The Uremic Toxins Inorganic Phosphate, Indoxylsulphate, p-Cresylsulphate, and TMAO Induce the Generation of Sulphated Glycosaminoglycans in Aortic Tissue and Vascular Cells via pAKT Signaling: A Missing Link in the “Gut–Matrix Axis”
Abstract
1. Introduction
2. Results
2.1. UTs Increase Contents of Sulphated GAGs (sGAGs) in Rat Aortic Ring Cultures
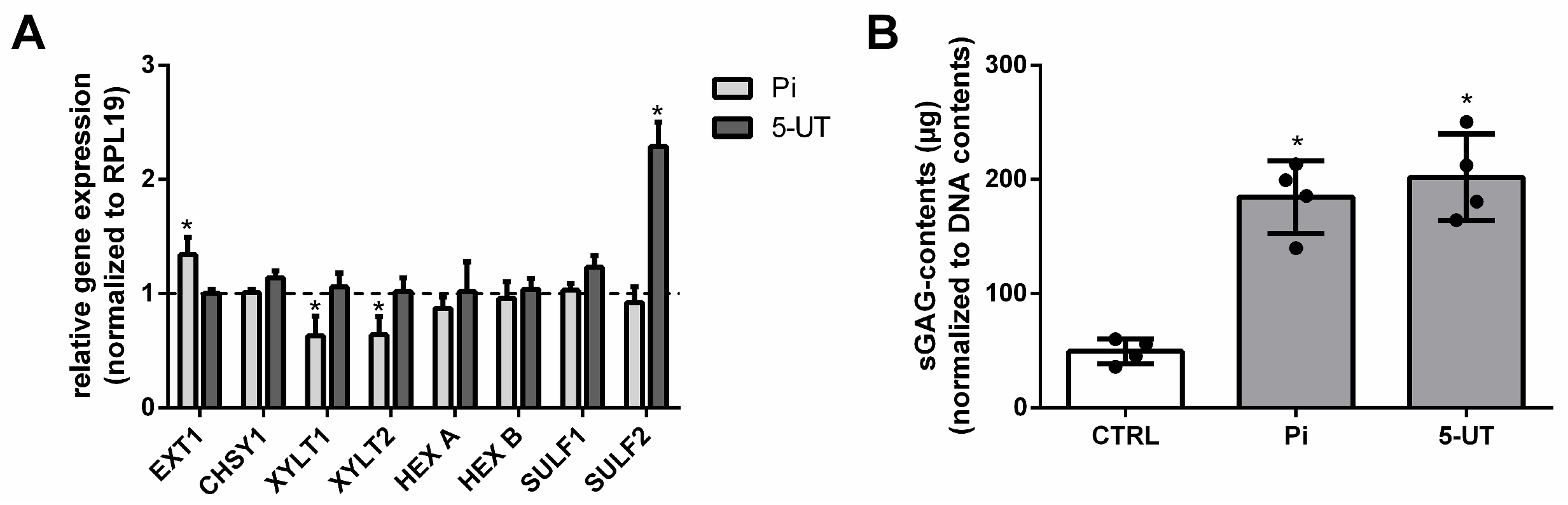
2.2. UTs Modify the Expression of GAG-Specific Genes and Increase Contents of sGAGs in Aortic Tissues
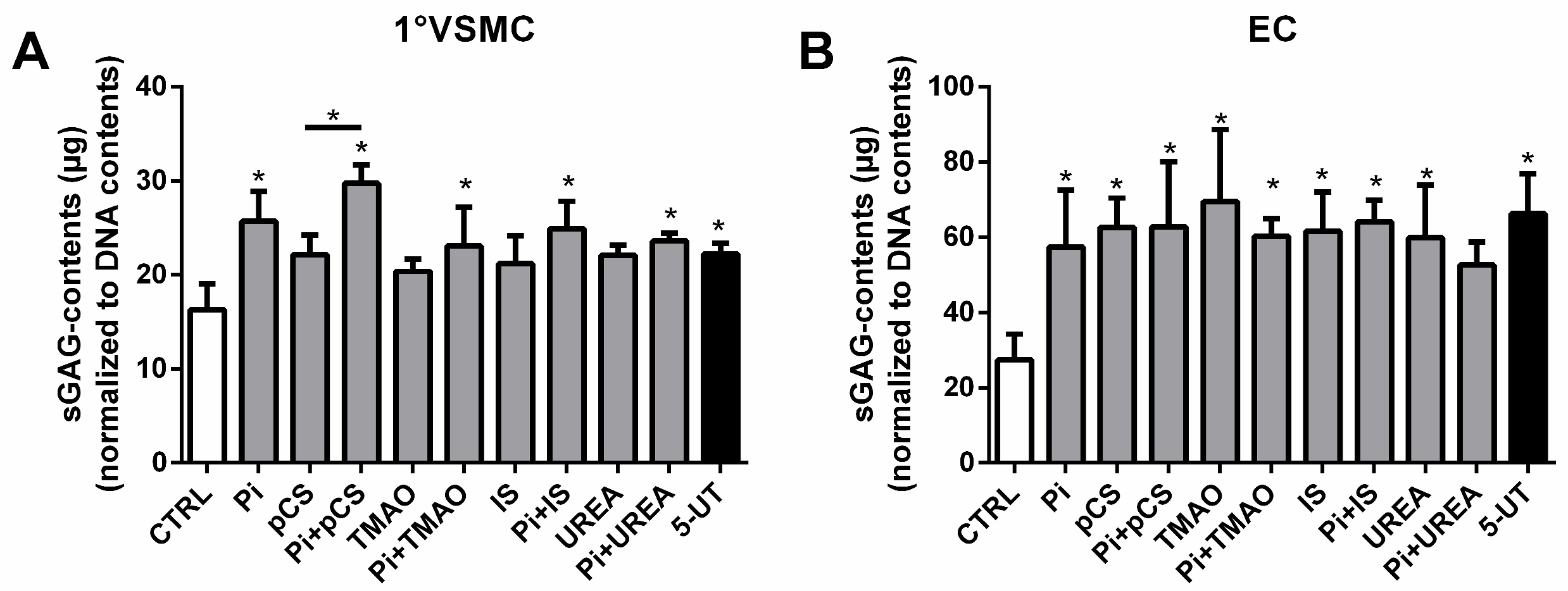
2.3. UTs Increase Contents of sGAGs Also in Vascular Smooth Muscle Cells and Endothelial Cells
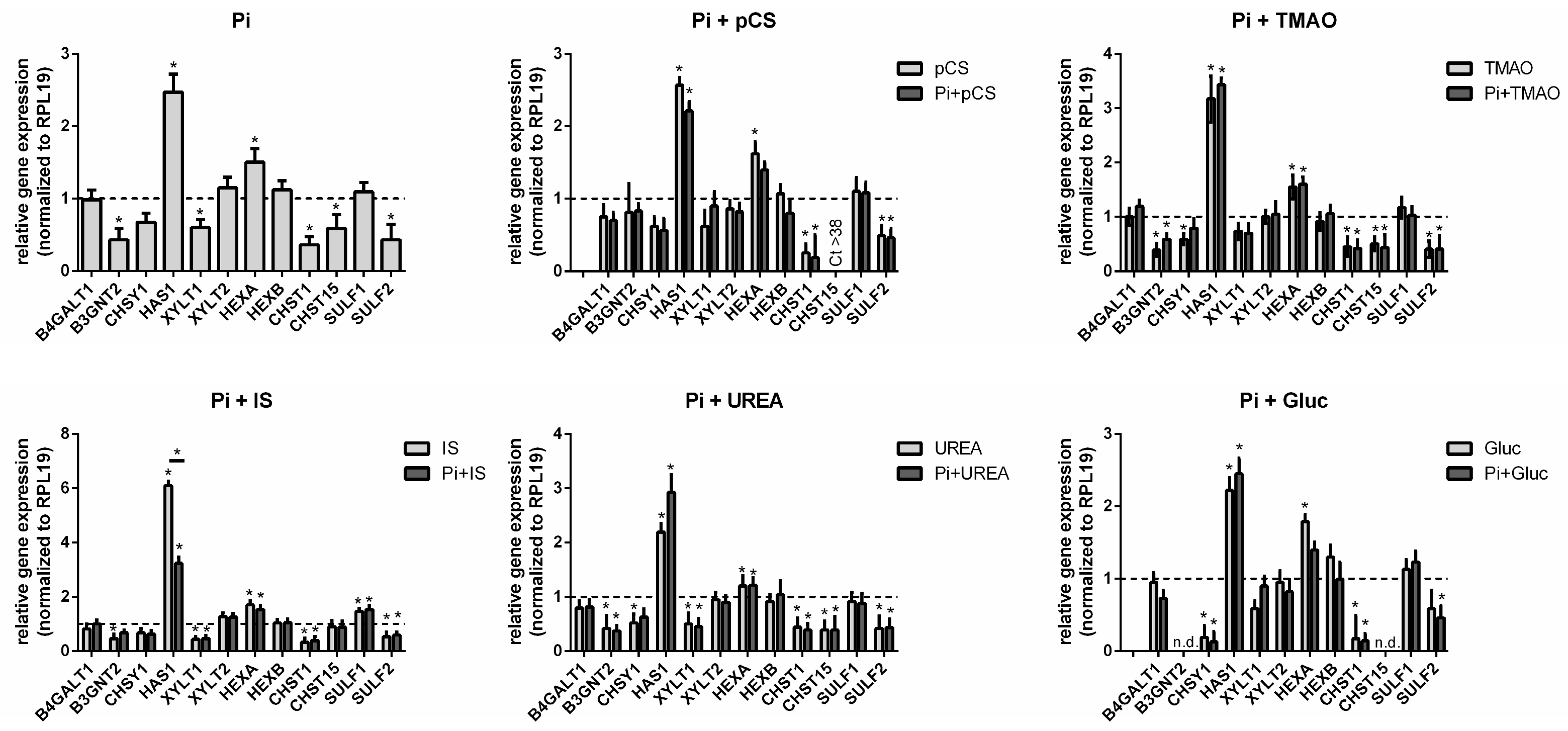
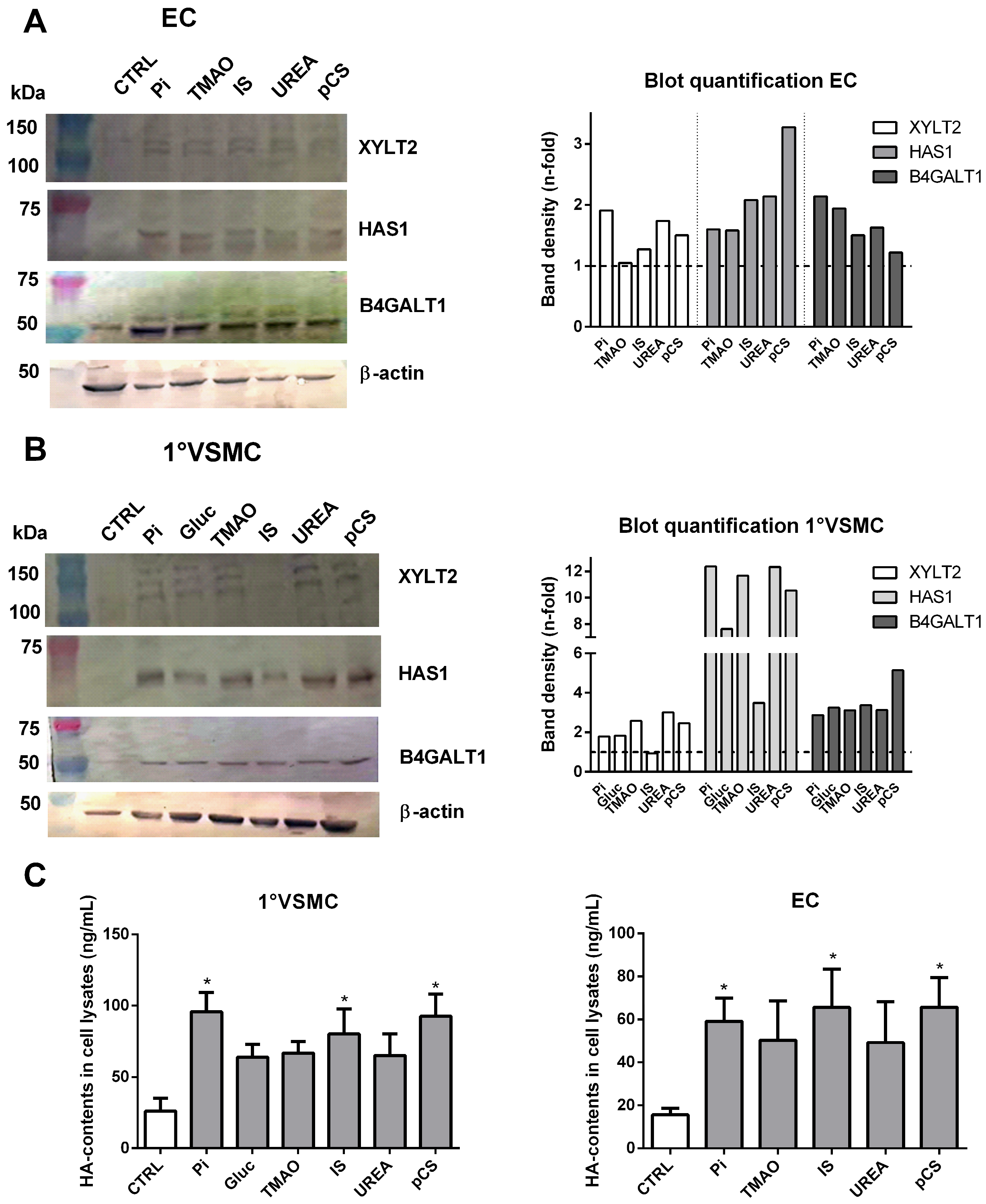
2.4. UTs Regulate GAG-Specific Genes and Proteins in Vascular Cells
2.5. Treatment of Vascular Cells with UTs Influences the Degree of Sulphation of Cellular GAGs
2.6. The GAG-Specific Effects of the UTs in Vascular Cells Involve Activation of the PI3K/AKT Pathway and Activation of NF-κB Signaling
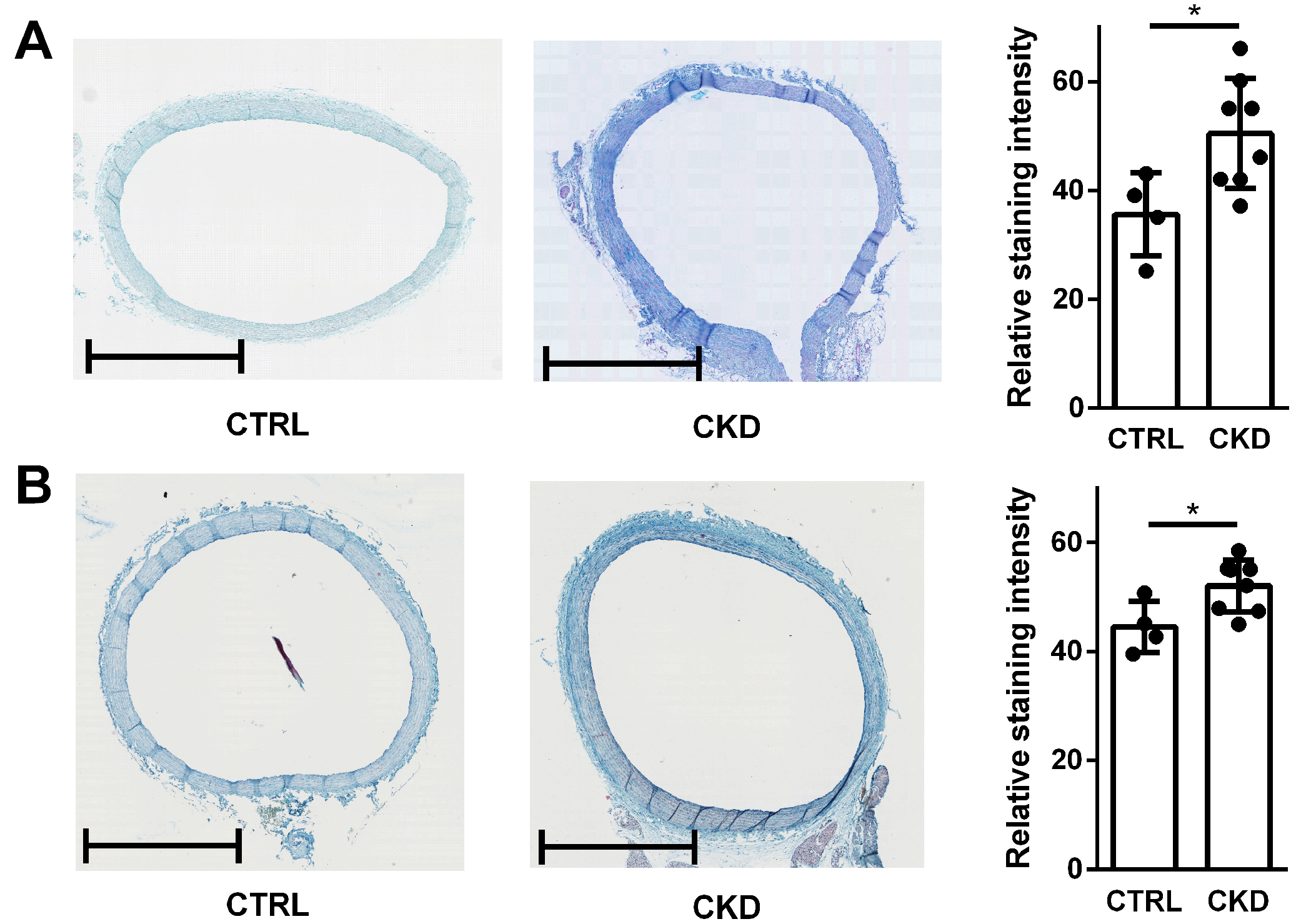
2.7. In Vivo-Derived Aortic Rings from CKD Rats Contain More sGAGs Compared to Healthy Controls
3. Discussion
4. Limitations
5. Conclusions
6. Materials and Methods
6.1. Cell Culture
6.2. Rat Model of CKD
6.3. Aortic Ring Culture
6.4. Histological Staining of Aortic Rings
6.5. Treatment of Cells with UTs
6.6. PCR Measurements
6.7. Determination of sGAG Contents in Aortic Rings and Cells
6.8. Determination of HA Contents in Cells
6.9. Western-Blot Analyses
6.10. Inhibition of AKT Signaling in Cultured Cells
6.11. HS and CS/DS Disaccharide Analysis of Vascular Cells
6.12. Measurement of NF-κB Activation in Vascular Cells
6.13. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-UT | mixture of the five uremic toxins (indoxylsulphate, p-cresylsulphate, trimethylamine N-oxide, inorganic phosphate and urea) |
| CKD | chronic kidney disease |
| CS | chondroitin sulphate |
| ECs | endothelial cells |
| GAGs | glycosaminoglycans |
| HA | hyaluronic acid |
| HS | heparan sulphate |
| IS | indoxylsulphate |
| pCS | p-cresylsulphate |
| Pi | inorganic phosphate |
| sGAGs | sulphated glycosaminoglycans |
| TMAO | trimethylamine N-oxide |
| UTs | uremic toxins |
| VSMCs | vascular smooth muscle cells |
References
