Abstract
Parkinson’s disease (PD) is the second most common neurodegenerative disorder worldwide, affecting over 8.5 million people as of 2019. While standard pharmacological treatments help alleviate symptoms, their long-term use can lead to side effects such as dyskinesia. Bee venom acupuncture (BVA) involves the use of a natural toxin derived from bees that can be used for pain relief and treating neurological disorders. This study aimed to review the efficacy and safety of BVA for the treatment of PD. This review protocol was prospectively registered with PROSPERO (CRD420251000577). We searched eight databases in February 2025 and selected 12 studies involving 215 PD patients treated with BVA. Idiopathic Parkinson’s disease (IPD) is the most common diagnosis. The concentration and dosage per session ranged from 0.03 to 0.1 mg/mL and from 0.1 to 1.0 mL, respectively. Twenty-four different outcome measures were used, with the Unified PD Rating Scale employed in 91.7% of the studies. All studies reported improvements in outcomes. Mild adverse effects such as swelling and itching were noted in four studies (33.3%); however, no severe reactions such as anaphylactic shock occurred. These findings suggest that BVA has the potential for broader clinical applications in the treatment of PD.
Key Contribution:
This study reviewed and summarized clinical studies applying bee venom acupuncture for Parkinson’s disease. Bee venom acupuncture may be beneficial in improving symptoms of Parkinson’s disease.
1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder globally, affecting over 8.5 million individuals as of 2019 [1]. In the United States, approximately one million people live with PD, and ~90,000 new cases are diagnosed annually [2].
Standard PD treatment strategy involves medications, such as levodopa, dopamine agonists, and MAO-B (Monoamine Oxidase B) inhibitors [1]. However, long-term use of these pharmacotherapies can lead to motor complications and dyskinesia, and some patients may experience insufficient symptom control or adverse effects that necessitate discontinuation.
Recent studies have explored alternative therapies, including acupuncture and bee venom acupuncture (BVA), as adjunctive treatments for PD. BVA is a widely used animal-derived toxin that is particularly common in East Asian countries, such as Korea, where it is processed into injectable forms [3]. Although it has demonstrated strong analgesic and anti-inflammatory effects and is widely applied under various conditions [4,5], there is a risk of anaphylaxis, a potentially fatal allergic reaction [6].
A pilot study demonstrated that both acupuncture and BVA showed promising results in improving motor function and quality of life in patients with PD [7]. Another study suggested that BVA may exert neuroprotective effects owing to its anti-inflammatory properties [8]. A previous systematic review examined the effects of acupuncture on idiopathic Parkinson’s disease (IPD) [9]. However, only three studies were included, one of which included BVA; it was 8 years old and needed to be updated. Therefore, this review aimed to assess whether bee venom injection is an effective and safe treatment for managing Parkinson’s disease.
2. Results
2.1. Study Description
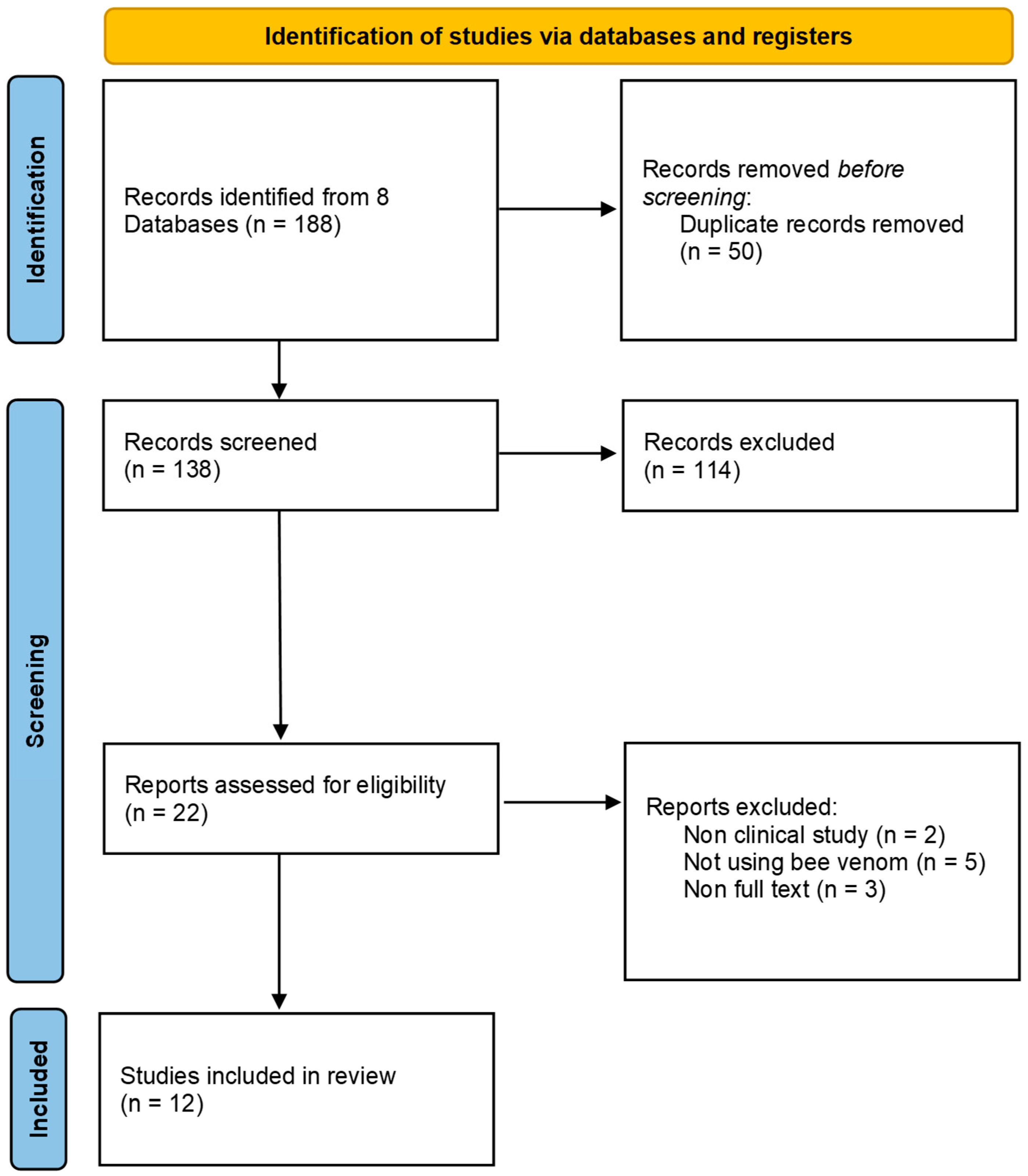
We selected 12 studies [7,8,9,10,11,12,13,14,15,16,17,18] that met our inclusion criteria (Figure 1 and Table 1).
