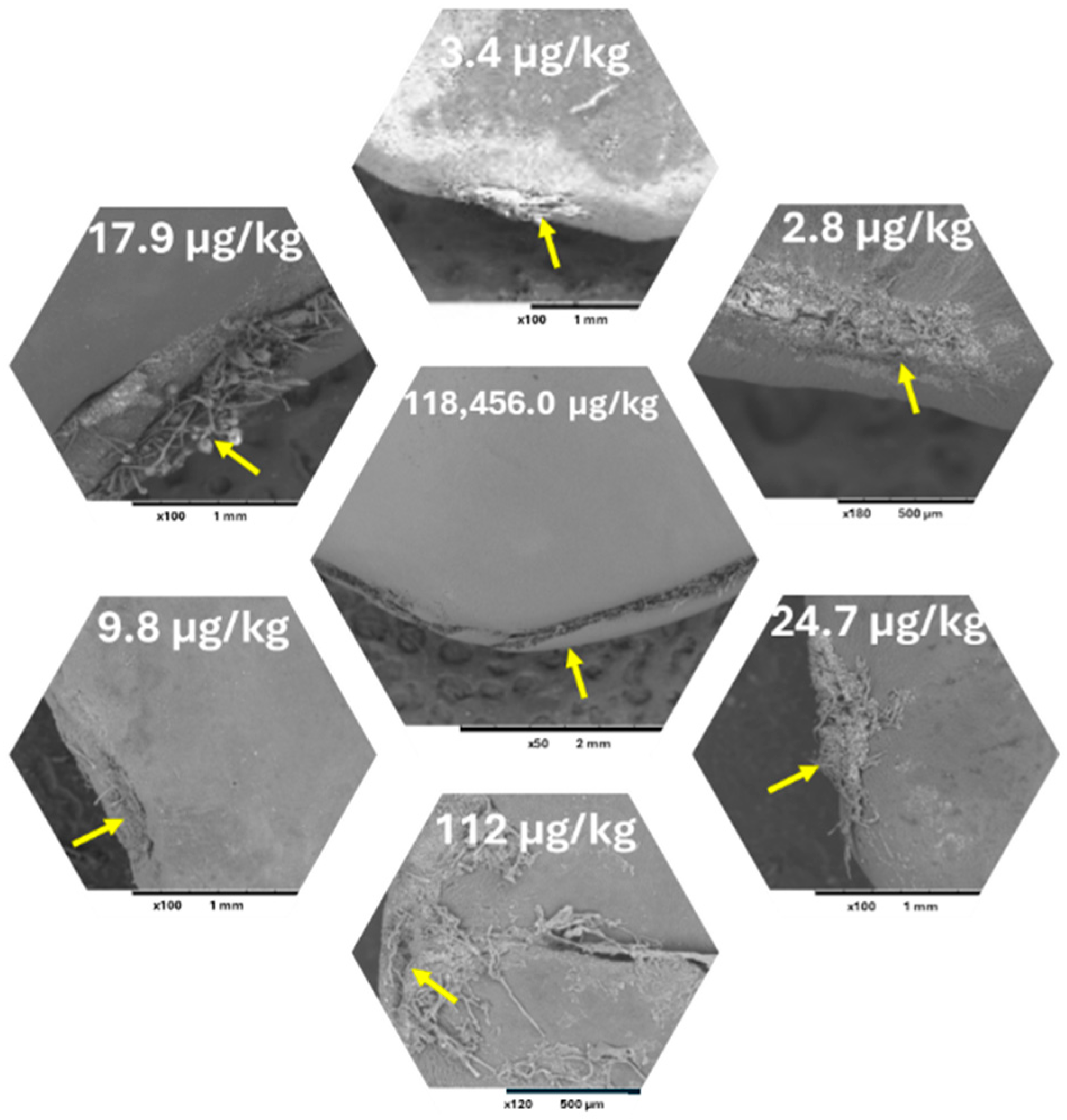
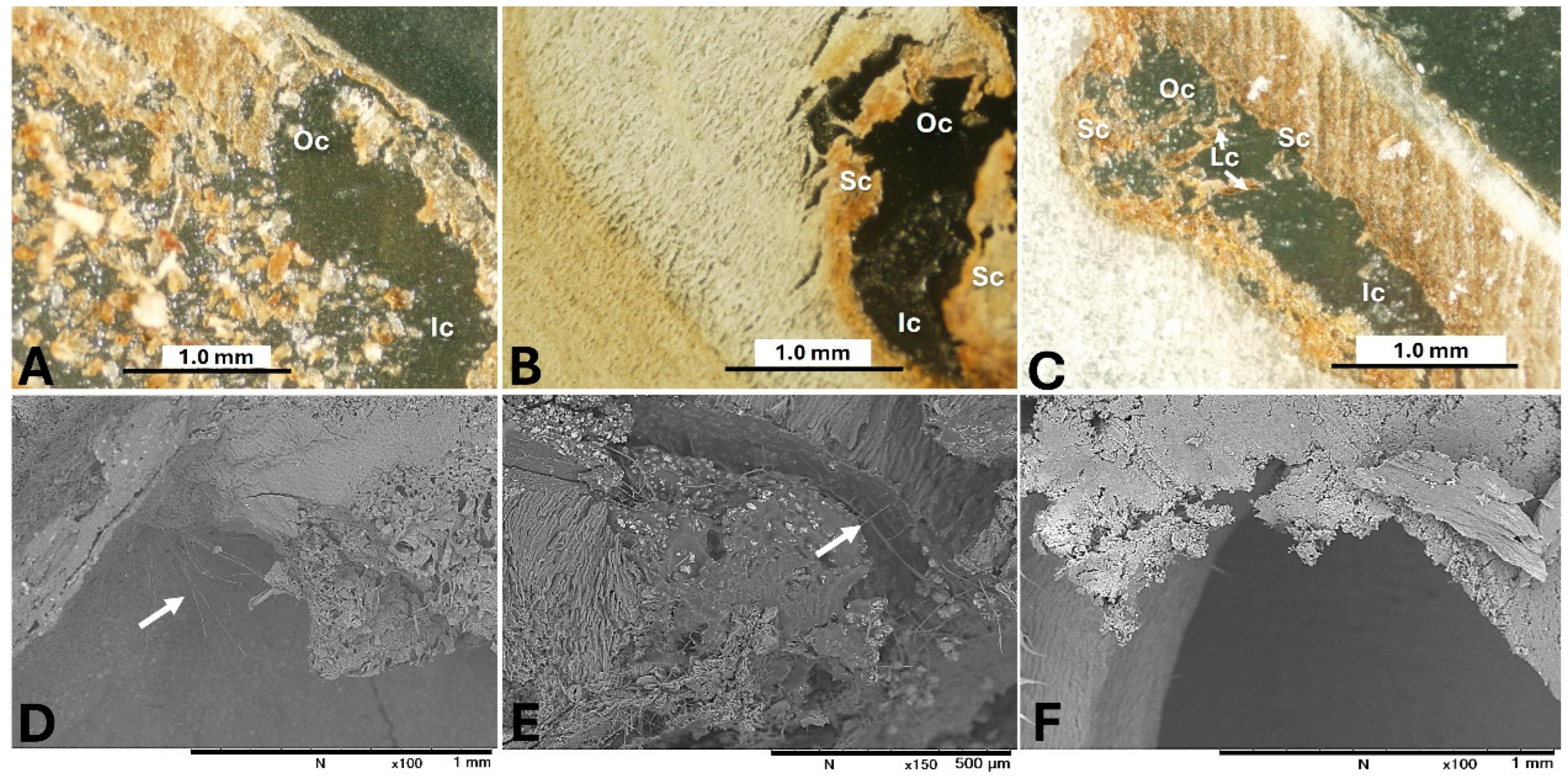
2. AF Contamination of Jujube
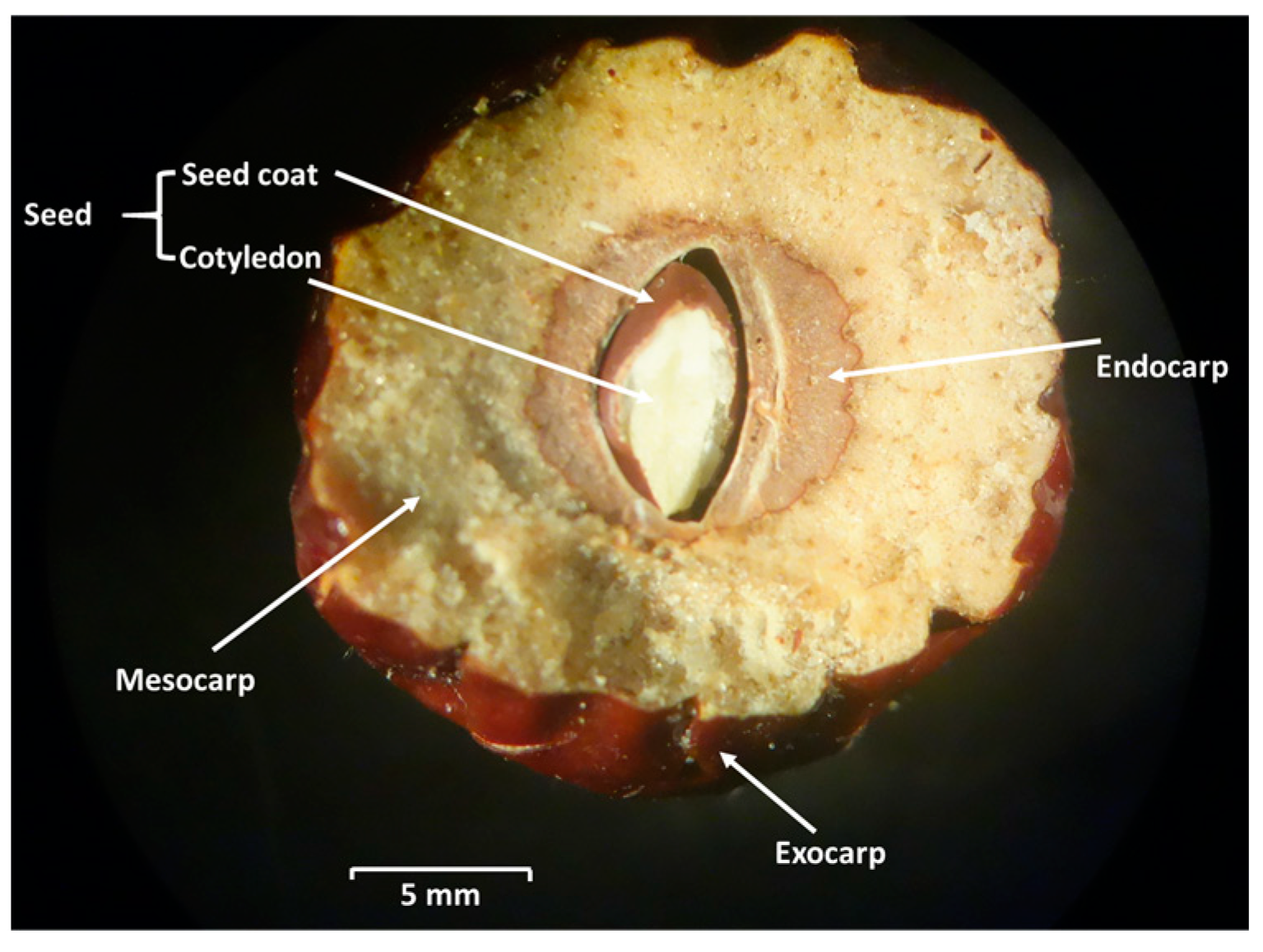
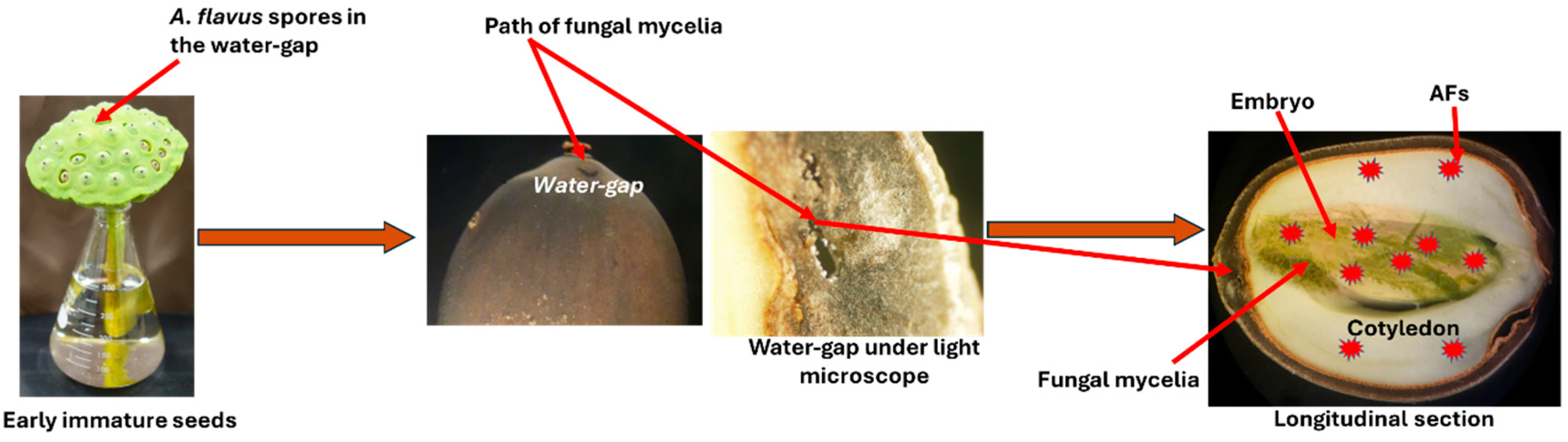
3. AF Contamination of Lotus (Nelumbo nucifera Gaertn.) Seeds
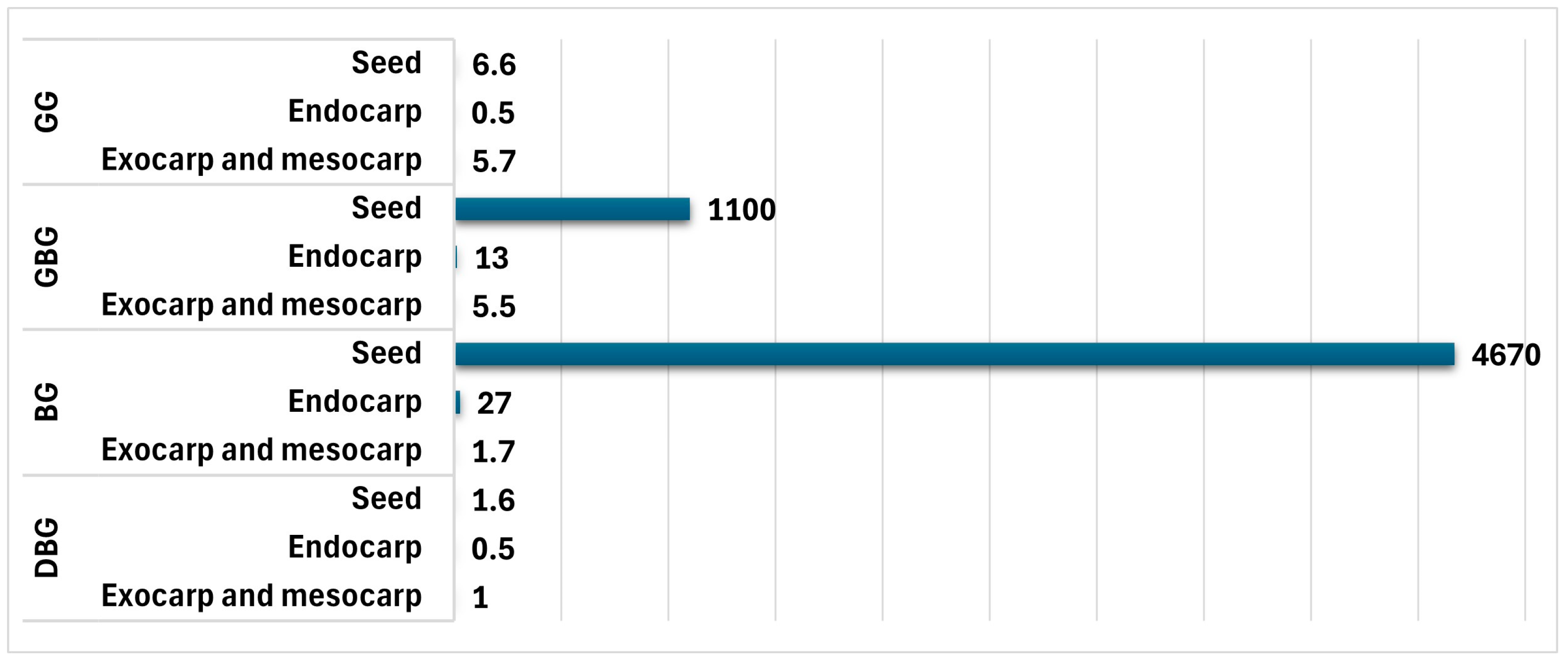
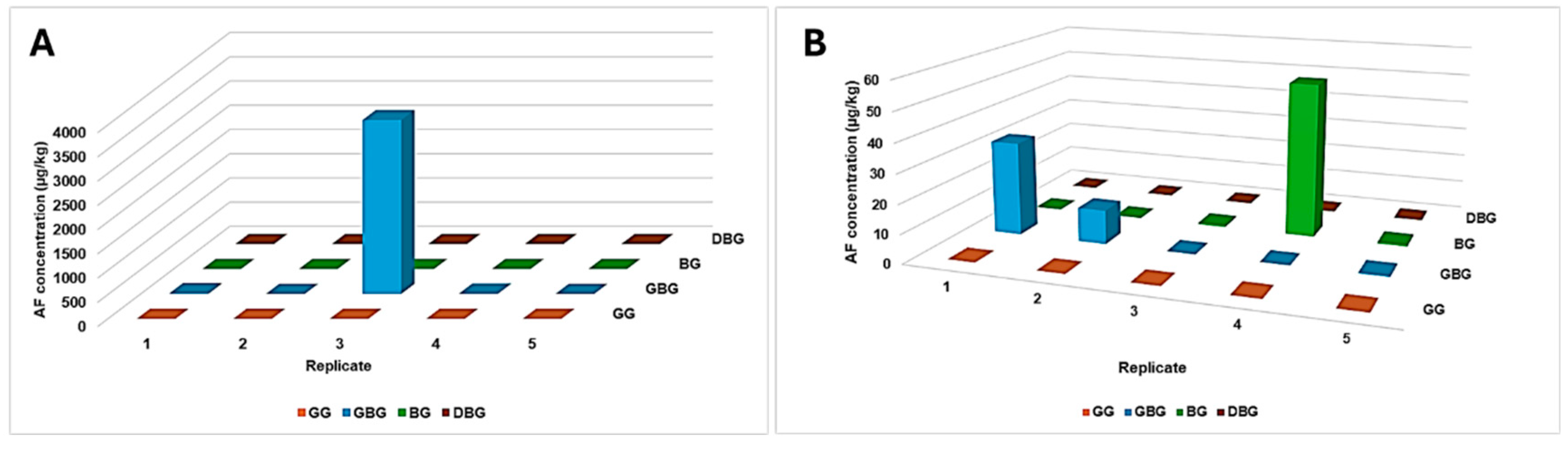
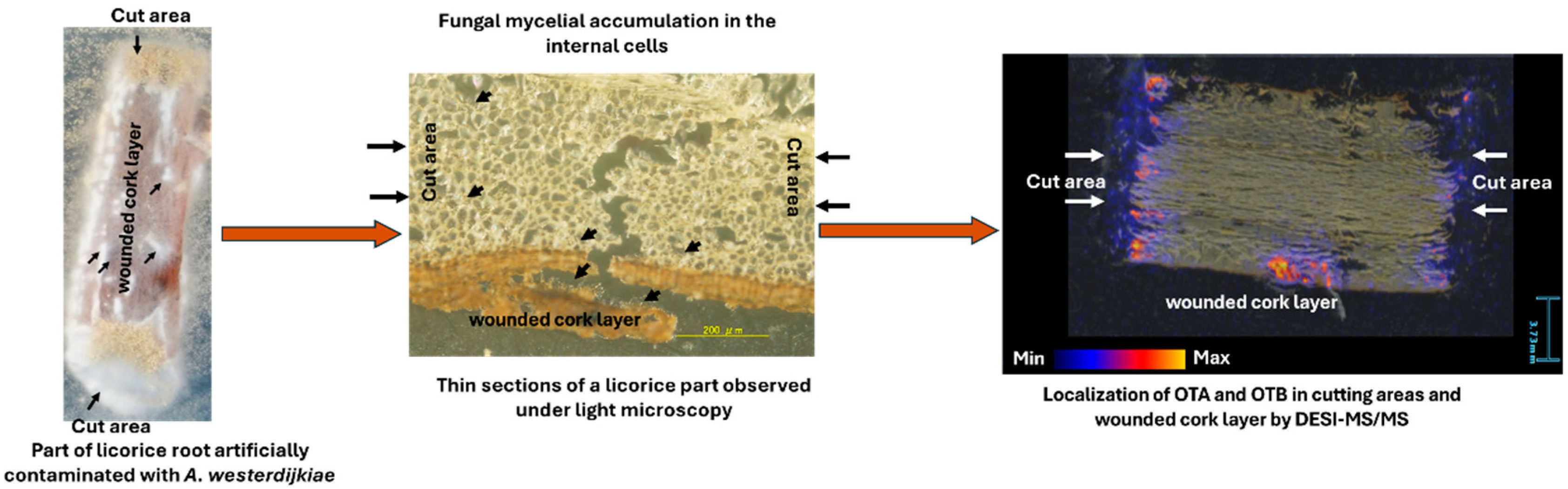
4. OTs Contamination of Licorice Roots
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide research trends on medicinal plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Choisy, P. Medicinal plants meet modern biodiversity science. Curr. Biol. 2024, 34, R158–R173. [Google Scholar] [CrossRef] [PubMed]
- Awuchi, C.G. The biochemistry, toxicology, and uses of the pharmacologically active phytochemicals: Alkaloids, terpenes, polyphenols, and glycosides. J. Food Pharm. Sci. 2019, 7, 131–150. [Google Scholar] [CrossRef]
- Pallarés, N.; Berrada, H.; Font, G.; Ferrer, E. Mycotoxins occurrence in medicinal herbs dietary supplements and exposure assessment. J. Food Sci. Technol. 2022, 59, 2830–2841. [Google Scholar] [CrossRef]
- Krsnik, S.; Erjavec, K. Factors influencing use of medicinal herbs. J. Patient Exp. 2024, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Qina, L.; Jianga, J.-Y.; Zhanga, L.; Doua, X.-W.; Ouyangc, Z.; Wanb, L.; Yanga, M.-H. Occurrence and analysis of mycotoxins in domestic Chinese herbal medicines. Mycology 2020, 11, 126–146. [Google Scholar] [CrossRef]
- Keter, L.; Too, R.; Mwikwabe, N.; Mutai, C.; Orwa, J.; Mwamburi, L.; Ndwigah, S.; Bii, C.; Korir, R. Risk of fungi associated with aflatoxin and fumonisin in medicinal herbal products in the Kenyan market. Sci. World J. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Ałtyn, I.; Twarużek, M. Review: Mycotoxin contamination concerns of herbs and medicinal plants. Toxins 2020, 12, 182. [Google Scholar] [CrossRef]
- Chen, L.; Guo, W.; Zheng, Y.; Zhou, J.; Liu, T.; Chen, W.; Liang, D.; Zhao, M.; Zhu, Y.; Wu, Q.; et al. Occurrence and characterization of fungi and mycotoxins in contaminated medicinal herbs. Toxins 2020, 12, 30. [Google Scholar] [CrossRef]
- Tulayakul, P.; Sugita-Konishi, Y. Mycotoxin contamination in foodstuffs and feeds-health concerns in Thailand. JJVR 2017, 65, 173–183. [Google Scholar]
- Su, C.; Hu, Y.; Gao, D.; Luo, Y.I.; Chen, A.J.; Jiao, X.; Gao, W. Occurrence of toxigenic fungi and mycotoxins on root herbs from Chinese markets. J. Food Prot. 2018, 81, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dou, X.W.; Zhang, C.; Logrieco, A.F.; Yang, M.H. A review of current methods for analysis of mycotoxins in herbal medicines. Toxins 2018, 10, 65. [Google Scholar] [CrossRef]
- Ab-Dullah, S.S.; Sabran, M.R.; Hasiah, A.H.; Abdullah, R. Risk assessment of aflatoxin B1 in herbal medicines and plant food supplements marketed in Malaysia using margin of exposure and RISK21 approaches. Genes Environ. 2023, 45, 31. [Google Scholar] [CrossRef] [PubMed]