- Zhang, L.S.; Davies, S.S. Microbial metabolism of dietary components to bioactive metabolites: Opportunities for new therapeutic interventions. Genome Med. 2016, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Six, I.; Flissi, N.; Lenglet, G.; Louvet, L.; Kamel, S.; Gallet, M.; Massy, Z.A.; Liabeuf, S. Uremic Toxins and Vascular Dysfunction. Toxins 2020, 12, 404. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Sidor, N.A.; Tonial, N.C.; Che, A.; Urquhart, B.L. Uremic Toxins in the Progression of Chronic Kidney Disease and Cardiovascular Disease: Mechanisms and Therapeutic Targets. Toxins 2021, 13, 142. [Google Scholar] [CrossRef]
- Nakamura, T.; Kawagoe, Y.; Matsuda, T.; Ueda, Y.; Shimada, N.; Ebihara, I.; Koide, H. Oral ADSORBENT AST-120 decreases carotid intima-media thickness and arterial stiffness in patients with chronic renal failure. Kidney Blood Press. Res. 2004, 27, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J. Am. Soc. Nephrol. JASN 2014, 25, 1897–1907. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, F.; Tang, H.; Zhang, X.; Hu, J.; Zhong, X.; Gong, N.; Lai, Y.; Zhou, M.; Tian, J.; et al. Gut microbial metabolite TMAO increases peritoneal inflammation and peritonitis risk in peritoneal dialysis patients. Transl. Res. 2022, 240, 50–63. [Google Scholar] [CrossRef]
- Tonelli, M.; Sacks, F.; Pfeffer, M.; Gao, Z.; Curhan, G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005, 112, 2627–2633. [Google Scholar] [CrossRef]
- Cancela, A.L.; Santos, R.D.; Titan, S.M.; Goldenstein, P.T.; Rochitte, C.E.; Lemos, P.A.; dos Reis, L.M.; Graciolli, F.G.; Jorgetti, V.; Moysés, R.M. Phosphorus is associated with coronary artery disease in patients with preserved renal function. PLoS ONE 2012, 7, e36883. [Google Scholar] [CrossRef]
- Mair, R.D.; Sirich, T.L.; Plummer, N.S.; Meyer, T.W. Characteristics of Colon-Derived Uremic Solutes. Clin. J. Am. Soc. Nephrol. 2018, 13, 1398–1404. [Google Scholar] [CrossRef]
- Gryp, T.; De Paepe, K.; Vanholder, R.; Kerckhof, F.M.; Van Biesen, W.; Van de Wiele, T.; Verbeke, F.; Speeckaert, M.; Joossens, M.; Couttenye, M.M.; et al. Gut microbiota generation of protein-bound uremic toxins and related metabolites is not altered at different stages of chronic kidney disease. Kidney Int. 2020, 97, 1230–1242. [Google Scholar] [CrossRef]