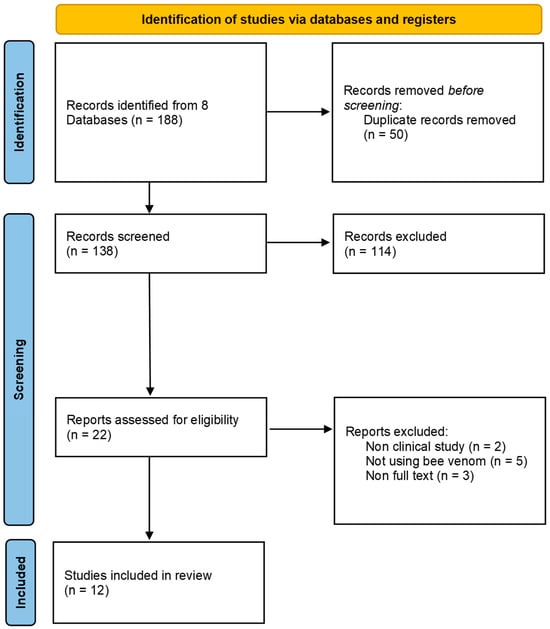
Figure 1.
PRISMA flow chart.

Table 1.
Characteristics of included clinical studies.
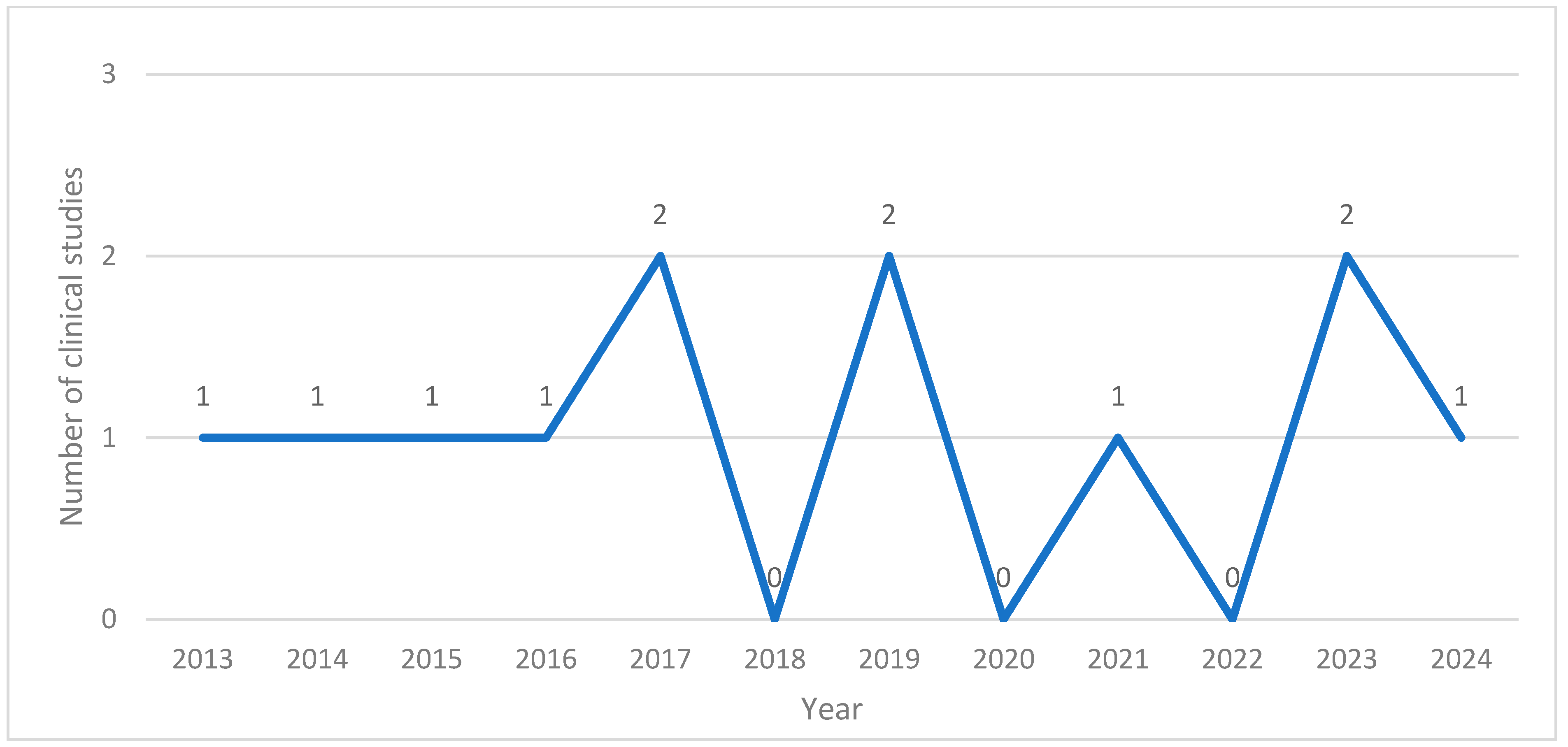
The application of BVA in research in South Korea was first documented in 2013. Between 2013 and 2024, the annual number of related publications ranged from none to two. Notably, no studies were published in 2018, 2020, or 2022. There were six case reports [12,13,15,16,17,18], one retrospective analysis [11], one prospective open-label investigation [10], and four randomized controlled trials (RCTs) [7,8,14,19] (Figure 2).