- Oni, E.O.; Oni, N.O.; Bamidele, J.; Taiwo, O.A.; Oyetibo, O.B.; Badmos, A.O.; Adeleye, T.M.; Olatunbosun, M.O.; Oluwafemi, F. Microbial load and aflatoxin contamination in locally formulated herbal mixtures obtained from Itoku market, Nigeria. J. Food Saf. Hyg. 2023, 9, 197–206. [Google Scholar] [CrossRef]
- Areoa, O.M.; Phokub, J.Z.; Gbashia, S.; Njobeha, P.B. A preliminary study of multi-mycotoxins contamination in some selected South Africa medicinal plants. Emir. J. Food Agric. 2020, 32, 426–433. [Google Scholar] [CrossRef]
- Kachapulula, P.W.; Bandyopadhyay, R.; Cotty, P.J. Aflatoxin contamination of non-cultivated fruits in Zambia. Front. Microbiol. 2019, 10, 1840. [Google Scholar] [CrossRef]
- Wei, F.; Liu, X.; Liao, X.; Shi, L.; Zhang, S.; Lu, J.; Zhou, L.; Kong, W. Simultaneous determination of 19 mycotoxins in lotus seed using a multimycotoxin UFLC-MS/MS method. J. Pharm. Pharmacol. 2019, 71, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Gherbawy, Y.; Shebany, Y. Mycobiota, total aflatoxins and ochratoxin a of cardamom pods. Food Sci. Technol. Res. 2018, 24, 87–96. [Google Scholar] [CrossRef]
- Özden, H.; Özden, S. Levels of heavy metals and ochratoxin a in medicinal plants commercialized in Turkey. Turk. J. Pharm. Sci. 2018, 15, 376–381. [Google Scholar] [CrossRef]
- Al-Meamar, T.S.; Al-Jassani, M.J.; Hamad, N.S. Aflatoxins and aflatoxigenic fungi contamination of dried fruits in Iraqi market. J. Glob. Pharma Technol. 2017, 9, 299–308. [Google Scholar]
- Rangsipanuratn, W.; Kammarnjassadakul, P.; Janwithayanuchit, I.; Paungmoung, P.; Ngamurulert, S.; Sriprapun, M.; Yangen, S.; Soottitantawat, V.; Sandee, A. Detection of microbes, aflatoxin and toxic heavy metals in Chinese medicinal herbs commonly consumed in Thailand. Pharm. Sci. Asia 2017, 44, 162–171. [Google Scholar] [CrossRef]
- Liu, S.; Qiu, F.; Kong, W.; Wei, J.; Xiao, X.; Yang, M. Development and validation of an accurate and rapid LC-ESI-MS/MS method for the simultaneous quantification of aflatoxin B1, B2, G1 and G2 in lotus seeds. Food Control 2013, 29, 156–161. [Google Scholar] [CrossRef]
- Santos, L.; Marín, S.; Sanchis, V.; Ramos, J.A. Screening of mycotoxin multicontamination in medicinal and aromatic herbs sampled in Spain. J. Sci. Food Agric. 2009, 89, 1802–1807. [Google Scholar] [CrossRef]
- Arino, A.; Herrera, M.; Estopanan, G.; Juan, T. High levels of ochratoxin A in licorice and derived products. Int. J. Food Microbiol. 2007, 114, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Bresch, H.; Urbanek, M.; Nusser, M. Ochratoxin A in food containing liquorice. Nahrung 2000, 44, 276–278. [Google Scholar] [CrossRef]
- Aziz, N.H.; Youssef, Y.A.; El-Fouly, M.Z.; Moussa, L.A. Contamination of some common medicinal plant samples and spices by fungi and their mycotoxins. Bot. Bull. Sin. 1998, 39, 279–285. [Google Scholar]
