Venomous Secretions from Marine Snails of the Terebridae Family Target Acetylcholine Receptors
Abstract
:1. Introduction
2. Results
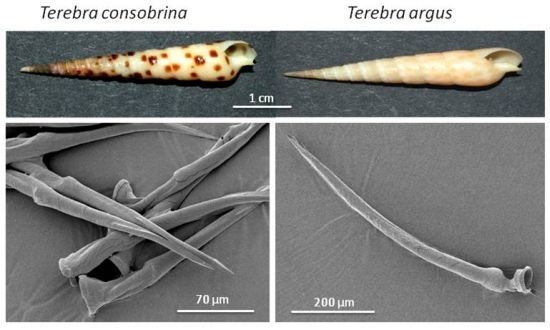
2.1. Venom Apparatus of the Terebridae

2.2. Electrophysiological Analysis of the Venom Gland Extracts
| Oxymeris maculata | Terebra argus | Terebra consobrina | |
|---|---|---|---|
| α3β2 | ++ | +/− | ++ |
| 1:10 | + | − | + |
| α3β4 | + | − | + |
| 1:10 | − | − | − |
| α4β2 | +++ | + | +++ |
| 1:10 | + | − | + |
| α4β4 | +++ | +/− | ++ |
| 1:10 | − | − | − |
| α7 | +++ | + | +++ |
| 1:10 | + | − | + |
| α1β1γδ | +++ | − | + |
| 1:10 | + | − | − |

3. Discussion
4. Experimental Section
4.1. Materials
4.2. Electrophysiology
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Taylor, J.D.; Kantor, Y.I.; Sysoev, A.V. Foregut anatomy, feeding mechanisms and classification of the Conoidea (=Toxoglossa) (Gastropoda). Bull. Nat. Hist. Muse. Zool. 1993, 59, 125–170. [Google Scholar]
- Terryn, Y.A. Collectors Guide to Recent Terebridae: (Mollusca: Neogastropoda). In Terebridae: A Collectors Guide; ConchBooks: Hackenheim, Germany, 2007. [Google Scholar]
- Puillandre, N.; Samadi, S.; Boisselier, M.C.; Sysov, A.V.; Kantor, Y.I.; Cruaud, C.; Couloux, A.; Bouchet, P. Starting to unravel the toxoglossan knot: Molecular phylogeny of the “turrids” (Neogastropoda: Conoidea). Mol. Phylogenet. Evol. 2008, 47, 1122–1134. [Google Scholar]
- Terlau, H.; Olivera, B.M. Conus venoms: A rich source of novel ion channel-targeted peptides. Physiol. Rev. 2004, 84, 41–68. [Google Scholar]
- Olivera, B.M. Conus peptides: Biodiversity-based discovery and exogenomics. J. Biol. Chem. 2006, 281, 31173–31177. [Google Scholar] [CrossRef]
- Olivera, B.M.; Teichert, R.W. Diversity of neurotoxic Conus peptides: A model for concerted pharmacological discovery. Mol. Interv. 2007, 7, 251–260. [Google Scholar] [CrossRef]
- Imperial, J.S.; Watkins, M.; Chen, P.; Hillyard, D.R.; Cruz, L.J.; Olivera, B.M. The augertoxins: Biochemical characterization of venom components from the toxoglossate, Terebra subulata. Toxicon 2003, 42, 391–398. [Google Scholar] [CrossRef]
- Imperial, J.S.; Kantor, Y.; Watkins, M.; Heralde, F.M.; Stevenson, B.; Chen, P.; Hansson, K.; Stenflo, J.; Ownby, J.-P.; Bouchet, P.; et al. Venomous auger snail Hastula (Impages) hectica (Linnaeus, 1758): Molecular phylogeny, foregut anatomy and comparative toxinology. J. Exp. Zool. B 2007, 308, 744–756. [Google Scholar]
- López-Vera, E.; Heimer de la Cotera, E.P.; Maillo, M.; Riesgo-Escovar, J.R.; Olivera, B.M.; Aguilar, M.B. A novel structural class of toxins: The methionine-rich peptides from the venoms of turrid marine snails (Mollusca, Conoidea). Toxicon 2004, 43, 365–374. [Google Scholar]
- Aguilar, M.B.; de la Rosa, R.A.; Falcón, A.; Olivera, B.M.; Heimer de la Cotera, E.P. Peptide pal9a from the venom of the turrid snail Polystira albida from the Gulf of Mexico: Purification, characterization, and comparison with P-conotoxin-like (framework IX) conoidean peptides. Peptide 2009, 30, 467–476. [Google Scholar] [CrossRef]
- Watkins, M.; Hillyard, D.R.; Olivera, B.M. Genes expressed in a turrid venom duct: Divergence and similarity to conotoxins. J. Mol. Evol. 2006, 62, 247–256. [Google Scholar] [CrossRef]