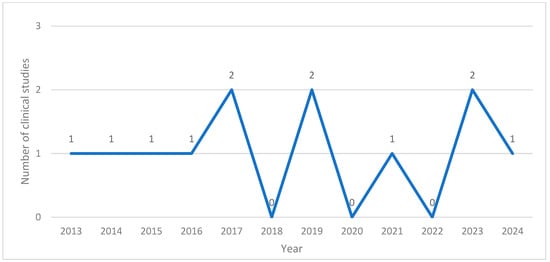
Figure 2.
Number of clinical studies published per year.
2.2. Medical Conditions
Twelve distinct clinical conditions were identified in 12 reviewed studies. They were grouped into three categories: IPD, PD with specific clinical characteristics, and secondary forms of Parkinsonism. IPD was the most explored condition, occurring in nine studies (75.0%) [8,9,10,12,13,14,15,18,19]. Two studies (16.7%) specifically focused on PD with additional features, such as postural instability, gait impairment, and camptocormia [11,16]. Secondary Parkinsonism, which includes conditions such as drug-induced Parkinsonism, was the subject of only one study (8.3%) [17].
2.3. Sample Size
A total of 215 participants were involved in the 12 articles. Sample size varied significantly between the studies, with the smallest involving a single participant and the largest including 63 patients.
2.4. Overview of BVA Treatment
BVA therapy was delivered via syringe-based injections into specific acupuncture points, with the concentration of the solution tailored according to the type of Parkinsonian condition. For individuals diagnosed with IPD, BV concentration ranges from 0.05 to 0.1 mg/mL. In these cases, the volume administered per session varied from 0.1 to 1.0 mL, with the cumulative dosage across sessions falling between 1.1 and 24 mL. In patients presenting with PD accompanied by distinct symptoms, such as postural instability or camptocormia, a standardized concentration of 0.05 mg/mL was used. The per-session volumes ranged from 0.2 to 1.0 mL, and the total administered dose was between 8.4 and 24 mL. For those with drug-induced Parkinsonism, treatment was conducted at lower concentrations, between 0.03 and 0.05 mg/mL. Each session involved a 1.0 mL injection, with the entire course amounting to 19 mL. However, there have been several instances where specific dosage data were not disclosed. In one study that used BVA named ‘eBV’ for PD [13], the venom concentration was not reported. In another case, ref. [10] the per-session dosage was not specified. Furthermore, the total dosage administered was omitted in two studies, one involving general Parkinson’s, and the other in which the number of sessions was not documented [12,13] (Table 2).

Table 2.
BVA concentrations and dosages according to the medical conditions of patients.
2.5. Outcome Measures
Clinical Outcomes
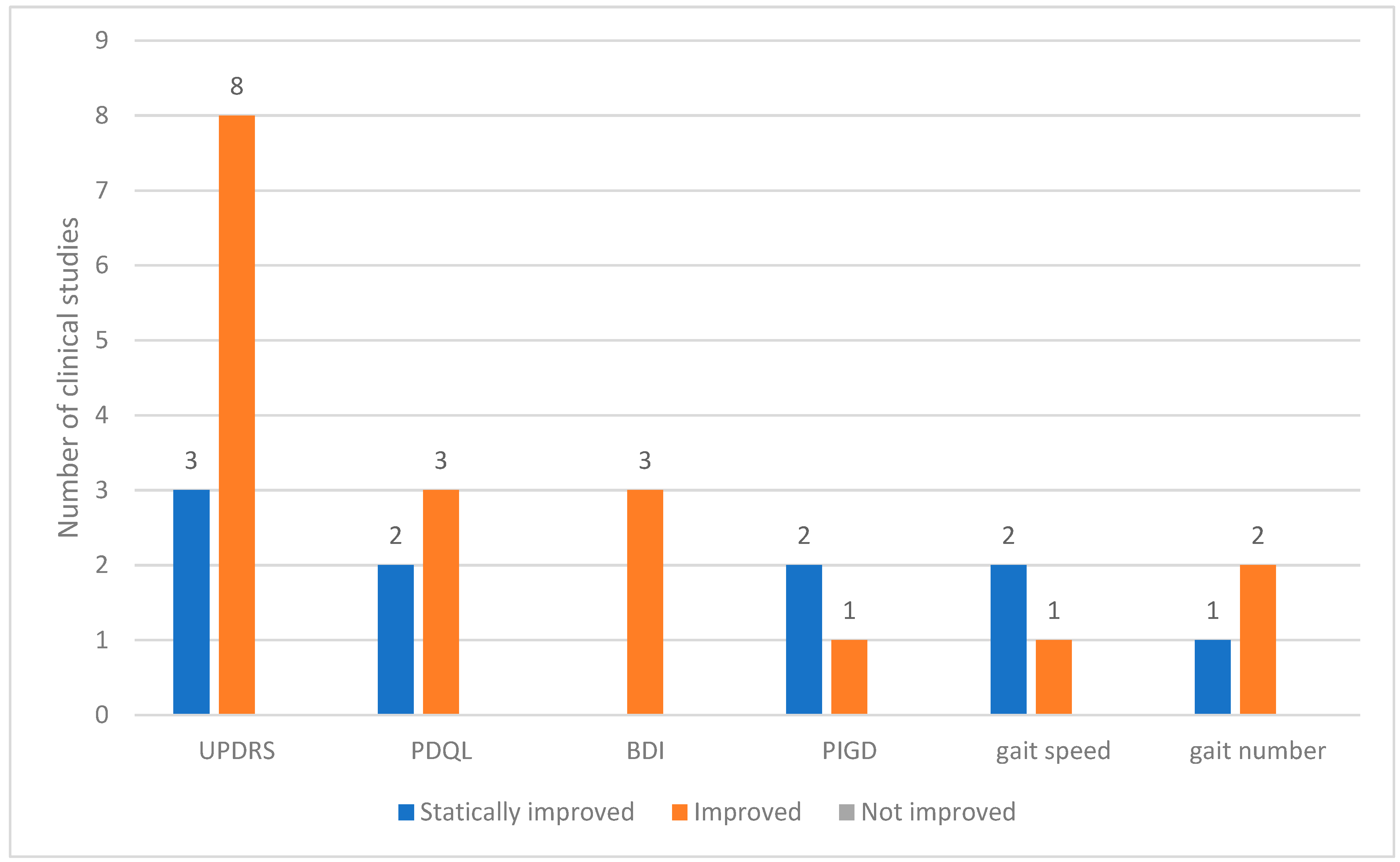
Across the 12 clinical studies reviewed, 24 distinct outcome measures were analyzed. These outcomes were categorized based on the degree of change observed as statistically improved, improved, or not improved. The Unified PD Rating Scale (UPDRS) is a commonly used assessment tool. Among the studies utilizing the UPDRS, eight reported general improvements and three demonstrated statistically significant improvements. Other frequently assessed indicators included the PD quality of life questionnaire (PDQL) and Beck depression inventory (BDI). In all instances in which these tools were applied, the outcomes showed consistent improvements. Measures related to motor function, such as postural instability and gait disorder (PIGD), gait speed, and gait step count, also showed improved results (Figure 3).