- Szeto, C.C.; Kwan, B.C.; Chow, K.M.; Lai, K.B.; Chung, K.Y.; Leung, C.B.; Li, P.K. Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin. J. Am. Soc. Nephrol. 2008, 3, 431–436. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.W.; Harrison, L.E.; Eldehni, M.T.; Jefferies, H.J.; Szeto, C.C.; John, S.G.; Sigrist, M.K.; Burton, J.O.; Hothi, D.; Korsheed, S.; et al. Circulating endotoxemia: A novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.L.; Kalantar-Zadeh, K.; Vaziri, N.D. The Gut as a Source of Inflammation in Chronic Kidney Disease. Nephron 2015, 130, 92–98. [Google Scholar] [CrossRef]
- Popkov, V.A.; Zharikova, A.A.; Demchenko, E.A.; Andrianova, N.V.; Zorov, D.B.; Plotnikov, E.Y. Gut Microbiota as a Source of Uremic Toxins. Int. J. Mol. Sci. 2022, 23, 483. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Kounatidis, D.; Panagopoulos, F.; Evangelopoulos, A.; Stamatopoulos, V.; Papagiorgos, A.; Geladari, E.; Dalamaga, M. Gut Microbiota and Its Role in the Brain-Gut-Kidney Axis in Hypertension. Curr. Hypertens. Rep. 2023, 25, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.Y.; Ge, J.; Huang, G.X.; Liu, K.G.; Yue, Y.; Li, H.; Lin, H.G.; Zhang, T.; Yan, H.F.; Xu, B.X.; et al. New insights into the intestinal barrier through “gut-organ” axes and a glimpse of the microgravity’s effects on intestinal barrier. Front. Physiol. 2024, 15, 1465649. [Google Scholar] [CrossRef]
- Freise, C.; Querfeld, U.; Ludwig, A.; Hamm, B.; Schnorr, J.; Taupitz, M. Uraemic extracellular vesicles augment osteogenic transdifferentiation of vascular smooth muscle cells via enhanced AKT signalling and PiT-1 expression. J. Cell. Mol. Med. 2021, 25, 5602–5614. [Google Scholar] [CrossRef]
- Freise, C.; Zappe, A.; Löwa, N.; Schnorr, J.; Pagel, K.; Wiekhorst, F.; Taupitz, M. Uremic Toxin-Induced Exosome-like Extracellular Vesicles Contain Enhanced Levels of Sulfated Glycosaminoglycans which Facilitate the Interaction with Very Small Superparamagnetic Iron Oxide Particles. Int. J. Mol. Sci. 2023, 24, 14253. [Google Scholar] [CrossRef]
- Freise, C.; Biskup, K.; Blanchard, V.; Schnorr, J.; Taupitz, M. Inorganic Phosphate-Induced Extracellular Vesicles from Vascular Smooth Muscle Cells Contain Elevated Levels of Hyaluronic Acid, Which Enhance Their Interaction with Very Small Superparamagnetic Iron Oxide Particles. Int. J. Mol. Sci. 2024, 25, 2571. [Google Scholar] [CrossRef]
- Caterson, B.; Melrose, J. Keratan sulfate, a complex glycosaminoglycan with unique functional capability. Glycobiology 2018, 28, 182–206. [Google Scholar] [CrossRef]