- Kumar, S.; Roy, A.K. Occurrence of aflatoxin in some liver curative herbal medicines. Lett. Appl. Microbiol. 1993, 17, 112–114. [Google Scholar] [CrossRef]
- Balekundri, A.; Mannur, V. Quality control of the traditional herbs and herbal products: A review. Future J. Pharm. Sci. 2020, 6, 67. [Google Scholar] [CrossRef]
- Elamin, A.; Enomoto, H.; Watanabe, M.; Sakuda, S. The Mechanism of ochratoxin contamination of artificially inoculated licorice roots. Toxins 2023, 15, 219. [Google Scholar] [CrossRef]
- Louppis, A.P.; Constantinou, M.S. Application of a validated method for the identification and quantification of mycotoxins in wines using UPLC-MS/MS. Separations 2022, 9, 102. [Google Scholar] [CrossRef]
- Scott, P.M.; Truckses, M.W. Application of immunoaffinity columns to mycotoxin analysis. J. AOAC Int. 1997, 80, 941–949. [Google Scholar] [CrossRef]
- Santos, L.; Marín, S.; Sanchis, V.; Ramos, J.A. Mycotoxin in Medicinal/Aromatic Herbs—A Review. Bol. Latinoam. Caribe Plantas Med. Aromat. 2013, 12, 119–142. [Google Scholar]
- Namjoo, M.; Salamat, F.; Rajabli, N.; Hajihoseeini, R.; Niknejad, F.; Kohsar, F.; Joshaghani, H. Quantitative determination of aflatoxin by high performance liquid chromatography in wheat silos in Golestan province, north of Iran. Iran. J. Public Health 2016, 45, 905–910. [Google Scholar] [PubMed]
- Alshannaq, A.F.; Yu, J.-H. A liquid chromatographic method for rapid and sensitive analysis of aflatoxins in laboratory fungal cultures. Toxins 2020, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-P.; Zhang, D.; Tan, L.-H.; Yu, B.; Cao, W.-G. Analysis of aflatoxins in traditional Chinese medicines: Classification of analytical method on the basis of matrix variations. Sci. Rep. 2016, 6, 30822. [Google Scholar] [CrossRef]
- Gil-Serna, J.; Patiño, B.; Cortes, L.; González-Jaén, M.A.; Vazquez, C. Aspergillus steynii and Aspergillus westerdijkiae as potential risk of OTA contamination in food products in warm climates. Food Microbiol. 2015, 46, 168–175. [Google Scholar] [CrossRef]
- Lerda, D.; Ambrosio, M.; Kunsagi, Z.; Stroka, J. Determination of ochratoxin A in licorice and licorice extracts by high-performance liquid chromatography coupled with fluorescence detection: Collaborative study. J. AOAC Int. 2013, 96, 331–340. [Google Scholar] [CrossRef]
- Gurikar, C.; Shivaprasad, P.D.; Sabillón, L.; Nanje Gowda, N.A.; Siliveru, K. Impact of mycotoxins and their metabolites associated with food grains. GOST 2023, 6, 1–9. [Google Scholar] [CrossRef]
- Chen, A.J.; Tang, D.; Zhou, Y.Q.; Sun, B.D.; Li, X.J.; Wang, L.Z.; Gao, W.W. Identification of ochratoxin A producing fungi associated with fresh and dry liquorice. PLoS ONE 2013, 8, e78285. [Google Scholar] [CrossRef]