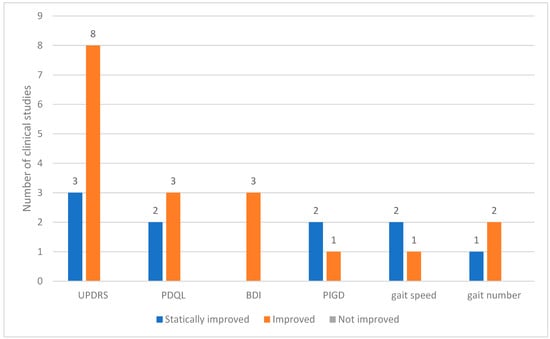
Figure 3.
Outcomes used in clinical studies on bee venom acupuncture for Parkinson’s Disease. Abbreviations: BDI, Beck Depression Inventory; PDQL, Parkinson’s Disease Quality of Life Questionnaire; PIGD, postural instability and gait disorder; UPDRS, Unified Parkinson’s Disease Rating Scale.
2.6. Summary of Adverse Events
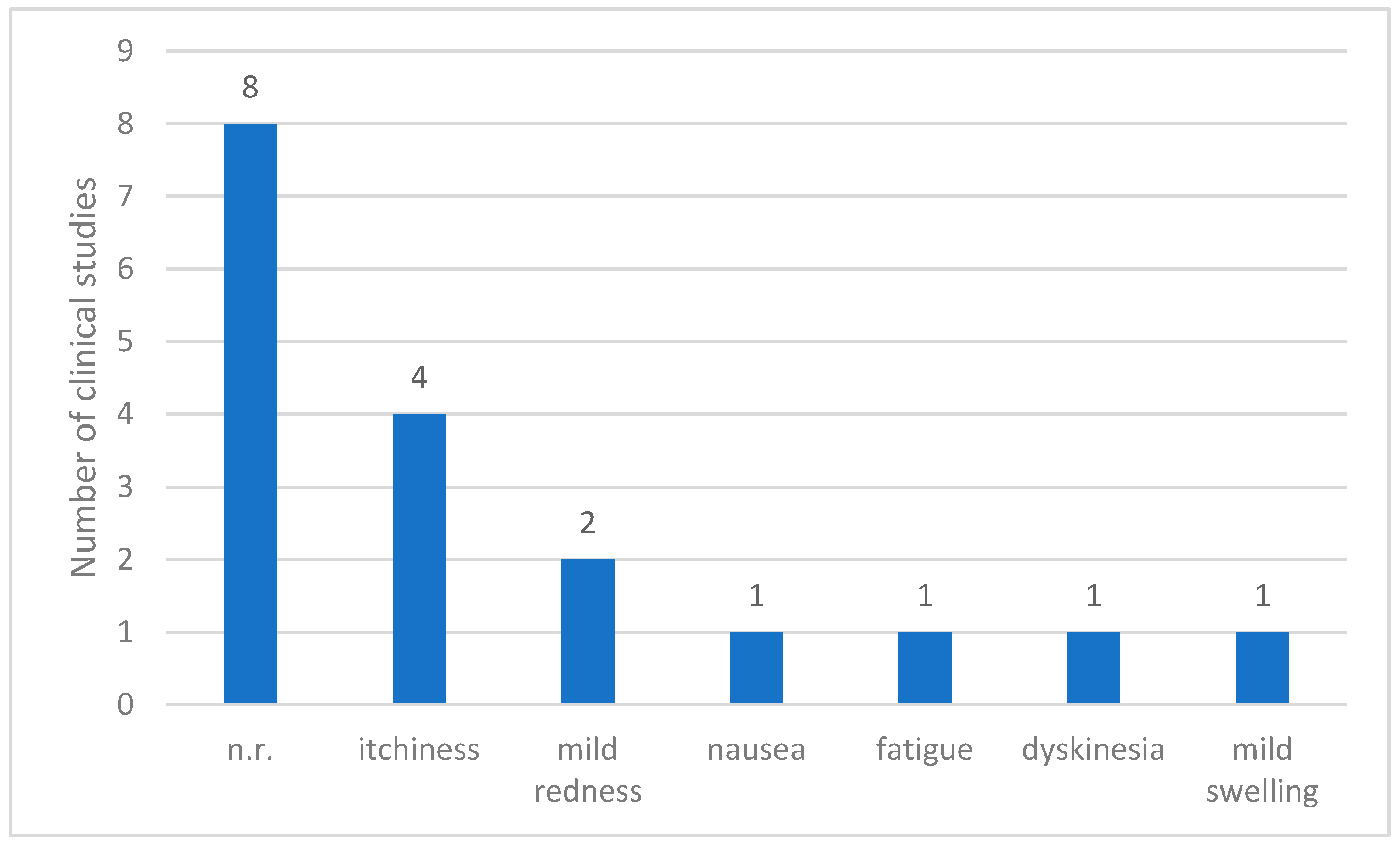
Of the 12 clinical studies reviewed, adverse events were not reported in 8 studies. However, the remaining four studies [7,8,9,10] provided records of the side effects associated with treatment. The most commonly observed adverse reaction was itching, which occurred in four patients. Mild skin redness was noted in two patients, whereas nausea, fatigue, dyskinesia, and mild swelling were reported once. Overall, the reported adverse effects were generally minor and localized. Most adverse events involved temporary irritation or discomfort at the injection site, with no evidence of serious systemic complications (Figure 4).