- Garantziotis, S.; Savani, R.C. Hyaluronan biology: A complex balancing act of structure, function, location and context. Matrix Biol. 2019, 78–79, 1–10. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Piperigkou, Z.; Theocharis, A.D.; Watanabe, H.; Franchi, M.; Baud, S.; Brézillon, S.; Götte, M.; Passi, A.; Vigetti, D.; et al. Proteoglycan Chemical Diversity Drives Multifunctional Cell Regulation and Therapeutics. Chem. Rev. 2018, 118, 9152–9232. [Google Scholar] [CrossRef] [PubMed]
- Kastana, P.; Choleva, E.; Poimenidi, E.; Karamanos, N.; Sugahara, K.; Papadimitriou, E. Insight into the role of chondroitin sulfate E in angiogenesis. FEBS J. 2019, 286, 2921–2936. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, A.; Sugahara, K.; Faissner, A. Chondroitin sulfate “wobble motifs” modulate maintenance and differentiation of neural stem cells and their progeny. J. Biol. Chem. 2012, 287, 2935–2942. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.R.; Schuksz, M.; Esko, J.D. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 2007, 446, 1030–1037. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef]
- Proudfoot, A.E.I.; Johnson, Z.; Bonvin, P.; Handel, T.M. Glycosaminoglycan Interactions with Chemokines Add Complexity to a Complex System. Pharmaceuticals 2017, 10, 70. [Google Scholar] [CrossRef]
- Lara-Prado, J.I.; Pazos-Pérez, F.; Méndez-Landa, C.E.; Grajales-García, D.P.; Feria-Ramírez, J.A.; Salazar-González, J.J.; Cruz-Romero, M.; Treviño-Becerra, A. Acute Kidney Injury and Organ Dysfunction: What Is the Role of Uremic Toxins? Toxins 2021, 13, 551. [Google Scholar] [CrossRef]
- Grist, M.; Chakraborty, J. Identification of a mucin layer in the urinary bladder. Urology 1994, 44, 26–33. [Google Scholar] [CrossRef]
- Layton, C.; Bancroft, J.D. 12—Carbohydrates. In Bancroft’s Theory and Practice of Histological Techniques, 7th ed.; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Churchill Livingstone: Oxford, UK, 2013; pp. 215–238. [Google Scholar]
- Movat, H.Z. Demonstration of all connective tissue elements in a single section; pentachrome stains. AMA Arch. Pathol. 1955, 60, 289–295. [Google Scholar]
- Lillie, R.D. Histopathologic Technic and Practical Histochemistry; Blakiston Division, McGraw-Hill: New York, NY, USA, 1965. [Google Scholar]
- Sugahara, K.; Kitagawa, H. Recent advances in the study of the biosynthesis and functions of sulfated glycosaminoglycans. Curr. Opin. Struct. Biol. 2000, 10, 518–527. [Google Scholar] [CrossRef]
