Release of Cytokines in the Peritoneal Fluid of C57BL/6 Mice After Bothrops jararaca and Bothrops atrox Venom Injection
Abstract
:1. Introduction
2. Results
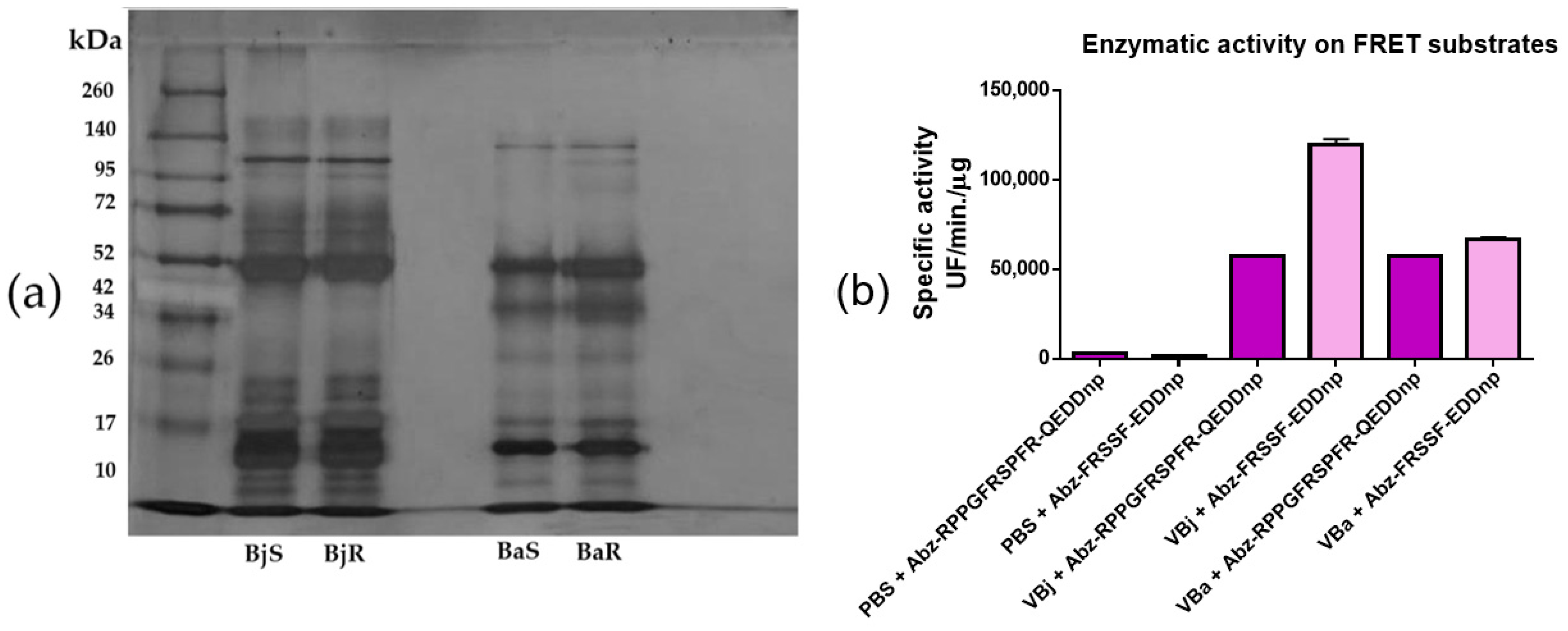
2.1. Evaluation of the Electrophoretic and Enzymatic Profile of Venoms
2.2. Evaluation of Pro- and Anti-Inflammatory Cytokines After 30% of the LD50 of Bothrops jararaca and Bothrops atrox Venoms
2.3. Evaluation of MCPT-1 Levels in Mice Treated with 30% of the LD50 of Bothrops jararaca and Bothrops atrox Venoms Using the ELISA Method
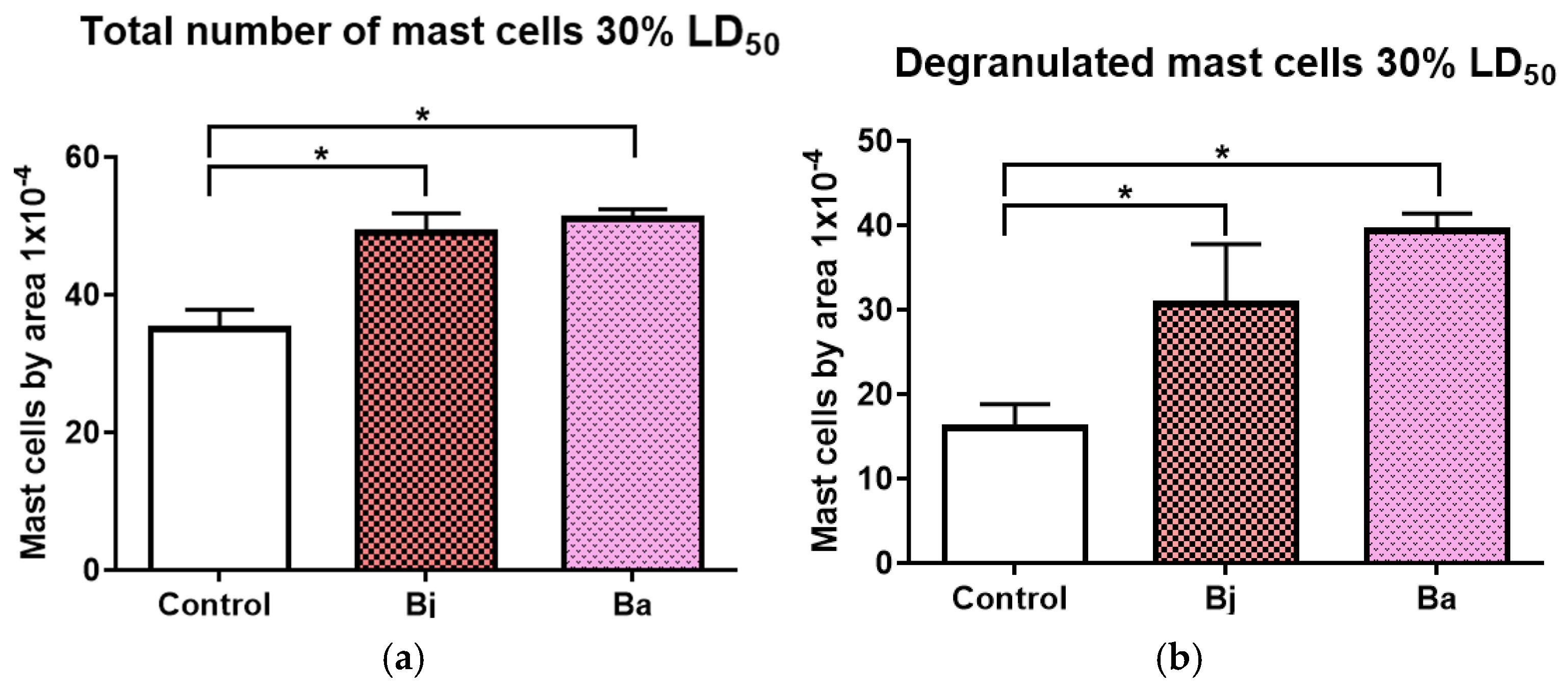
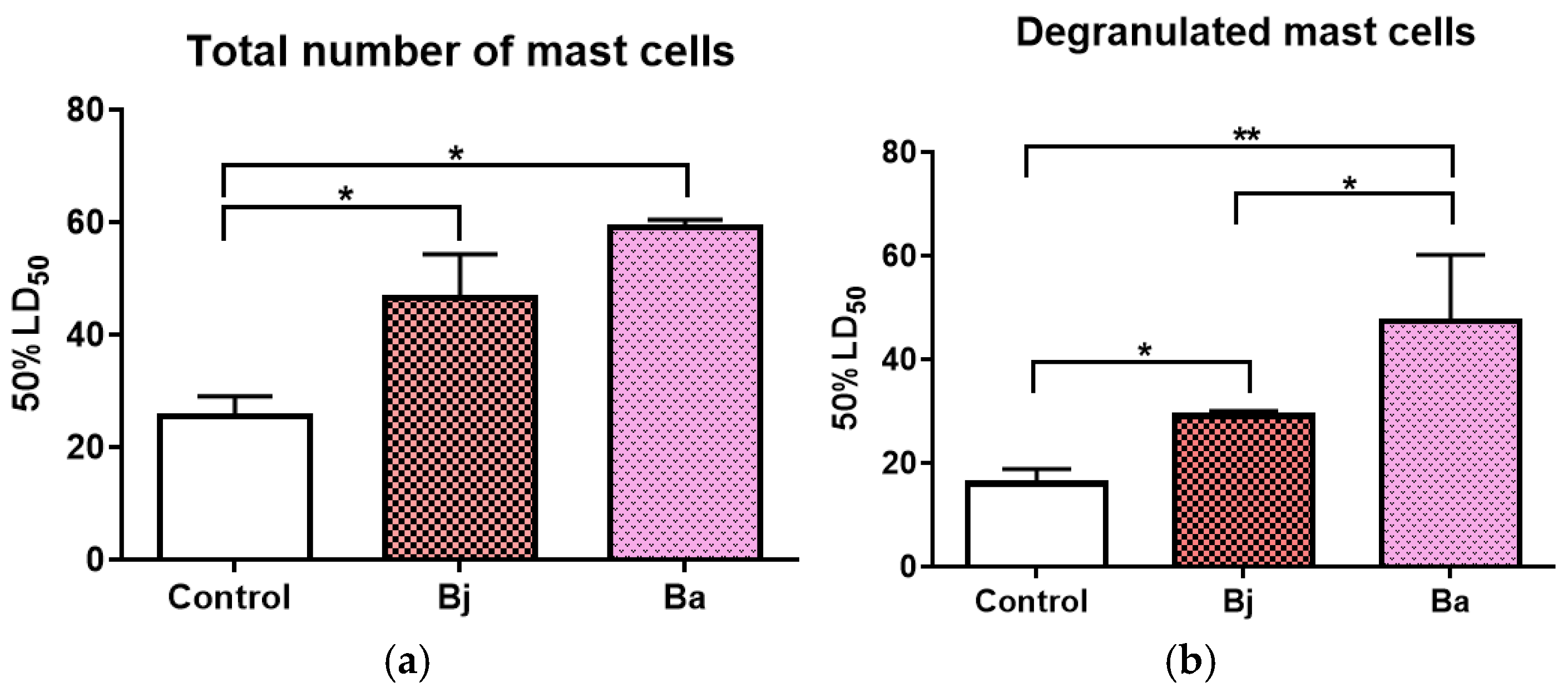
2.4. Mast Cell Activation Caused by 30% of the LD50 of Bothrops jararaca and Bothrops atrox Venoms
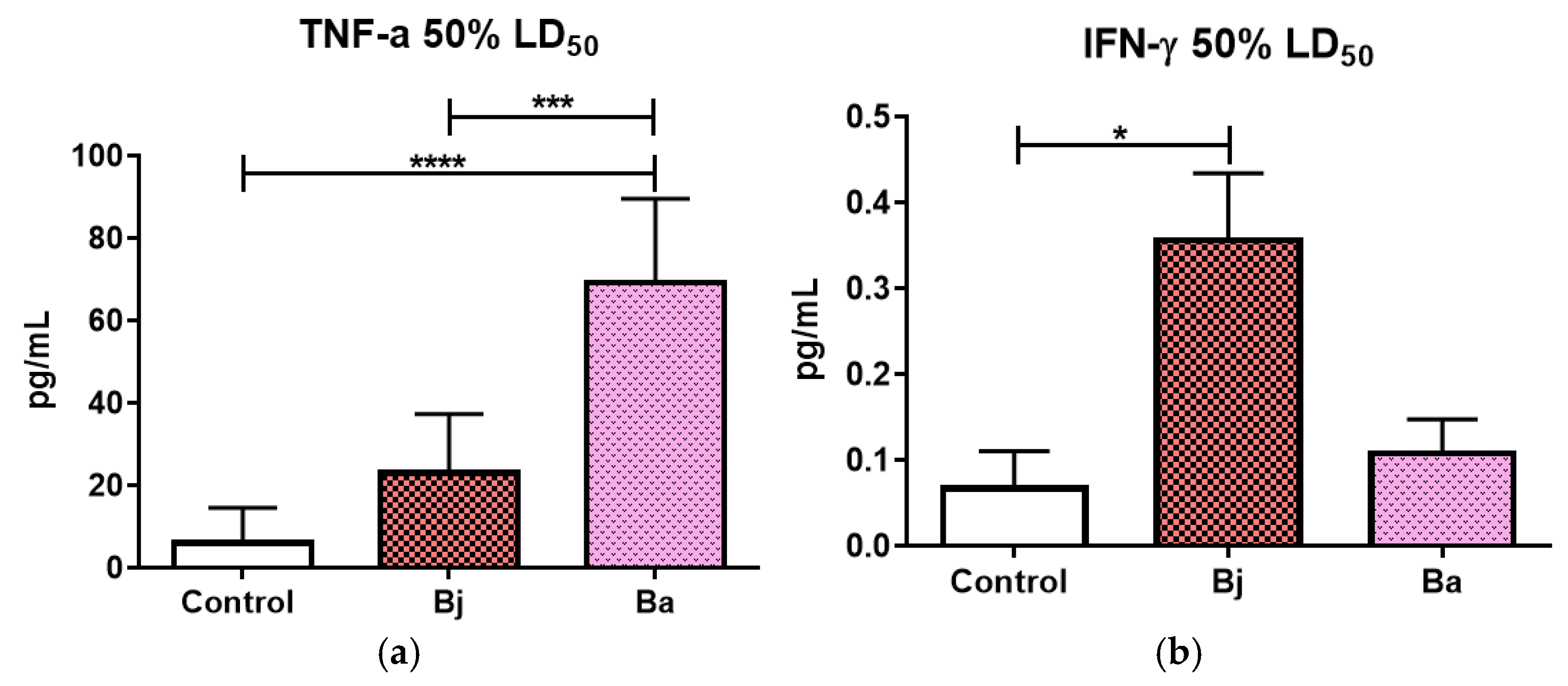
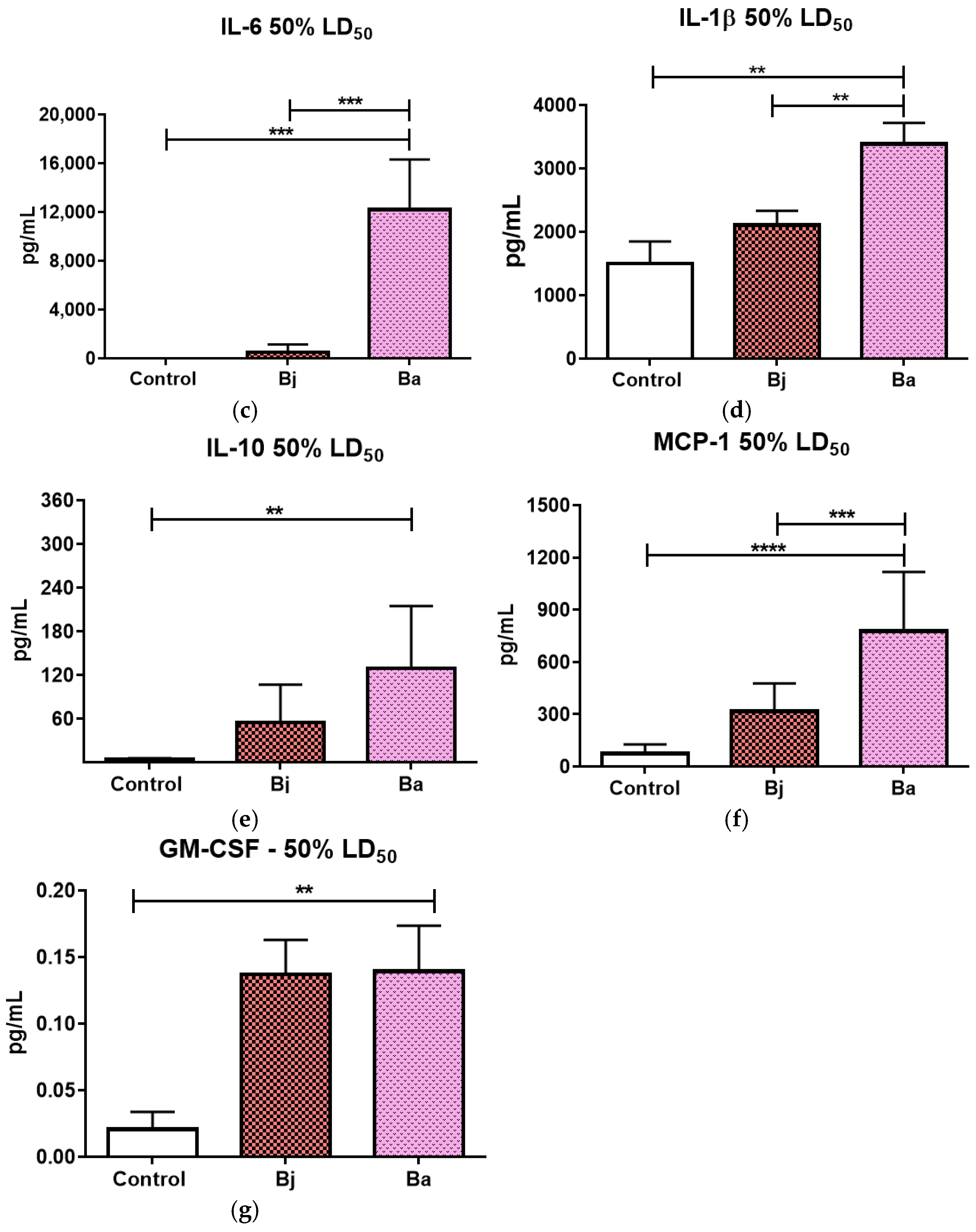
2.5. Evaluation of Pro- and Anti-Inflammatory Cytokines After 50% of the LD50 of Bothrops jararaca and Bothrops atrox Venoms
2.6. Evaluation of MCPT-1 Levels in Mice Treated with Bothropic Venoms Using the ELISA Method
2.7. Mast Cell Activation Caused by 50% of the LD50 of Bothrops jararaca and Bothrops atrox Venoms
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Venoms
5.2. Analysis of the Electrophoretic Profile
5.3. Proteinase Activity Test
5.4. Animals
5.5. Quantification of Chemokine and Inflammatory Cytokines
5.6. Quantification of IL-1β
5.7. Quantification of GM-CSF
5.8. Quantification of MCPT-1
5.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carr, I. The fine structure of the cells of the mouse peritoneum. Z. Für Zellforsch. Und Mikrosk. Anat. 1967, 80, 534–555. [Google Scholar] [CrossRef] [PubMed]