- Agrawal, P.; Singh, T.; Pathak, D.; Chopra, H. An updated review of Ziziphus jujube: Major focus on its phytochemicals and pharmacological properties. Pharmacol. Res. Mod. Chin. Med. 2023, 8, 100297. [Google Scholar] [CrossRef]
- Singh, Y.P.; Sumbali, G. Natural incidence of toxigenic Aspergillus flavus strain on the surface of pre-harvest jujube fruits. Indian Phytopathol. 2000, 53, 404–406. [Google Scholar]
- Elamin, A.; Sakuda, S. Susceptibility and mechanism of aflatoxin contamination of Ziziphus jujuba var. spinosa. Toxins 2025, 17, 113. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Delgado, A.D.; González, M.C.M.; Isasa, M.E.T. Fatty acids and carotenes in some ber (Ziziphus jujuba Mill) varieties. Plant Foods Hum. Nutr. 2004, 59, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Bi, J.; Chen, Q.; Wu, X.; Lyu, Y.; Meng, X. Assessment of sugar content, fatty acids, free amino acids, and volatile profiles in jujube fruits at different ripening stages. Food Chem. 2019, 270, 344–352. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Tulpule, P.G. Effect of free fatty acids on aflatoxin production in a synthetic medium. Food Cosmet. Toxicol. 1980, 18, 367–369. [Google Scholar] [CrossRef]
- Choi, S.-H.; Ahn, J.-B.; Kim, H.-J.; Im, N.-K.; Kozukue, N.; Levin, C.E.; Friedman, M. Changes in free amino acid, protein, and flavonoid content in jujube (Ziziphus jujube) fruit during eight stages of growth and antioxidative and cancer cell inhibitory effects by extracts. J. Agric. Food Chem. 2012, 60, 10245–10255. [Google Scholar] [CrossRef]
- Payne, G.A.; Hagler, W.M.J. Effect of specific amino acids on growth and aflatoxin production by Aspergillus parasiticus and Aspergillus flavus in defined media. Appl. Environ. Microbiol. 1983, 46, 805–812. [Google Scholar] [CrossRef]
- Uppala, S.S.; Bowen, K.L.; Woods, F.M. Pre-harvest aflatoxin contamination and soluble sugars of peanut. Peanut Sci. 2013, 40, 40–51. [Google Scholar] [CrossRef]
- Sapkota, S.; Sapkota, S.; Wang, S.; Liu, Z. Phenological study of Chinese jujube trees using biologische Bundesanstalt, Bundessortenamt and Chemische Industrie (BBCH) scale. J. Hortic. Sci. Res. 2020, 3, 68–73. [Google Scholar]
- Zhang, D.; Liu, T.; Sheng, J.; Lv, S.; Ren, L. TMT-based quantitative proteomic analysis reveals the physiological regulatory networks of embryo dehydration protection in lotus (Nelumbo nucifera). Front. Plant Sci. 2021, 12, 792057. [Google Scholar] [CrossRef]
- Elamin, A.; Sakuda, S. Evaluation of the susceptibility of Ziziphus jujuba var. spinosa fruit to aflatoxin contamination and infection of aflatoxigenic fungus based on ripening stages and fruit parts. JSM Mycotoxins 2021, 71, 63–67. [Google Scholar]
- Baskin, C.C. Breaking physical dormancy in seeds—focussing on the lens. New Phytol. 2003, 158, 229–232. [Google Scholar] [CrossRef]