- Götting, C.; Kuhn, J.; Zahn, R.; Brinkmann, T.; Kleesiek, K. Molecular Cloning and Expression of Human UDP-d-Xylose: Proteoglycan Core Protein β-d-Xylosyltransferase and its First Isoform XT-II. J. Mol. Biol. 2000, 304, 517–528. [Google Scholar] [CrossRef]
- Parenti, G.; Meroni, G.; Ballabio, A. The sulfatase gene family. Curr. Opin. Genet. Dev. 1997, 7, 386–391. [Google Scholar] [CrossRef]
- Krog, M.; Ejerblad, S.; Johansson, H. The aortic content of glycosaminoglycans, hydroxyproline and calcium in experimental uraemia with special reference to parathyroidectomy and vitamin-D treatment. Scand. J. Urol. Nephrol. 1984, 18, 241–247. [Google Scholar] [CrossRef]
- Liew, H.; Roberts, M.A.; Pope, A.; McMahon, L.P. Endothelial glycocalyx damage in kidney disease correlates with uraemic toxins and endothelial dysfunction. BMC Nephrol. 2021, 22, 21. [Google Scholar] [CrossRef]
- Wang, X.; He, B. Endothelial dysfunction: Molecular mechanisms and clinical implications. MedComm 2024, 5, e651. [Google Scholar] [CrossRef]
- Foudi, N.; Palayer, M.; Briet, M.; Garnier, A.S. Arterial Remodelling in Chronic Kidney Disease: Impact of Uraemic Toxins and New Pharmacological Approaches. J. Clin. Med. 2021, 10, 3803. [Google Scholar] [CrossRef]
- Togayachi, A.; Sato, T.; Narimatsu, H. Comprehensive enzymatic characterization of glycosyltransferases with a beta3GT or beta4GT motif. Methods Enzymol. 2006, 416, 91–102. [Google Scholar] [CrossRef]
- Izumikawa, T.; Koike, T.; Shiozawa, S.; Sugahara, K.; Tamura, J.; Kitagawa, H. Identification of chondroitin sulfate glucuronyltransferase as chondroitin synthase-3 involved in chondroitin polymerization: Chondroitin polymerization is achieved by multiple enzyme complexes consisting of chondroitin synthase family members. J. Biol. Chem. 2008, 283, 11396–11406. [Google Scholar] [CrossRef]
- Kitagawa, H.; Fujita, M.; Ito, N.; Sugahara, K. Molecular cloning and expression of a novel chondroitin 6-O-sulfotransferase. J. Biol. Chem. 2000, 275, 21075–21080. [Google Scholar] [CrossRef]
- Sainio, A.; Takabe, P.; Oikari, S.; Salomäki-Myftari, H.; Koulu, M.; Söderström, M.; Pasonen-Seppänen, S.; Järveläinen, H. Metformin decreases hyaluronan synthesis by vascular smooth muscle cells. J. Investig. Med. 2020, 68, 383–391. [Google Scholar] [CrossRef]