- Farsky, S.H.; Gonçalves, L.R.C.; Gutiérrez, J.M.; Correa, A.P.; Rucavado, A.; Gasque, P.; Tambourgi, D.V. Bothrops asper snake venom and its metalloproteinase BaP-1 activate the complement system. Role in lekocyte recruitment. J. Immunol. 2000, 164, 6533–6541. [Google Scholar]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, 15. [Google Scholar] [CrossRef]
- Teixeira, C.D.F.P.; Landucci, E.C.T.; Antunes, E.; Chacur, M.; Cury, Y. Inflammatory effects of snake venom metalloproteinases. Toxicon 2003, 42, 947–962. [Google Scholar] [PubMed]
- Opal, S.M.; Deppalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef]
- Ettarh, R.R.; Carr, K.E. Ultrastructural observations on the peritoneum in the mouse. J. Anat. 1996, 188, 211–215. [Google Scholar]
- Xing, D.; Feng, W.; Miller, A.P.; Weathington, N.M.; Chen, Y.F.; Novak, L.; Blalock, O. Estrogen modulates TNF-alpha-induced inflammatory responses in rat aortic smooth muscle cells through estrogen receptor-beta activation. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, 2607–2612. [Google Scholar] [CrossRef]
- Kondo, F.V.; Cabrera, W.H.K.; Ribeiro, O.G.; De-Franco, M.; Jensen, J.R.; Picolo, G.; Santa’anna, M.B.; Spafa-dora-Ferreira, M.; Borrego, A.; Ibanêz, O.M.; et al. Pain and Cellular Migration Induced by Bothrops jararaca Venom in Mice Selected for an Acute Inflammatory Response: Involvement of Mast Cells. Front. Immunol. 2022, 12, 779473. [Google Scholar] [CrossRef]