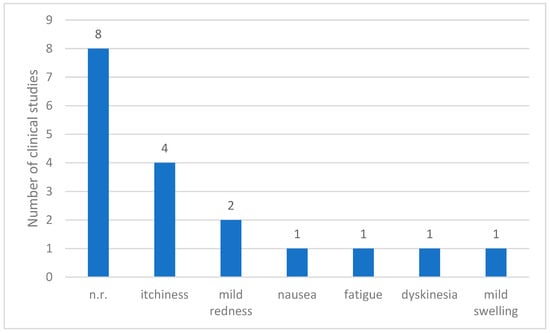
Figure 4.
Reported adverse events in clinical studies on Parkinson’s Disease. Abbreviations: n.r.: not reported.
2.7. Types of Co-Interventions Used in Clinical Studies on PD
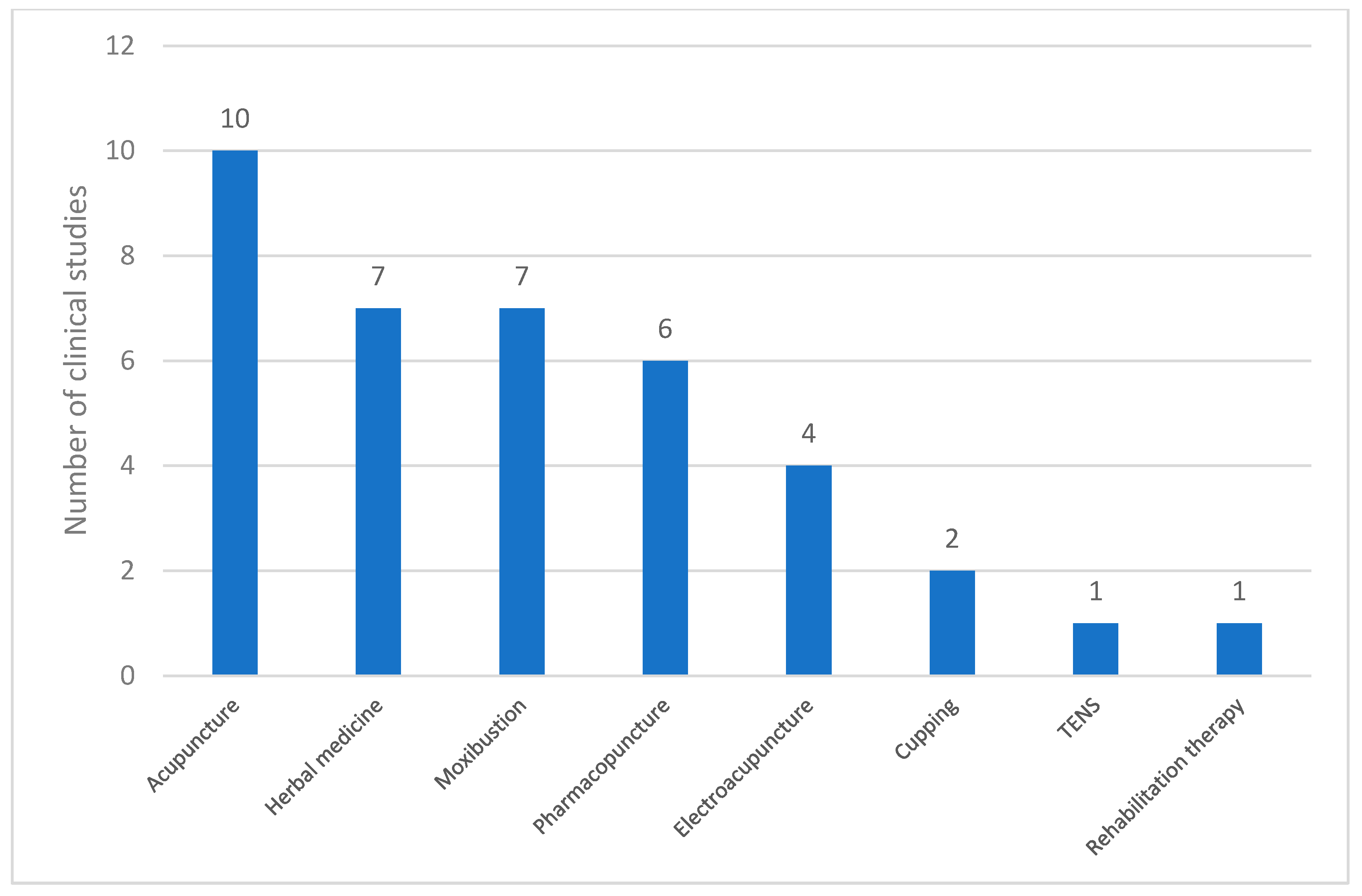
In the 12 studies that incorporated co-interventions, various complementary therapies were used. The data presented in Figure 5 illustrate the frequency of these co-interventions. Acupuncture was the most commonly employed co-intervention (n = 10), followed by herbal medicine (n = 7) and moxibustion (n = 7). Pharmacopuncture (n = 6) and electroacupuncture (n = 4) were frequently used. Less frequently reported interventions included cupping therapy (n = 2), TENS (n = 1), and rehabilitation therapy (n = 1). These findings indicate that acupuncture-based therapies were the predominant co-interventions in the analyzed studies, and other techniques, such as herbal medicine and moxibustion, also played a significant role.