- Golusda, L.; Kühl, A.A.; Lehmann, M.; Dahlke, K.; Mueller, S.; Boehm-Sturm, P.; Saatz, J.; Traub, H.; Schnorr, J.; Freise, C.; et al. Visualization of Inflammation in Experimental Colitis by Magnetic Resonance Imaging Using Very Small Superparamagnetic Iron Oxide Particles. Front. Physiol. 2022, 13, 862212. [Google Scholar] [CrossRef]
- Mohamed, R.; Dayati, P.; Mehr, R.N.; Kamato, D.; Seif, F.; Babaahmadi-Rezaei, H.; Little, P.J. Transforming growth factor-β1 mediated CHST11 and CHSY1 mRNA expression is ROS dependent in vascular smooth muscle cells. J. Cell Commun. Signal. 2019, 13, 225–233. [Google Scholar] [CrossRef]
- Chacko, B.K.; Scott, D.W.; Chandler, R.T.; Patel, R.P. Endothelial surface N-glycans mediate monocyte adhesion and are targets for anti-inflammatory effects of peroxisome proliferator-activated receptor γ ligands. J. Biol. Chem. 2011, 286, 38738–38747. [Google Scholar] [CrossRef]
- Mohamadzadeh, M.; DeGrendele, H.; Arizpe, H.; Estess, P.; Siegelman, M. Proinflammatory stimuli regulate endothelial hyaluronan expression and CD44/HA-dependent primary adhesion. J. Clin. Investig. 1998, 101, 97–108. [Google Scholar] [CrossRef]
- Natarajan, R.; Gonzales, N.; Xu, L.; Nadler, J.L. Vascular smooth muscle cells exhibit increased growth in response to elevated glucose. Biochem. Biophys. Res. Commun. 1992, 187, 552–560. [Google Scholar] [CrossRef]
- Yasunari, K.; Kohno, M.; Kano, H.; Yokokawa, K.; Minami, M.; Yoshikawa, J. Antioxidants improve impaired insulin-mediated glucose uptake and prevent migration and proliferation of cultured rabbit coronary smooth muscle cells induced by high glucose. Circulation 1999, 99, 1370–1378. [Google Scholar] [CrossRef]
- Meng, L.; Park, J.; Cai, Q.; Lanting, L.; Reddy, M.A.; Natarajan, R. Diabetic conditions promote binding of monocytes to vascular smooth muscle cells and their subsequent differentiation. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H736–H745. [Google Scholar] [CrossRef]
- de Sousa Mesquita, A.P.; de Araújo Lopes, S.; Pernambuco Filho, P.C.A.; Nader, H.B.; Lopes, C.C. Acquisition of anoikis resistance promotes alterations in the Ras/ERK and PI3K/Akt signaling pathways and matrix remodeling in endothelial cells. Apoptosis Int. J. Program. Cell Death 2017, 22, 1116–1137. [Google Scholar] [CrossRef]