- Cavalcante, T.T.A.; De-Souza, M.B.S.; Neves, J.C.F.; Ibiapina, H.N.S.; Barbosa, F.B.A.; Bentes, K.O.; Alves, E.C.; Marques, H.O.; Colombini, M.; Sampaio, S.V.; et al. Inflammatory Profile Associated with Secondary Infection from Bothrops atrox Snakebites in the Brazilian Amazon. Toxins 2023, 15, 524. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Rucavado, A. Snake venom metalloproteinases: Their role in the pathogenesis of local tissue damage. Biochimie 2000, 82, 841–850. [Google Scholar] [CrossRef]
- Moura-da-Silva, A.M.; Butera, D.; Tanjoni, I. Importance of snake venom metalloproteinases in cell biology: Effects on platelets, in-flammatory and endothelial cells. Curr. Pharm. Des. 2007, 13, 2893–2905. [Google Scholar] [CrossRef]
- Farsky, S.H.; Gonçalves, L.R.; Cury, Y. Characterization of local tissue damage evoked by Bothrops jararaca venom in the rat connective tissue micro-circulation: An intravital microscopic study. Toxicon 1999, 37, 1079–1083. [Google Scholar] [CrossRef]
- Ribeiro, L.A.; Jorge, M.T.; Lebrão, M.L. Prognostic factors for local necrosis in Bothrops jararaca (Brazilian pit viper) bites. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 630–634. [Google Scholar] [CrossRef]
- Patiño, R.S.P.; Salazar-Valenzuela, D.; Medina-Villamizar, E.; Mendes, B.; Proaño-Bolaños, C.; da Silva, S.L.; Almeida, J.R. Bothrops atrox from Ecuado-rian Amazon: Initial analyses of venoms from individuals. Toxicon 2021, 193, 63–72. [Google Scholar] [CrossRef]
- Moreira, V.; Dos-Santos, M.C.; Nascimento, N.G.; Borges da Silva, H.; Fer-nandes, C.M.; D’Império Lima, M.R.; Teixeira, C. Local inflammatory events induced by Bothrops atrox snake venom and the release of distinct clas-ses of inflammatory mediators. Toxicon 2012, 60, 12–20. [Google Scholar] [CrossRef]
