Milk Thistle Extract and Silymarin Inhibit Lipopolysaccharide Induced Lamellar Separation of Hoof Explants in Vitro
Abstract
:1. Introduction
2. Results
2.1. In Vitro Endotoxin Neutralization Tests

2.2. Viability

| Number of explants | ||
|---|---|---|
| Separated/total | Separated/total | |
| LPS (µg/mL) | 24 h | 48 h |
| 0 | 3/18 a | 0/32 a |
| 1.25 | n.d. | 0/8 a |
| 2.5 | 5/10 a | 0/12 a |
| 5 | 16/18 b | 4/12 a |
| 10 | 18/18 b | 6/12 a |
| 20 | 16/17 b | 11/11 b |
| 40 | 18/18 b | 12/12 b |
| 60 | n.d. | 8/8 b |
| 80 | n.d. | 9/9 b |
| 100 | 18/18 b | 12/12 b |
| 120 | n.d. | 9/9 b |
| 140 | n.d. | 9/9 b |
| 160 | n.d. | 9/9 b |
| 180 | n.d. | 9/9 b |
| 200 | 18/18 b | 12/12 b |
2.3. Studies with LPS
2.4. Studies with LPS in Combination with PMB
| Treatment | Number of explants | |
|---|---|---|
| LPS (µg/mL) | PMB (µg/mL) | Separated/total |
| 0 | 0 | 3/30 a |
| 10 | 0 | 28/30 b |
| 10 | 50 | 2/8 a |
| 10 | 100 | 0/9 a |
| 10 | 200 | 6/20 a |
| 10 | 500 | 3/26 a |
2.5. Studies with LPS in Combination with MT and Silymarin
2.5.1. Results of Forceps Testing
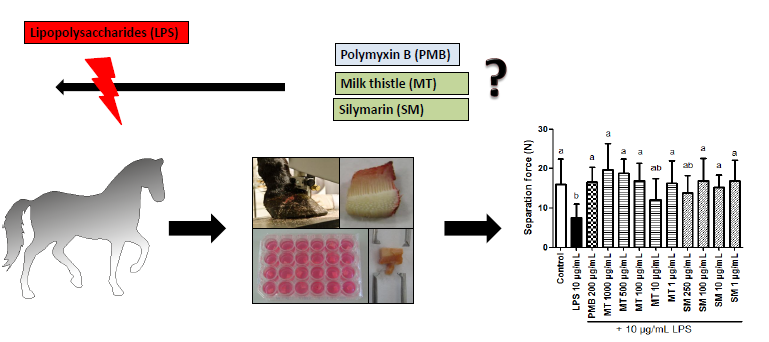
2.5.2. Force Transducer Results
| Treatment | Number of explants | |
|---|---|---|
| LPS (µg/mL) | MT, silymarin (µg/mL) | Separated/total |
| 0 | 0 | 0/8 a |
| 10 | 0 | 9/9 b |
| 10 | MT 10 | 4/6 b |
| 10 | MT 100 | 3/9 b |
| 10 | MT 500 | 0/9 a |
| 10 | MT 1000 | 2/9 a |
| 10 | Silymarin 100 | 0/3 a |
| 10 | Silymarin 250 | 0/5 a |

3. Discussion
4. Experimental Section
4.1. In Vitro LPS Neutralization Assay and Limulus Amebocyte Lysate (LAL) Assay
4.2. Animals
4.3. Preparation of Explants
4.4. Preparation of Endotoxin, PMB, MT and Silymarin Stock Solutions
4.5. Cultivation
4.6. Viability
4.7. Separation
4.8. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- USDA. Lameness and Laminitis in U.S. Horses; USDA Rural Development: Denver, CO, USA, 2000. [Google Scholar]
- Mungall, B.A.; Kyaw-Tanner, M.; Pollitt, C.C. In vitro evidence for a bacterial pathogenesis of equine laminitis. Vet. Microbiol. 2001, 79, 209–223. [Google Scholar]
- Mungall, B.A.; Pollitt, C.C. Thermolysin activates equine lamellar hoof matrix metalloproteinases. J. Comp. Pathol. 2002, 126, 9–16. [Google Scholar]
- Bailey, S.R.; Adair, H.S.; Reinemeyer, C.R.; Morgan, S.J.; Brooks, A.C.; Longhofer, S.L.; Elliott, J. Plasma concentrations of endotoxin and platelet activation in the developmental stage of oligofructose-induced laminitis. Vet. Immunol. Immunopathol. 2009, 129, 167–173. [Google Scholar]
- Katz, L.M.; Bailey, S.R. A review of recent advances and current hypotheses on the pathogenesis of acute laminitis. Equine Vet. J. 2012, 44, 752–761. [Google Scholar]
- Sprouse, R.F.; Garner, H.E.; Green, E.M. Plasma endotoxin levels in horses subjected to carbohydrate induced laminitis. Equine Vet. J. 1987, 19, 25–28. [Google Scholar]
- Patan-Zugaj, B.; Gauff, F.C.; Licka, T.F. Effects of the addition of endotoxin during perfusion of isolated forelimbs of equine cadavers. Am. J. Vet. Res. 2012, 73, 1462–1468. [Google Scholar]