- Souza, F.H.D.; Marcos-Filho, J. The seed coat as a modulator of seed–environment relationships in Fabaceae. Braz. J. Bot. 2001, 24, 365–375. [Google Scholar] [CrossRef]
- Fouda, T.; Abdelsalam, A.; Swilam, A.; El-Didamony, M. Determination of physical properties of some seeds. Sci. Pap. Ser. Manag. Econom. Eng. Agric. Rural Dev. 2022, 22, 223–238. [Google Scholar]
- Debeaujon, I.; Léon-Kloosterziel, K.M.; Koornneef, M. Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol. 2000, 122, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Jayasuriya, K.M.G.G.; Baskin, J.M.; Geneve, R.L.; Baskin, C.C. Morphology and anatomy of physical dormancy in Ipomoea lacunosa: Identification of the water gap in seeds of Convolvulaceae (Solanales). Ann. Bot. 2007, 100, 13–22. [Google Scholar] [CrossRef]
- Jaganathan, G.K.; Yule, K.J.; Biddick, M. Determination of the water gap and the germination ecology of Adenanthera pavonina (Fabaceae, Mimosoideae); the adaptive role of physical dormancy in mimetic seeds. AoB Plants 2018, 10, ply048. [Google Scholar] [CrossRef] [PubMed]
- Mendgen, K.; Hahn, M.; Deising, H. Morphogenesis and mechanisms of penetration by plant pathogenic fungi. Annu. Rev. Phytopathol. 1996, 34, 364–386. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, X.; Zeng, S.; Huang, X.; Guo, Z.; Zheng, Y.; Tian, Y.; Zheng, B. Nutritional composition, physiological functions and processing of lotus (Nelumbo nucifera Gaertn.) seeds: A review. Phytochem. Rev. 2015, 14, 321–334. [Google Scholar] [CrossRef]
- Ashoka, S.; Revann, M.L. Physico chemical and functional properties of lotus (Nelumbo nucifera) Seed. Mysore J. Agric. Sci. 2022, 56, 61–67. [Google Scholar]
- Kakar, M.U.; Karim, H.; Shabir, G.; Iqbal, I.; Akram, M.; Ahmad, S.; Shafi, M.; Gul, P.; Riaz, S.; Ur-Rehman, R.; et al. A review on extraction, composition, structure, and biological activities of polysaccharides from different parts of Nelumbo nucifera. Food Sci Nutr. 2023, 11, 3655–3674. [Google Scholar] [CrossRef]
- Bangar, S.P.; Dunno, K.; Kumar, M.; Mostafa, H.; Maqsood, S. A comprehensive review on lotus seeds (Nelumbo nucifera Gaertn.): Nutritional composition, health-related bioactive properties, and industrial applications. J. Funct. Foods 2022, 89, 104937. [Google Scholar] [CrossRef]
- Bhat, R.; Sridhar, K.R.; Karim, A.A. Microbial quality evaluation and effective decontamination of nutraceutically valued lotus seeds by electron beams and gamma irradiation. Radiat. Phys. Chem. 2010, 79, 976–981. [Google Scholar] [CrossRef]
- Liao, X.; Sun, C.; Wei, F.; Zhou, L.; Kong, W. Exploration of the safe water content and activity control points for medicinal and edible lotus seeds from mildew. AMB Expr. 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.J.; Jiao, X.; Hu, Y.; Lu, X.; Gao, W. Mycobiota and mycotoxins in traditional medicinal seeds from China. Toxins 2015, 7, 3858–3875. [Google Scholar] [CrossRef]