- Zamuner, S.R.; Zuliani, J.P.; Fernandes, C.M.; Gutiérrez, J.M.; de Fátima Pereira Teixeira, C. Inflammation induced by Bothrops asper venom: Release of proinflammatory cytokines and eicosanoids, and role of adhesion molecules in leukocyte infiltration. Toxicon 2005, 46, 806–813. [Google Scholar] [CrossRef]
- Da-Silva, R.J.; da Silva, M.G.; Vilela, L.C.; Fecchio, D. Cytokine profile of Ehr-lich ascites tumor treated with Bothrops jararaca venom. Mediat. Inflamm. 2002, 11, 197–201. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Zamuner, S.R.; Zuliani, J.P.; Rucavado, A.; Gutiérrez, J.M.; Teixeira Cde, F. Inflammatory effects of BaP1 a metalloproteinase isolated from Bothrops asper snake venom: Leukocyte recruitment and release of cytokines. Toxicon 2006, 47, 549–559. [Google Scholar] [CrossRef]
- Shivers, K.Y.; Amador, N.; Abrams, L.; Hunter, D.; Jenab, S.; Quiñones-Jenab, V. Estrogen alters baseline and inflammatory-induced cytokine levels in-dependent from hypothalamic-pituitary-adrenal axis activity. Cytokine 2015, 72, 121–129. [Google Scholar] [CrossRef]
- Corcoran, M.P.; Meydani, M.; Lichtenstein, A.H.; Schaefer, E.J.; Dillard, A.; Lamon-Fava, S. Sex hormone modulation of proinflammatory cytokine and C-reactive protein expression in macrophages from older men and postmenopausal women. J. Endocrinol. 2010, 206, 217–224. [Google Scholar] [CrossRef]
- Lomonte, B.; Tarkowski, A.; Hanson, L.A. Host response to Bothrops asper snake venom. Analysis of edema formation, inflammatory cells, and cytokine release in a mouse model. Inflammation 1993, 17, 93–105. [Google Scholar] [CrossRef]
- Moulton, V.R. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front. Immunol. 2018, 9, 2279. [Google Scholar] [CrossRef]