- Hunt, R.J.; Allen, D.; Moore, J.N. Effect of endotoxin administration on equine digital hemodynamics and starling forces. Am. J. Vet. Res. 1990, 51, 1703–1707. [Google Scholar]
- Tóth, F.; Frank, N.; Elliott, S.B.; Geor, R.J.; Boston, R.C. Effects of an intravenous endotoxin challenge on glucose and insulin dynamics in horses. Am. J. Vet. Res. 2008, 69, 82–88. [Google Scholar]
- Pass, M.A.; Pollitt, S.; Pollitt, C.C. Decreased glucose metabolism causes separation of hoof lamellae in vitro: A trigger for laminitis? Equine Vet. J. Suppl. 1998, 30, 133–138. [Google Scholar]
- Pollitt, C.C.; Pass, M.A.; Pollitt, S. Batimastat (bb-94) inhibits matrix metalloproteinases of equine laminitis. Equine Vet. J. Suppl. 1998, 30, 119–124. [Google Scholar]
- Danner, R.L.; Joiner, K.A.; Rubin, M.; Patterson, W.H.; Johnson, N.; Ayers, K.M.; Parrillo, J.E. Purification, toxicity, and antiendotoxin activity of polymyxin b nonapeptide. Antimicrob. Agents Chemother. 1989, 33, 1428–1434. [Google Scholar]
- Abenavoli, L.; Capasso, R.; Milic, N.; Capasso, F. Milk thistle in liver diseases: Past, present, future. Phytother. Res. 2010, 24, 1423–1432. [Google Scholar]
- Saller, R.; Meier, R.; Brignoli, R. The use of silymarin in the treatment of liver diseases. Drugs 2001, 61, 2035–2063. [Google Scholar]
- Kang, J.S.; Jeon, Y.J.; Park, S.K.; Yang, K.H.; Kim, H.M. Protection against lipopolysaccharide-induced sepsis and inhibition of interleukin-1β and prostaglandin e2 synthesis by silymarin. Biochem. Pharmacol. 2004, 67, 175–181. [Google Scholar]
- Youn, C.K.; Park, S.J.; Lee, M.Y.; Cha, M.J.; Kim, O.H.; You, H.J.; Chang, I.Y.; Yoon, S.P.; Jeon, Y.J. Silibinin inhibits lps-induced macrophage activation by blocking p38 mapk in raw 264.7 cells. Biomol. Ther. 2013, 21, 258–263. [Google Scholar]
- Tóth, F.; Frank, N.; Chameroy, K.A.; Boston, R.C. Effects of endotoxaemia and carbohydrate overload on glucose and insulin dynamics and the development of laminitis in horses. Equine Vet. J. 2009, 41, 852–858. [Google Scholar]
- MacKay, R.J.; Clark, C.K.; Logdberg, L.; Lake, P. Effect of a conjugate of polymyxin b-dextran 70 in horses with experimentally induced endotoxemia. Am. J. Vet. Res. 1999, 60, 68–75. [Google Scholar]
- Duncan, S.G.; Meyers, K.M.; Reed, S.M.; Grant, B. Alterations in coagulation and hemograms of horses given endotoxins for 24 h via hepatic portal infusions. Am. J. Vet. Res. 1985, 46, 1287–1293. [Google Scholar]
- Ingle-Fehr, J.E.; Baxter, G.M. Evaluation of digital and laminar blood flow in horses given a low dose of endotoxin. Am. J. Vet. Res. 1998, 59, 192–196. [Google Scholar]
- Leise, B.S.; Yin, C.; Pettigrew, A.; Belknap, J.K. Proinflammatory cytokine responses of cultured equine keratinocytes to bacterial pathogen-associated molecular pattern motifs. Equine Vet. J. 2010, 42, 294–303. [Google Scholar]
- Brooks, A.C.; Menzies-Gow, N.J.; Wheeler-Jones, C.; Bailey, S.R.; Cunningham, F.M.; Elliott, J. Endotoxin-induced activation of equine digital vein endothelial cells: Role of p38 mapk. Vet. Immunol. Immunopathol. 2009, 129, 174–180. [Google Scholar]