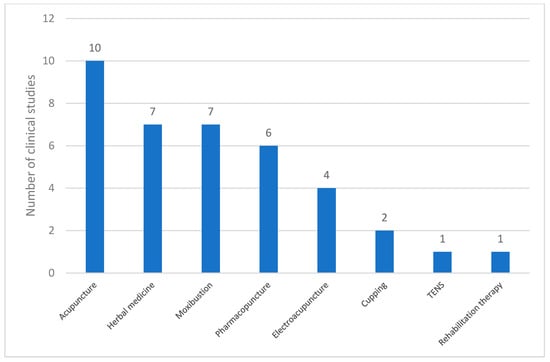
Figure 5.
Types of co-interventions used in clinical studies on PD. Abbreviations: TENS, transcutaneous electrical nerve stimulation.
3. Discussion
BVA is an alternative therapy that combines traditional acupuncture techniques with the injection of purified, diluted bee venom into specific acupuncture points. The main component of BVA is melittin, along with allergenic substances such as protease inhibitors and peptides [20]. While these components may have therapeutic effects, they can also cause side effects and allergic reactions [21]. Although anaphylactic reactions are rare, they can still occur and may lead to fatal outcomes [6].
PD is a debilitating neurodegenerative disease. Despite the availability of pharmacological treatments, they are not effective in all patients and may cause significant adverse effects. Consequently, alternative treatment options such as BVA have been explored for PD management. This study aimed to evaluate the applicability and safety of BVA for the treatment of PD.
The application of BVA in PD has been steadily published since 2013, in contrast to studies applying BVA to other conditions that began in the early 2000s [4,5,22]. A review of BVA in PD animal models noted a marked increase in studies since 2010, with more than 20 papers published annually and more than 50 published by 2016 [23]. Although BVA has traditionally been used for its anti-inflammatory and analgesic effects in joint and pain-related conditions, its application is now expanding to neurological diseases such as PD.
Among the 12 studies reviewed, 4 were RCTs, while the remaining included case reports (6), one retrospective analysis, and one prospective open-label investigation. Notably, RCTs will continue until 2023, indicating an ongoing effort to establish higher levels of evidence for the use of BVA in PD.
In the PD, the BVA concentration generally ranged from 0.03 to 0.1 mg/mL. While some studies did not specify the exact concentration used per session, the manufacturers (e.g., eBV made by Jaseng) did not disclose the precise dilution ratio. Compared to concentrations used for neck pain (0.05–0.5 mg/mL) and shoulder pain (0.005–1.0 mg/mL), BVA for PD is typically used at concentrations from 5 to 10 times lower. This is likely because most BVA indications involve musculoskeletal conditions, in which high-concentration injections directly stimulate the affected muscles or ligaments. In contrast, PD is a neurological condition. Therefore, the therapeutic focus is on stimulating specific acupuncture points using a standard clinical concentration, typically approximately 0.05 mg/mL.
The volume per session ranged from 0.2 to 1.0 mL, which is comparable to dosages used in neck pain (0.1–1.0 mL) and shoulder pain (0.1–2.0 mL). In Korean clinical practice, practitioners often use insulin syringes to draw insulin from BVA vials, with a maximum volume of 1.0 mL per treatment. As injections are distributed across multiple acupuncture points, the volume per point is typically approximately 0.1 mL.
Twenty-four outcome measures were used in the twelve included studies. Except for one study, the remaining 11 used the UPDRS, and all reported improvements. Other commonly used tools include the PDQL, BDI, and PIGD scores, and gait parameters such as speed and step count.
The UPDRS [24] is a comprehensive tool for assessing both motor and non-motor symptoms of PD and comprises four parts. Part 3 of UPDRS evaluated motor functions, including tremor, rigidity, and balance. Most studies focused on parts 2 and 3, whereas only five studies [7,8,12,13,14] used the full version, including parts 1 and 4. Notably, all were RCTs except for two studies [12,13] that were RCTs. Given the length and complexity of the full UPDRS, it is likely that partial versions were used in case-based studies to reduce evaluation time.
The PDQL [25] was employed to assess the quality of life in patients with PD, and a similar tool, the PDQ-39, was used in one study [8]. These instruments are important because patients with PD generally report a poorer physical and mental quality of life than healthy controls [26]. Since PD remains incurable, improving the overall quality of life may be a more realistic treatment goal than symptom relief alone. Studies on consensus outcome measures for PD recommend the MDS-UPDRS as the standard tool for both motor and non-motor assessments and suggest using the shortened PDQ-8 version for quality-of-life evaluation [27]. Notably, most studies included in this review used approximately 40-item tools, which were longer than the PDQ-8. The original version was used instead of the shortened PDQ-8, as a longer version of the questionnaire can provide more detailed and comprehensive information, if feasible.
BVA-related adverse effects were reported in four studies. These symptoms were all mild, including itching, burning sensations, and localized pain, with no cases of severe reactions such as anaphylaxis. This may be attributed to the routine use of skin tests before treatment in most studies and the exclusion of participants with positive skin test results from the RCTs.
Except for one study [17], all other studies included the concomitant use of Western medications and other complementary therapies, such as acupuncture, herbal medicine, and moxibustion. While most studies have reported favorable outcomes without serious side effects, the lack of studies specifically focusing on safety remains a limitation.
This review has some limitations. It is unclear whether the absence of adverse event reports in some studies is due to the actual absence of such events or underreporting. Therefore, accurately assessing the safety profile of BVA remains challenging. However, according to a previous meta-analysis on BVA, the estimated incidence of severe reactions, such as anaphylaxis, is relatively low (0.045%) [6]. Of the twelve included studies, six were case reports and four were RCTs. More RCTs are required to improve the level of evidence. Most studies have combined BVA with other treatments and Western medicine, making it difficult to isolate the specific effects of BVA. Several case reports have been published in Korea, where BVA is commonly used alongside acupuncture or moxibustion in clinical practice. Therefore, future high-quality RCTs should assess the efficacy of BVA as a standalone therapy.
4. Conclusions
BVA shows promising potential as an adjunct treatment alongside conventional Western medicine for the disease. Large-scale multicenter trials and randomized controlled studies are warranted to further validate its efficacy and safety.