- Harding, A.T.; Heaton, N.S. The Impact of Estrogens and Their Receptors on Immunity and Inflammation during Infection. Cancers 2022, 14, 909. [Google Scholar] [CrossRef]
- Lang, T.J. Estrogen as an immunomodulator. Clin. Immunol. 2004, 113, 224–230. [Google Scholar] [CrossRef]
- Sciarra, F.; Campolo, F.; Franceschini, E.; Carlomagno, F.; Venneri, M.A. Gender-Specific Impact of Sex Hormones on the Immune System. Int. J. Mol. Sci. 2023, 24, 6302. [Google Scholar] [CrossRef]
- Roved, J.; Westerdahl, H.; Hasselquist, D. Sex differences in immune responses: Hormonal effects, antagonistic selection, and evolutionary consequences. Horm. Behav. 2017, 88, 95–105. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Rucavado, A.; Chaves, F.; Díaz, C.; Escalante, T. Experimental pathology of local tissue damage induced by Bothrops asper snake venom. Toxicon 2009, 54, 958–975. [Google Scholar] [CrossRef]
- Bit-tenbinder, M.A.; van Thiel, J.; Cardoso, F.C.; Casewell, N.R.; Gutiérrez, J.M.; Kool, J.; Vonk, F.J. Tissue damaging toxins in snake venoms: Mechanisms of action, pathophysiology and treatment strategies. Commun. Biol. 2024, 7, 358. [Google Scholar] [CrossRef]
- e Silva, M.R.; Beraldo, W.T.; Rosenfeld, G. Bradykinin, a hypotensive and smooth muscle stimulating factor released from plasma globulin by snake venoms and by trypsin. Am. J. Physiol. 1949, 156, 261–273. [Google Scholar] [CrossRef]
- Chaves, F.; Barboza, M.; Gutiérrez, J. Pharmacological study of edema induced by venom of the snake Bothrops asper (terciopelo) in mice. Toxicon 1995, 3, 31–39. [Google Scholar]