- Gama-Arachchige, N.S.; Baskin, J.M.; Geneve, R.L.; Baskin, C.C. Identification and characterization of ten new water gaps in seeds and fruits with physical dormancy and classification of water-gap complexes. Ann. Bot. 2013, 112, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Elamin, A.; Sultana, S.; Sakuda, S. Evaluation of the susceptibility of lotus seeds (Nelumbo nucifera Gaertn.) to Aspergillus flavus infection and aflatoxin contamination. Toxins 2024, 16, 29. [Google Scholar] [CrossRef]
- Dam, S.; Laursen, B.S.; Ørnfelt, J.H.; Jochimsen, B.; Stærfeldt, H.H.; Friis, C.; Nielsen, K.; Goffard, N.; Besenbacher, S.; Krusell, L.; et al. The proteome of seed development in the model legume Lotus japonicus. Plant Physiol. 2009, 149, 1325–1340. [Google Scholar] [CrossRef]
- Jaganathan, G.K.; Song, D.; Liu, W.; Han, Y.; Liu, B. Relationship between seed moisture content and acquisition of impermeability in Nelumbo nucifera (Nelumbonaceae). Acta Bot. Bras. 2017, 31, 639–644. [Google Scholar] [CrossRef]
- Shen-Miller, J.; Aung, L.H.; Turek, J.; Schopf, J.W.; Tholandi, M.; Yang, M.; Czaj, A. Centuries-old viable fruit of sacred lotus Nelumbo nucifera Gaertn var. China antique. Trop. Plant Biol. 2013, 6, 53–68. [Google Scholar] [CrossRef]
- Moro, C.F.; Fukao, Y.; Shibato, J.; Rakwal, R.; Agrawal, G.K.; Shioda, S.; Kouzuma, K.; Yonekura, M. Immature seed endosperm and embryo proteomics of the lotus (Nelumbo Nucifera Gaertn.) by one-dimensional gel-based tandem mass spectrometry and a comparison with the mature endosperm proteome. Proteomes 2015, 3, 184–235. [Google Scholar] [CrossRef] [PubMed]
- Reda, F.M.; El-Saadony, M.T.; El-Rayes, T.K.; Farahat, M.; Attia, G.; Alagawany, M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021, 100, 101266. [Google Scholar] [CrossRef]
- Cerulli, A.; Masullo, M.; Montoro, P.; Piacent, S. Licorice (Glycyrrhiza glabra, G. uralensis, and G. inflata) and their constituents as active cosmeceutical ingredients. Cosmetics 2022, 9, 7. [Google Scholar] [CrossRef]
- Chen, A.J.; Huang, L.F.; Wang, L.Z.; Tang, D.; Cai, F.; Gao, W.W. Occurrence of toxigenic fungi in ochratoxin A contaminated liquorice root. Food Addit. Contam. 2011, 28, 1091–1097. [Google Scholar] [CrossRef]
- Kitagawa, I. Licorice root. A natural sweetener and an important ingredient in Chinese medicine. Pure Appl. Chem. 2002, 74, 1189–1198. [Google Scholar] [CrossRef]
- Pietri, A.; Rastelli, S.; Terenzio Bertuzzi, T. Ochratoxin A and aflatoxins in liquorice products. Toxins 2010, 2, 758–770. [Google Scholar] [CrossRef]
- Schrenk, D.; Bodin, L.; Chipman, J.K.D.; Mazoc, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.-C.; Nebbia, C.S.; Nielsen, E.; et al. Risk assessment of ochratoxin A in food. EFSA J. 2020, 18, 6113. [Google Scholar]