5. Materials and Methods
5.1. Search Methods for Identification of Studies
This systematic review was registered in the PROSPERO International Prospective Register of Systematic Reviews (registration number: CRD420251000577). The following databases were searched from their inception until February 2025: PubMed, Embase, Cochrane Library, Chinese National Knowledge Infrastructure (CNKI), ScienceON (https://scienceon.kisti.re.kr), KISS (https://kiss.kstudy.com/), RISS (http://www.riss.or.kr), and OASIS (https://oasis.kiom.re.kr). Studies involving human subjects, including RCTs, case reports, and cohort studies on bee venom injection for Parkinson’s disease, will be included without language restrictions. The search strategy for each database is presented in Supplementary File S1.
5.2. Study Selection
5.2.1. Types of Studies
Studies involving human subjects, including RCTs, case reports, and cohort studies on bee venom injections for PD, were included.
5.2.2. Types of Participants
We included trials in which participants were diagnosed with PD. The study population included individuals of all ages, races, and sexes.
5.3. Types of Intervention
5.3.1. Experimental Interventions
The experimental group received BVA acupuncture. BVA involves the injection of diluted bevacizumab into specific acupuncture points associated with PD management.
5.3.2. Control Interventions
The control group received standard Western medical treatment for PD, which may include medications such as levodopa or dopamine agonists. Alternatively, the control group may have received traditional body acupuncture therapy without bee venom. Studies comparing combined BVA and Western medicine versus Western medicine alone were excluded to isolate the effects of BVA.
5.4. Data Collection and Analysis
Data Extraction and Management
Two independent reviewers conducted this study. They assessed the studies for inclusion by reviewing titles, abstracts, and full texts to determine their eligibility based on predefined criteria. Any disagreements or discrepancies between the two reviewers were resolved through discussion or by involving a third reviewer. The entire screening process was documented and presented using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram, which provides a transparent overview of the study selection process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxins17040204/s1.
Author Contributions
Conceptualization, H.J.; investigation, H.J. and K.H.K.; writing—original draft preparation, H.J., and K.H.K.; writing—review and editing, S.-g.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Research Foundation of Korea grant funded by the Korean Government (MSIT) (No. 2020R1A5A2019413).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Atx | Acupuncture |
| BBS | Berg balance scale |
| BDI | Beck depression inventory |
| BREF | Batterie rapide d’évaluation frontale |
| BVA | Bee venom acupuncture |
| C | Control group |
| DCL | Directional control |
| IPD | Idiopathic Parkinson’s disease |
| I | Intervention group |
| KPPS | King’s Parkinson’s disease pain scale |
| LED | Levodopa equivalent dose |
| MMSE | Mini-mental state examination |
| MRI | Magnetic resonance imaging |
| MXE | Maximum excursion |
| n.r | Not reported |
| NRS | Numerical rating scale |
| PD | Parkinson’s disease |
| PDQ-39 | 39-item Parkinson’s disease questionnaire |
| PDQL | Parkinson’s disease quality of life questionnaire |
| PET | Positron emission tomography |
| PIGD | Postural instability and gait disorder |
| RCT | Randomized controlled trial |
| TENS | Transcutaneous electrical nerve stimulation |
| UPDRS | Unified Parkinson’s disease rating scale |
| 123I]-FP-CIT SPECT | Single photon emission computed tomography using [123I]-FP-CIT |
References
- World Health Organization. Parkinson Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/parkinson-disease? (accessed on 24 March 2025).
- Marras, C.; Beck, J.C.; Bower, J.H.; Roberts, E.; Ritz, B.; Ross, G.W.; Abbott, R.D.; Savica, R.; Van Den Eeden, S.K.; Willis, A.W.; et al. Prevalence of Parkinson’s Disease across North America. NPJ Park. Dis. 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.-H.; Kim, J.-W.; Han, J.-E.; Shin, B.-C.; Park, J.-K.; Lee, G. Animal Venom for Medical Usage in Pharmacopuncture in Korean Medicine: Current Status and Clinical Implication. Toxins 2021, 13, 105. [Google Scholar] [CrossRef]
- Jeong, H.; Jang, S.; Park, J.-K.; Kim, K.H.; Park, J.H.; Lee, G.; Sung, S.-H. Bee Venom Acupuncture for Shoulder Pain: A Literature Review of Clinical Studies. Toxins 2024, 16, 501. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.-H.; Lee, H.-J.; Han, J.-E.; Sung, A.D.-M.; Park, M.; Shin, S.; Jeong, H.I.; Jang, S.; Lee, G. Bee Venom Acupuncture for Neck Pain: A Review of the Korean Literature. Toxins 2023, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-H.; Oh, H.-M.; Kwon, D.-Y.; Yang, J.-E.; Kim, B.-J.; Ha, H.-J.; Lim, E.-J.; Oh, M.-S.; Son, C.-G.; Lee, E.-J. Incidence Rate of Bee Venom Acupuncture Related Anaphylaxis: A Systematic Review. Toxins 2022, 14, 238. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Shim, S.-R.; Rhee, H.Y.; Park, H.-J.; Jung, W.-S.; Moon, S.-K.; Park, J.-M.; Ko, C.-N.; Cho, K.-H.; Park, S.-U. Effectiveness of Acupuncture and Bee Venom Acupuncture in Idiopathic Parkinson’s Disease. Park. Relat. Disord. 2012, 18, 948–952. [Google Scholar] [CrossRef]