- Trebien, H.A.; Calixto, J.B. Pharmacological evaluation of rat paw edema induced by Bothrops jararaca venom. Agents Actions 1989, 26, 292–300. [Google Scholar] [CrossRef]
- Eastmond, N.C.; Banks, E.M.; Coleman, J.W. Nitric oxide inhibits IgE-mediated degranulation of mast cells and is the principal intermediate in IFN-gamma-induced suppression of exocytosis. J. Immunol. 1997, 159, 1444–1450. [Google Scholar]
- Del-Rei, T.H.M.; Sousa, L.F.; Rocha, M.M.; Freitas-de-Sousa, L.A.; Travaglia-Cardoso, S.R.; Grego, K.; Sant’Anna, S.S.; Chalkidis, H.M.; Moura-da-Silva, A.M. Functional variability of Bothrops atrox venoms from three distinct areas across the Brazilian Amazon and consequences for human envenomings. Toxicon 2019, 164, 61–70. [Google Scholar] [CrossRef]
- sano-Martins, I.S.; Santoro, M.L. Distúrbios hemostáticos em envenenamentos por animais peçonhentos no Brasil. Sarvier 2009, 2, 57. [Google Scholar]
- Moura-Da-Silva, A.M.; Contreras-Bernal, J.C.; Gimenes, S.N.C.; Freitas-De-Sousa, L.A.; Portes-Junior, J.A.; da Silva Peixoto, P.; Iwai, L.K.; De Moura, V.M.; Bisneto, P.F.; Lacerda, M.; et al. The relationship between clinics and the venom of the causative Amazon pit viper (Bothrops atrox). PLoS Negl. Trop. Dis. 2020, 14, 325–369. [Google Scholar] [CrossRef]
- Van-Overmeire, E.; Stijlmans, B.; Heymann, F.; Keirsse, J.; Morias, Y.; Elkrim, Y.; Brys, L.; Abels, C.; Lahmar, Q.; Erggen, C.; et al. GM-CSF and GM-CSF Receptor Signaling Differentially Regulate Monocyte Maturation and Macrophage Polarization in the Tumor Microenvironment. Cancer Res. 2016, 76, 35–42. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Roberts, A. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: What we do and don’t know. Cell Res. 2006, 16, 126–133. [Google Scholar] [CrossRef]