- Toyoda, S.; Shin, J.; Fukuhara, A.; Otsuki, M.; Shimomura, I. Transforming growth factor β1 signaling links extracellular matrix remodeling to intracellular lipogenesis upon physiological feeding events. J. Biol. Chem. 2022, 298, 101748. [Google Scholar] [CrossRef]
- He, X.; Jiang, H.; Gao, F.; Liang, S.; Wei, M.; Chen, L. Indoxyl sulfate-induced calcification of vascular smooth muscle cells via the PI3K/Akt/NF-κB signaling pathway. Microsc. Res. Tech. 2019, 82, 2000–2006. [Google Scholar] [CrossRef]
- Shi, G.; Zeng, L.; Shi, J.; Chen, Y. Trimethylamine N-oxide Promotes Atherosclerosis by Regulating Low-Density Lipoprotein-Induced Autophagy in Vascular Smooth Muscle Cells Through PI3K/AKT/mTOR Pathway. Int. Heart J. 2023, 64, 462–469. [Google Scholar] [CrossRef]
- Kapetanaki, S.; Kumawat, A.K.; Persson, K.; Demirel, I. The Fibrotic Effects of TMAO on Human Renal Fibroblasts Is Mediated by NLRP3, Caspase-1 and the PERK/Akt/mTOR Pathway. Int. J. Mol. Sci. 2021, 22, 11864. [Google Scholar] [CrossRef]
- Shukla, S.; Maclennan, G.T.; Hartman, D.J.; Fu, P.; Resnick, M.I.; Gupta, S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int. J. Cancer 2007, 121, 1424–1432. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.C. NF-κB in inflammation and renal diseases. Cell Biosci. 2015, 5, 63. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Li, M.; Li, Y.; Luo, X.; Liu, Y.; Zhang, X.; Hocher, J.G.; Krämer, B.K.; Hocher, B.; et al. Both partial inactivation as well as activation of NF-κB signaling lead to hypertension and chronic kidney disease. Nephrol. Dial Transpl. 2024, 39, 1993–2004. [Google Scholar] [CrossRef]
- Poveda, J.; Tabara, L.C.; Fernandez-Fernandez, B.; Martin-Cleary, C.; Sanz, A.B.; Selgas, R.; Ortiz, A.; Sanchez-Niño, M.D. TWEAK/Fn14 and Non-Canonical NF-kappaB Signaling in Kidney Disease. Front. Immunol. 2013, 4, 447. [Google Scholar] [CrossRef]
- Lindner, M.; Laporte, A.; Elomaa, L.; Lee-Thedieck, C.; Olmer, R.; Weinhart, M. Flow-induced glycocalyx formation and cell alignment of HUVECs compared to iPSC-derived ECs for tissue engineering applications. Front. Cell Dev. Biol. 2022, 10, 953062. [Google Scholar] [CrossRef]
- Ross, R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J. Cell Biol. 1971, 50, 172–186. [Google Scholar] [CrossRef]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef]
- Farhat, Y. PicoGreen Cell Proliferation Assay Protocol. Available online: http://protocol-place.com (accessed on 25 July 2022).