- Heralde, F.M.; Imperial, J.; Bandyopadhyay, P.K.; Olivera, B.M.; Concepcion, G.P.; Santos, A.D. A rapidly diverging superfamily of peptide toxins in venomous Gemmula species. Toxicon 2008, 51, 890–897. [Google Scholar] [CrossRef]
- Seronay, R.A.; Fedosov, A.E.; Astilla, M.A.; Watkins, M.; Saguil, N.; Heralde, F.M.; Tagaro, S.; Poppe, G.T.; Aliño, P.M.; Oliverio, M.; et al. Accessing novel conoidean venoms: Biodiverse lumun-lumun marine communities, an untapped biological and toxinological resource. Toxicon 2010, 56, 1257–1266. [Google Scholar] [CrossRef]
- Santos, A.D.; McIntosh, J.M.; Hilliyard, D.R.; Cruz, L.J.; Olivera, B.M. The A-superfamily of conotoxins: Structural and functional divergence. J. Biol. Chem. 2004, 279, 17596–17606. [Google Scholar]
- Gray, W.R.; Luque, A.; Olivera, B.M.; Barrett, J.; Cruz, L.J. Peptide toxins from Conus geographus. J. Biol. Chem. 1981, 256, 4734–4740. [Google Scholar]
- Nicke, A.; Wonnacott, S.; Lewis, R.J. α-Conotoxins as tools for the elucidation of structure and function of neuronal nicotinic acetylcholine receptor subtypes. Eur. J. Biochem. 2004, 271, 2305–2319. [Google Scholar]
- Olivera, B.M.; Quick, M.; Vincler, M.; McIntosh, J.M. Subtype-selective conopeptides targeted to nicotinic receptors. Channels 2008, 2, 143–152. [Google Scholar] [CrossRef]
- Castelin, M.; Puillandre, N.; Kantor, Y.I.; Modica, M.V.; Terryn, Y.; Cruaud, C.; Bochet, P.; Holford, M. Mcroevolution of venom apparatus innovations in auger snails (Gastropoda; Conoidea; Terebridae). Mol. Phylogenet. Evol. 2012, 64, 21–44. [Google Scholar] [CrossRef]
- Biggs, J.S.; Olivera, B.M.; Kantor, Y. Alpha-conopeptides specifically expressed in the salivary gland of Conus pulicarius. Toxicon 2008, 52, 101–105. [Google Scholar] [CrossRef]
- Holford, M.; Puillandre, N.; Modica, M.V.; Watkins, M.; Collin, R.; Bermingham, E.; Olivera, B.M. Correlating molecular phylogeny with venom apparatus occurrence in Panamic auger snails (Terebridae). PLoS One 2009, 4, e7667. [Google Scholar]
- Puillandre, N.; Holford, M. The Terebridae and teretoxins: Combining phylogeny and anatomy for concerted discovery of bioactive compounds. BMC Chem. Biol. 2010, 10. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kendel, Y.; Melaun, C.; Kurz, A.; Nicke, A.; Peigneur, S.; Tytgat, J.; Wunder, C.; Mebs, D.; Kauferstein, S. Venomous Secretions from Marine Snails of the Terebridae Family Target Acetylcholine Receptors. Toxins 2013, 5, 1043-1050. https://doi.org/10.3390/toxins5051043
Kendel Y, Melaun C, Kurz A, Nicke A, Peigneur S, Tytgat J, Wunder C, Mebs D, Kauferstein S. Venomous Secretions from Marine Snails of the Terebridae Family Target Acetylcholine Receptors. Toxins. 2013; 5(5):1043-1050. https://doi.org/10.3390/toxins5051043
Chicago/Turabian StyleKendel, Yvonne, Christian Melaun, Alexander Kurz, Annette Nicke, Steve Peigneur, Jan Tytgat, Cora Wunder, Dietrich Mebs, and Silke Kauferstein. 2013. "Venomous Secretions from Marine Snails of the Terebridae Family Target Acetylcholine Receptors" Toxins 5, no. 5: 1043-1050. https://doi.org/10.3390/toxins5051043
APA StyleKendel, Y., Melaun, C., Kurz, A., Nicke, A., Peigneur, S., Tytgat, J., Wunder, C., Mebs, D., & Kauferstein, S. (2013). Venomous Secretions from Marine Snails of the Terebridae Family Target Acetylcholine Receptors. Toxins, 5(5), 1043-1050. https://doi.org/10.3390/toxins5051043