- Nagata, K.; Nishiyama, C. IL-10 in Mast Cell-Mediated Immune Responses: Anti- Inflammatory and Proinflammatory Roles. Int. J. Mol. Sci. 2021, 22, 4972. [Google Scholar] [CrossRef]
- Pejler, G.; Maccarana, M. Interaction of heparin with rat mast cell protease 1. J. Biol. Chem. 1961, 269, 14451–14456.70. [Google Scholar] [CrossRef]
- Rivera, J. Snake bites and bee stings: The mast cell strikes back. Nat. Med. 2006, 12, 999–1000. [Google Scholar] [CrossRef]
- Stone, S.F.; Isbister, G.K.; Shahmy, S.; Mohamed, F.; Abeysinghe, C.; Karunathilake, H.; Ariaratnam, A.; Jacoby-Alner, T.E.; Cotterell, C.L.; Brown, S.G. Immune response to snake envenoming and treatment with antivenom; complement activation, cytokine production and mast cell degranulation. PLoS Negl. Trop. Dis. 2013, 7, 2326. [Google Scholar] [CrossRef] [PubMed]
- Alberca, R.W.; Gomes, E.; Moretti, E.H.; Russo, M.; Steiner, A.A. Naturally occurring hypothermia promotes survival in severe anaphylaxis. Immunol. Lett. 2021, 237, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sánchez, F.; Diamond, C.; Zeitler, M.; Gomez, A.I.; Baroja-Mazo, A.; Bagmall, J.; Spiller, D.; White, M.; Daniels, M.J.; Mortellaro, A.; et al. Inflammasome-dependent IL-1β release depends upon membrane permeabilisation. Cell Death Differ. 2016, 23, 1219–1231. [Google Scholar] [CrossRef]
- Coelho, K.F.; Neves, J.C.F.; Ibiapina, H.N.S.; Magalhães-Gama, F.; Barbosa, F.B.A.; Silva, F.S.; Wellmann, I.A.M.; Sachett, J.A.G.; Tarragô, A.M.; Ferreira, L.C.L.; et al. Exploring the Profile of Cell Populations and Soluble Immunological Mediators in Bothrops atrox Envenomations. Toxins 2023, 15, 196. [Google Scholar] [CrossRef] [PubMed]