- Freise, C.; Querfeld, U. The lignan (+)-episesamin interferes with TNF-α-induced activation of VSMC via diminished activation of NF-ĸB, ERK1/2 and AKT and decreased activity of gelatinases. Acta Physiol. 2015, 213, 642–652. [Google Scholar] [CrossRef] [PubMed]




| Gene | Full Name | Assay-ID (Rat) | Assay-ID (Human) |
|---|---|---|---|
| B3GNT2 | Beta-1,3-N-acetylglucosaminyltransferase 2 | Rn02112835_s1 | Hs01935859_s1 |
| B4GALT1 | Beta-1,4-galactosyltransferase 1 | Rn01764643_m1 | Hs00419232_g1 |
| CHST1 | Carbohydrate sulfotransferase 1 | Rn01484520_m1 | Hs04972213_s1 |
| CHST15 | Carbohydrate sulfotransferase 15 | Rn00597859_m1 | Hs01031067_m1 |
| CHSY1 | Chondroitin sulphate synthase 1 | Rn01478125_m1 | Hs00208704_m1 |
| EXT1 | Exostosin Glycosyltransferase 1 | Rn00468764_m1 | Hs00609162_m1 |
| HAS1 | Hyaluronan synthase 1 | Rn01455687_g1 | Hs00608272_m1 |
| HEXA | Hexosaminidase subunit alpha | Rn01422539_m1 | Hs00942655_m1 |
| HEXB | Hexosaminidase subunit beta | Rn01493909_m1 | Hs01077594_m1 |
| RPL19 | Ribosomal Protein L19 | Rn00821265_g1 | Hs02338565_gH |
| SULF1 | Sulfatase 1 | Rn00592734_m1 | Hs00392834_m1 |
| SULF2 | Sulfatase 2 | Rn01423347_m1 | Hs01016480_m1 |
| XYLT1 | Xylosyltransferase 1 | Rn01755138_m1 | Hs00544498_m1 |
| XYLT2 | Xylosyltransferase 2 | Rn00574186_m1 | Hs01048792_m1 |
| Target | Dilution | Company | Product Number |
|---|---|---|---|
| AKT | 1:1000 | Cell signaling (Danvers, MA, USA) | #9272 |
| p-AKT | 1:1000 | Cell signaling | #4056 |
| B4GALT1 | 1:1000 | ThermoFisher | #PA5-52744 |
| HAS1 | 1:800 | ThermoFisher | #PA5-95599 |
| XYLT2 | 1:800 | ThermoFisher | #PA5-29127 |
| β-actin | 1:1000 | Sigma-Aldrich (Taufkirchen, Germany) | #A2228 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freise, C.; Metzkow, S.; Zappe, A.; Ebert, M.; Stolzenburg, N.; Hahndorf, J.; Schnorr, J.; Pagel, K.; Taupitz, M. The Uremic Toxins Inorganic Phosphate, Indoxylsulphate, p-Cresylsulphate, and TMAO Induce the Generation of Sulphated Glycosaminoglycans in Aortic Tissue and Vascular Cells via pAKT Signaling: A Missing Link in the “Gut–Matrix Axis”. Toxins 2025, 17, 217. https://doi.org/10.3390/toxins17050217
Freise C, Metzkow S, Zappe A, Ebert M, Stolzenburg N, Hahndorf J, Schnorr J, Pagel K, Taupitz M. The Uremic Toxins Inorganic Phosphate, Indoxylsulphate, p-Cresylsulphate, and TMAO Induce the Generation of Sulphated Glycosaminoglycans in Aortic Tissue and Vascular Cells via pAKT Signaling: A Missing Link in the “Gut–Matrix Axis”. Toxins. 2025; 17(5):217. https://doi.org/10.3390/toxins17050217
Chicago/Turabian StyleFreise, Christian, Susanne Metzkow, Andreas Zappe, Monika Ebert, Nicola Stolzenburg, Julia Hahndorf, Jörg Schnorr, Kevin Pagel, and Matthias Taupitz. 2025. "The Uremic Toxins Inorganic Phosphate, Indoxylsulphate, p-Cresylsulphate, and TMAO Induce the Generation of Sulphated Glycosaminoglycans in Aortic Tissue and Vascular Cells via pAKT Signaling: A Missing Link in the “Gut–Matrix Axis”" Toxins 17, no. 5: 217. https://doi.org/10.3390/toxins17050217
APA StyleFreise, C., Metzkow, S., Zappe, A., Ebert, M., Stolzenburg, N., Hahndorf, J., Schnorr, J., Pagel, K., & Taupitz, M. (2025). The Uremic Toxins Inorganic Phosphate, Indoxylsulphate, p-Cresylsulphate, and TMAO Induce the Generation of Sulphated Glycosaminoglycans in Aortic Tissue and Vascular Cells via pAKT Signaling: A Missing Link in the “Gut–Matrix Axis”. Toxins, 17(5), 217. https://doi.org/10.3390/toxins17050217