- Hartmann, A.; Müllner, J.; Meier, N.; Hesekamp, H.; van Meerbeeck, P.; Habert, M.-O.; Kas, A.; Tanguy, M.-L.; Mazmanian, M.; Oya, H.; et al. Bee Venom for the Treatment of Parkinson Disease—A Randomized Controlled Clinical Trial. PLoS ONE 2016, 11, e0158235. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, T.-H.; Jung, W.-S.; Moon, S.-K.; Ko, C.-N.; Cho, S.-Y.; Jeon, C.-Y.; Choi, T.Y.; Lee, M.S.; Lee, S.-H.; et al. Pharmacoacupuncture for Idiopathic Parkinson’s Disease: A Systematic Review of Randomized Controlled Trials. Evid. Based Complement. Altern. Med. 2018, 2018, 3671542. [Google Scholar] [CrossRef]
- Doo, K.-H.; Lee, J.-H.; Cho, S.-Y.; Jung, W.-S.; Moon, S.-K.; Park, J.-M.; Ko, C.-N.; Kim, H.; Park, H.-J.; Park, S.-U. A Prospective Open-Label Study of Combined Treatment for Idiopathic Parkinson’s Disease Using Acupuncture and Bee Venom Acupuncture as an Adjunctive Treatment. J. Altern. Complement. Med. 2015, 21, 598–603. [Google Scholar] [CrossRef]
- Yang, S.-B.; Kim, Y.-J.; Lee, H.-M.; Lee, S.-H.; Cho, S.-Y.; Park, J.-M.; Ko, C.-N.; Park, S.-U. Effects of Korean Medicine on Postural Instability and Gait Difficulty in Patient with Parkinsonism: Retrospective Study. J. Korean Med. 2017, 38, 96–102. [Google Scholar] [CrossRef]
- Lee, H.; Hwang, Y.; Lee, K.; Kim, D.; Cho, S.; Park, J.; Ko, C.; Park, S. Two Cases of Korean Medicine Treatment for Patients with Parkinson’s Disease Evaluated Using a Three-Dimensional Gait Analysis System. J. Intern. Korean Med. 2023, 44, 774–790. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, D.-H.; Kang, M.S.; Song, H.-S. Drug-Induced Gastrointestinal Dysfunction in Parkinson’s Disease: Treatment with Korean Medicine. J. Acupunct. Res. 2019, 36, 113–117. [Google Scholar] [CrossRef]
- Lee, Y.-E.; Cho, S.-Y.; Lee, H.-G.; Kwon, S.; Jung, W.-S.; Moon, S.-K.; Park, J.-M.; Ko, C.-N.; Park, S.-U. Neuroimaging Assessment of the Therapeutic Mechanism of Acupuncture and Bee Venom Acupuncture in Patients with Idiopathic Parkinson’s Disease: A Double-Blind Randomized Controlled Trial. J. Korean Med. 2023, 44, 104–120. [Google Scholar] [CrossRef]
- Kwak, M.; Kim, D.; Lee, K.; Cho, S.; Park, J.; Ko, C.; Park, S. A Case Report of Korean Medicine in the Treatment of Idiopathic Parkinson’s Disease with Chronic Pain and Gait Disturbance. J. Intern. Korean Med. 2024, 45, 839–849. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, H.; Shin, H.; Choi, J.; Yang, S.; Cho, S.; Park, J.; Ko, C.; Park, S. A Case of Korean Medical Treatment on Parkinson’s Disease Patient with Postural Instability, Presenting as Camptocormia. J. Intern. Korean Med. 2019, 40, 220–227. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S.; Jun, G.; Hwang, Y.; Cho, S.; Park, J.; Ko, C.; Park, S. A Case Report of Persistent Drug-Induced Parkinsonism After Drug Discontinuation. J. Intern. Korean Med. 2021, 42, 1356–1365. [Google Scholar] [CrossRef]
- Lee, Y.-E.; Lee, D.-H.; Lee, J.-H.; Lu, H.-Y.; Cho, S.-Y.; Park, J.-M.; Ko, C.-N.; Bae, H.-S.; Park, S.-U. Three Case Reports of Postural Instability and Gait Difficulty in Parkinson’s Disease Patients Treated with Korean and Western Medicine. Korean J. Acupunct. 2014, 31, 40–47. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Lee, Y.-E.; Doo, K.-H.; Lee, J.-H.; Jung, W.-S.; Moon, S.-K.; Park, J.-M.; Ko, C.-N.; Kim, H.; Rhee, H.Y.; et al. Efficacy of Combined Treatment with Acupuncture and Bee Venom Acupuncture as an Adjunctive Treatment for Parkinson’s Disease. J. Altern. Complement. Med. 2018, 24, 25–32. [Google Scholar] [CrossRef]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.-M.; Fajloun, Z. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef]
- Yoo, J.; Lee, G. Adverse Events Associated with the Clinical Use of Bee Venom: A Review. Toxins 2022, 14, 562. [Google Scholar] [CrossRef]
- Sung, S.-H.; Lee, G. Bee Venom Acupuncture Effects on Pain and Its Mechanisms: An Updated Review. Toxins 2021, 13, 608. [Google Scholar] [CrossRef] [PubMed]
- Awad, K.; Abushouk, A.I.; AbdelKarim, A.H.; Mohammed, M.; Negida, A.; Shalash, A.S. Bee Venom for the Treatment of Parkinson’s Disease: How Far Is It Possible? Biomed. Pharmacother. 2017, 91, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and Recommendations. Mov. Disord. 2003, 18, 738–750. [Google Scholar] [CrossRef] [PubMed]
- de Boer, A.G.; Wijker, W.; Speelman, J.D.; de Haes, J.C. Quality of Life in Patients with Parkinson’s Disease: Development of a Questionnaire. J. Neurol. Neurosurg. Psychiatry 1996, 61, 70–74. [Google Scholar] [CrossRef]
- Zhao, N.; Yang, Y.; Zhang, L.; Zhang, Q.; Balbuena, L.; Ungvari, G.S.; Zang, Y.-F.; Xiang, Y.-T. Quality of Life in Parkinson’s Disease: A Systematic Review and Meta-Analysis of Comparative Studies. CNS Neurosci. Ther. 2021, 27, 270–279. [Google Scholar] [CrossRef]
- de Roos, P.; Bloem, B.R.; Kelley, T.A.; Antonini, A.; Dodel, R.; Hagell, P.; Marras, C.; Martinez-Martin, P.; Mehta, S.H.; Odin, P.; et al. A Consensus Set of Outcomes for Parkinson’s Disease from the International Consortium for Health Outcomes Measurement. J. Park. Dis. 2017, 7, 533–543. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).