- Escocard, R.d.C.M.; Kanashiro, M.M.; Petretski, J.H.; Azevedo-Silva, J.; de Carvalho, E.C.Q.; da Silva, W.D.; Kipnis, T.L. Neutrophils regulate the expression of cytokines, chemokines and nitric oxide synthase/nitric oxide in mice injected with Bothrops atrox venom. Immunobiology 2006, 211, 37–46. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Fernandes Ribas, A.; de Godoi, K.S.; Sant’Anna, S.S.; da Rocha, M.M.T.; da Silva, W.D. Release of Cytokines in the Peritoneal Fluid of C57BL/6 Mice After Bothrops jararaca and Bothrops atrox Venom Injection. Toxins 2025, 17, 164. https://doi.org/10.3390/toxins17040164
da Silva Fernandes Ribas A, de Godoi KS, Sant’Anna SS, da Rocha MMT, da Silva WD. Release of Cytokines in the Peritoneal Fluid of C57BL/6 Mice After Bothrops jararaca and Bothrops atrox Venom Injection. Toxins. 2025; 17(4):164. https://doi.org/10.3390/toxins17040164
Chicago/Turabian Styleda Silva Fernandes Ribas, Adriana, Kemily Stephanie de Godoi, Sávio Stefanini Sant’Anna, Marisa Maria Teixeira da Rocha, and Wilmar Dias da Silva. 2025. "Release of Cytokines in the Peritoneal Fluid of C57BL/6 Mice After Bothrops jararaca and Bothrops atrox Venom Injection" Toxins 17, no. 4: 164. https://doi.org/10.3390/toxins17040164
APA Styleda Silva Fernandes Ribas, A., de Godoi, K. S., Sant’Anna, S. S., da Rocha, M. M. T., & da Silva, W. D. (2025). Release of Cytokines in the Peritoneal Fluid of C57BL/6 Mice After Bothrops jararaca and Bothrops atrox Venom Injection. Toxins, 17(4), 164. https://doi.org/10.3390/toxins17040164