- Morresey, P.R.; Mackay, R.J. Endotoxin-neutralizing activity of polymyxin b in blood after IV administration in horses. Am. J. Vet. Res. 2006, 67, 642–647. [Google Scholar]
- Barton, M.H.; Parviainen, A.; Norton, N. Polymyxin b protects horses against induced endotoxaemia in vivo. Equine Vet. J. 2004, 36, 397–401. [Google Scholar]
- Schwarz, B.; Anen, C.; van den Hoven, R. Preliminary data of a retrospective study on neurological side effects after administration of polymyxin b to endotoxaemic horses. Equine Vet. J. 2013, 45, 18–19. [Google Scholar]
- Saller, R.; Brignoli, R.; Melzer, J.; Meier, R. An updated systematic review with meta-analysis for the clinical evidence of silymarin. Forsch. Komplementmed. 2008, 15, 9–20. [Google Scholar]
- Hackett, E.S. Silibinin Pharmacology and Opportunities for Therapy in Horses. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2011. [Google Scholar]
- Sonnenbichler, J.; Zetl, I. Mechanism of action of silibinin. V. Effect of silibinin on the synthesis of ribosomal RNA, mRNA and tRNA in rat liver in vivo. Hoppe Seylers Z. Physiol. Chem. 1984, 365, 555–566. [Google Scholar]
- Favari, L.; Perez-Alvarez, V. Comparative effects of colchicine and silymarin on ccl4-chronic liver damage in rats. Arch. Med. Res. 1997, 28, 11–17. [Google Scholar]
- Lieber, C.S.; Leo, M.A.; Cao, Q.; Ren, C.; DeCarli, L.M. Silymarin retards the progression of alcohol-induced hepatic fibrosis in baboons. J. Clin. Gastroenterol. 2003, 37, 336–339. [Google Scholar]
- Singh, D.; Singh, R.; Singh, P.; Gupta, R.S. Effects of embelin on lipid peroxidation and free radical scavenging activity against liver damage in rats. Basic Clin. Pharmacol. Toxicol. 2009, 105, 243–248. [Google Scholar]
- Pollitt, C.C. Basement membrane pathology: A feature of acute equine laminitis. Equine Vet. J. 1996, 28, 38–46. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reisinger, N.; Schaumberger, S.; Nagl, V.; Hessenberger, S.; Schatzmayr, G. Milk Thistle Extract and Silymarin Inhibit Lipopolysaccharide Induced Lamellar Separation of Hoof Explants in Vitro. Toxins 2014, 6, 2962-2974. https://doi.org/10.3390/toxins6102962
Reisinger N, Schaumberger S, Nagl V, Hessenberger S, Schatzmayr G. Milk Thistle Extract and Silymarin Inhibit Lipopolysaccharide Induced Lamellar Separation of Hoof Explants in Vitro. Toxins. 2014; 6(10):2962-2974. https://doi.org/10.3390/toxins6102962
Chicago/Turabian StyleReisinger, Nicole, Simone Schaumberger, Veronika Nagl, Sabine Hessenberger, and Gerd Schatzmayr. 2014. "Milk Thistle Extract and Silymarin Inhibit Lipopolysaccharide Induced Lamellar Separation of Hoof Explants in Vitro" Toxins 6, no. 10: 2962-2974. https://doi.org/10.3390/toxins6102962
APA StyleReisinger, N., Schaumberger, S., Nagl, V., Hessenberger, S., & Schatzmayr, G. (2014). Milk Thistle Extract and Silymarin Inhibit Lipopolysaccharide Induced Lamellar Separation of Hoof Explants in Vitro. Toxins, 6(10), 2962-2974. https://doi.org/10.3390/toxins6102962