- Gil-Serna, J.; González-Salgado, A.; González-Jaén, M.A.; Vázquez, C.; Patiño, B. ITS-based detection and quantification of Aspergillus ochraceus and Aspergillus westerdijkiae in grapes and green coffee beans by real-time quantitative PCR. Int. J. Food Microbiol. 2009, 131, 162–167. [Google Scholar] [CrossRef]
- Davis, N.; Searcy, J.; Diener, U. Production of ochratoxin A by Aspergillus ochraceus in a semisynthetic medium. Appl. Microbiol. 1969, 17, 742–744. [Google Scholar] [CrossRef]
- Heussner, A.H.; Bingle, L.E.H. Comparative ochratoxin toxicity: A review of the available data. Toxins 2015, 7, 4253–4282. [Google Scholar] [CrossRef]
- Remiro, R.; Ibáñez-Vea, M.; González-Peñas, E.; Lizarraga, E. Validation of a liquid chromatography method for the simultaneous quantification of ochratoxin A and its analogues in red wines. J. Chromatogr. A 2010, 1217, 8249–8256. [Google Scholar] [CrossRef] [PubMed]
- Parussoloa, G.; Oliveirab, M.S.; Garciaa, M.V.; Bernardia, A.O.; Lemosa, J.G.; Stefanelloa, A.; Mallmannb, C.A.; Copetti, M.V. Ochratoxin A production by Aspergillus westerdijkiae in Italian-type salami. Food Microbiol. 2019, 83, 134–140. [Google Scholar] [CrossRef]
- Coton, M.; Auffret, A.; Poirier, E.; Debaets, S.; Coton, E.; Dantigny, P. Production and migration of ochratoxin A and citrinin in Comté cheese by an isolate of Penicillium verrucosum selected among Penicillium spp. mycotoxin producers in YES medium. Food Microbiol. 2019, 82, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Szulc, J.; Ruman, T. Laser ablation remote-electrospray ionisation mass spectrometry (LARESI MSI) imaging-new method for detection and spatial localization of metabolites and mycotoxins produced by moulds. Toxins 2020, 12, 720. [Google Scholar] [CrossRef]
- Hickert, S.; Cramer, B.; Letzel, M.C.; Humpf, H.-U. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry imaging of ochratoxin A and fumonisins in mold-infected food. Rapid Commun. Mass Spectrom. 2016, 30, 2508–2516. [Google Scholar] [CrossRef]
- Bani, M.; Pérez-De-Luque, A.; Rubiales, D.; Rispail, N. Physical and chemical barriers in root tissues contribute to quantitative resistance to Fusarium oxysporum f. sp. pisi in Pea. Front. Plant Sci. 2018, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Gupta, V.K.; Luqman, S.; Negi, A.S.; Kumar, J.K.; Shanker, K.; Saikia, D.; Srivastava, S.; Darokar, M.P.; Khanuja, P.S. Antifungal activity of Glycyrrhiza glabra extracts and its active constituent glabridin. Phytother. Res. 2009, 23, 1190–1193. [Google Scholar] [CrossRef]
- Teixeira, R.T. Review: Cork development: What lies within. Plants 2022, 11, 2671. [Google Scholar] [CrossRef]


| Herb | Country | Mycotoxin | Concentration (μg/kg) | Fungi | Year | Ref. |
|---|---|---|---|---|---|---|
| Plant food supplements: 1. Centella Asiatica (L.) Urb., 2. Hippocratea indica Willd., Piper nigrum L., Trachyspermum ammi Trachyspermum ammi (L.) Sprague, Quercus infectoria Olivier, Labisia pumillia (Blume) Fern.-Vill., 3. Phoenix dactylifera L., Nigella sativa L., Piper betle L., Crocus sativus L., and 4. Punica granatum L., Zingiber officinale Roscoe, Quercus infectoria Olivier. Elephantopus scaber L., Plectranthus L., Labisia pumila (Blume) Fern.-Vill. | Malaysia | AFB1 | 5.905–13.941 | - | 2023 | [13] |
| typhoid herbs: Bark of Enantia chlorantha Oliv., Sarcocephalus latifolium (Sm.) E.A. Bruce, Garcinia kola Heckel and Cocos nucifera L. | Nigeria | Total AFs | ≤7600.0 | A. flavus | 2023 | [14] |
| Codonopsis radix, Scutellariae radix (the dried roots of Scutellaria baicalensis Georgi) and Tremella fuciformis Berk. | China | AFB1 OTA | 0.9–3.05 37.0–515.0 | A. flavus and Penicillium spp. | 2020 | [9] |
| Harmbstaedti aodorata (Burch.) T. Cooke, Vachellia karroo (Hayne) Banfi & Galasso, and Cyperus rotundus L. | South Africa | Total AFs OTA | 2.0–31.46 2.4–10.09 | - | 2020 | [15] |
| Ziziphus spp. | Zambia | Total AFs | ND-24.4 | A. parasiticus and A. flavus | 2019 | [16] |
| Nelumbo nucifera Gaertn. | China | Total AFs | 45.6–275.6 | - | 2019 | [17] |
| Elettaria cardamomum (L.) Maton. | Saudi Arabia | Total AFs OTA | 42.0–164.7 30.0–78.0 | A. flavus, A. parasiticus, A. niger, A. ochraceus and P. verrucosum | 2018 | [18] |
| Matricaria chamomilla L. | Turkey | OTA | 0.034 (below LOD) | - | 2018 | [19] |
| Ziziphus jujuba Mill. | Iraq | Total AFs | 144.0 | A. flavus | 2017 | [20] |
| Ziziphus jujuba Mill. | Thailand | Total AFs | 2.5–6.1 | - | 2017 | [21] |
| Nelumbo nucifera Gaertn. | China | Total AFs | ND- 688.4 | - | 2013 | [22] |
| Salvia officinalis L. | Spain | Total AFs OTA | 23.8–25.2 0.1.1–17.3 | Aspergillus spp., Penicillium spp. | 2009 | [23] |
| Glycyrrhiza sp. | Spain | OTA | ≤152.8 | - | 2007 | [24] |
| Glycyrrhiza sp. | Germany | OTA | 0.3–216.0 | - | 2000 | [25] |
| Zingiber officinale Rosoe, Foeniculum vulgare Miller, and Artemisia absinthium L. | India | AFB1 OTA | 25.0–160.0 20.0–80.0 | A. flavus, A. parasiticus, A. niger, and P. viridicatum | 1998 | [26] |
| Phyllanthus emblica L., and Asparagus racemosus Willd. | India | AFB1 | 280.0–2230.0 | A. flavus | 1993 | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elamin, A.; Sakuda, S. Mechanism of Mycotoxin Contamination of Medicinal Herbs. Toxins 2025, 17, 139. https://doi.org/10.3390/toxins17030139
Elamin A, Sakuda S. Mechanism of Mycotoxin Contamination of Medicinal Herbs. Toxins. 2025; 17(3):139. https://doi.org/10.3390/toxins17030139
Chicago/Turabian StyleElamin, Abdelrahman, and Shohei Sakuda. 2025. "Mechanism of Mycotoxin Contamination of Medicinal Herbs" Toxins 17, no. 3: 139. https://doi.org/10.3390/toxins17030139
APA StyleElamin, A., & Sakuda, S. (2025). Mechanism of Mycotoxin Contamination of Medicinal Herbs. Toxins, 17(3), 139. https://doi.org/10.3390/toxins17030139





