The Venom of the Spider Selenocosmia Jiafu Contains Various Neurotoxins Acting on Voltage-Gated Ion Channels in Rat Dorsal Root Ganglion Neurons
Abstract
:1. Introduction

2. Results and Discussion
2.1. Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC), Matrix-Assisted Laser-Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF-MS) and Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analyses of the Venom
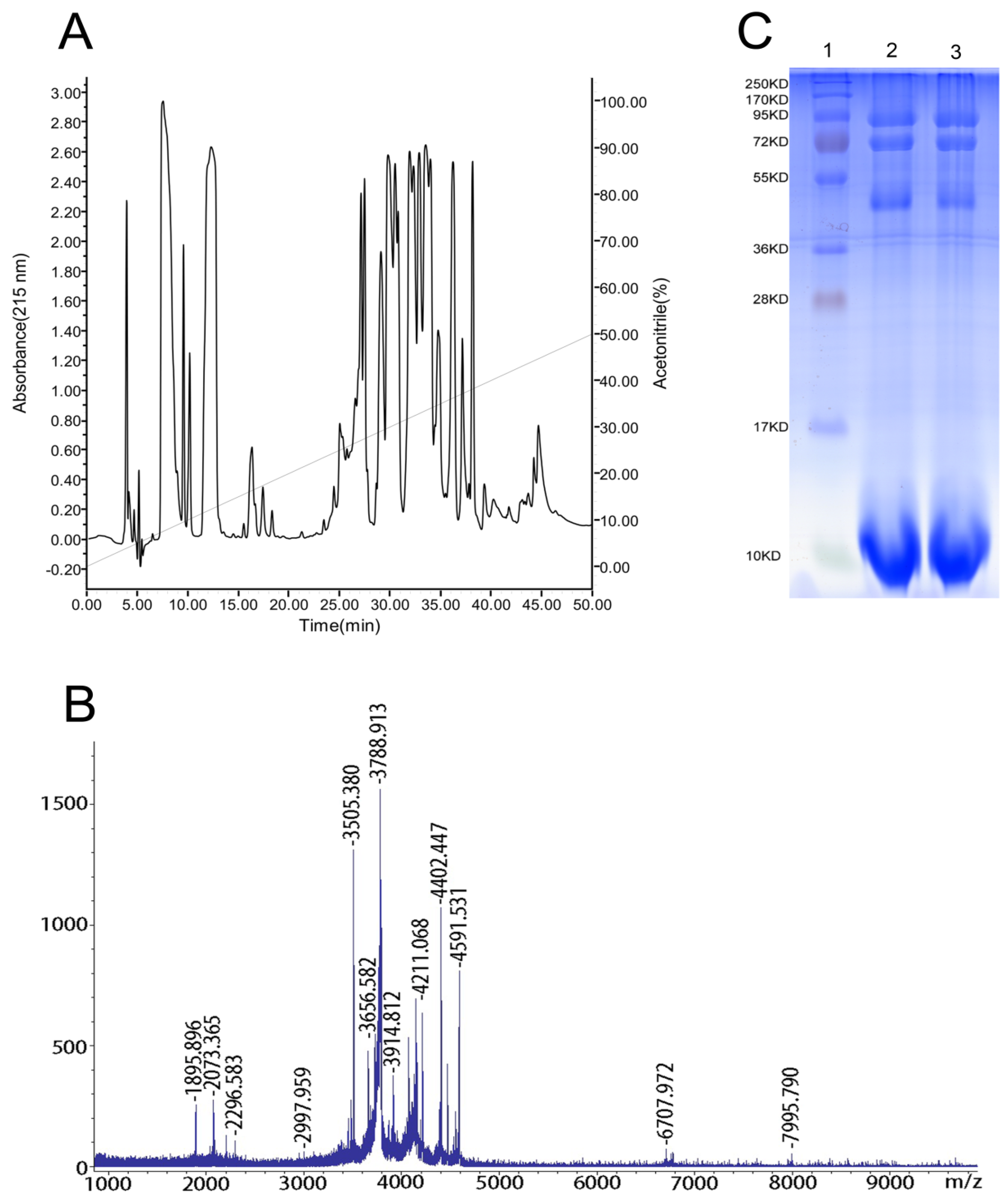

2.2. Effects of the Venom on Voltage-Gated Sodium Channels (VGSCs)
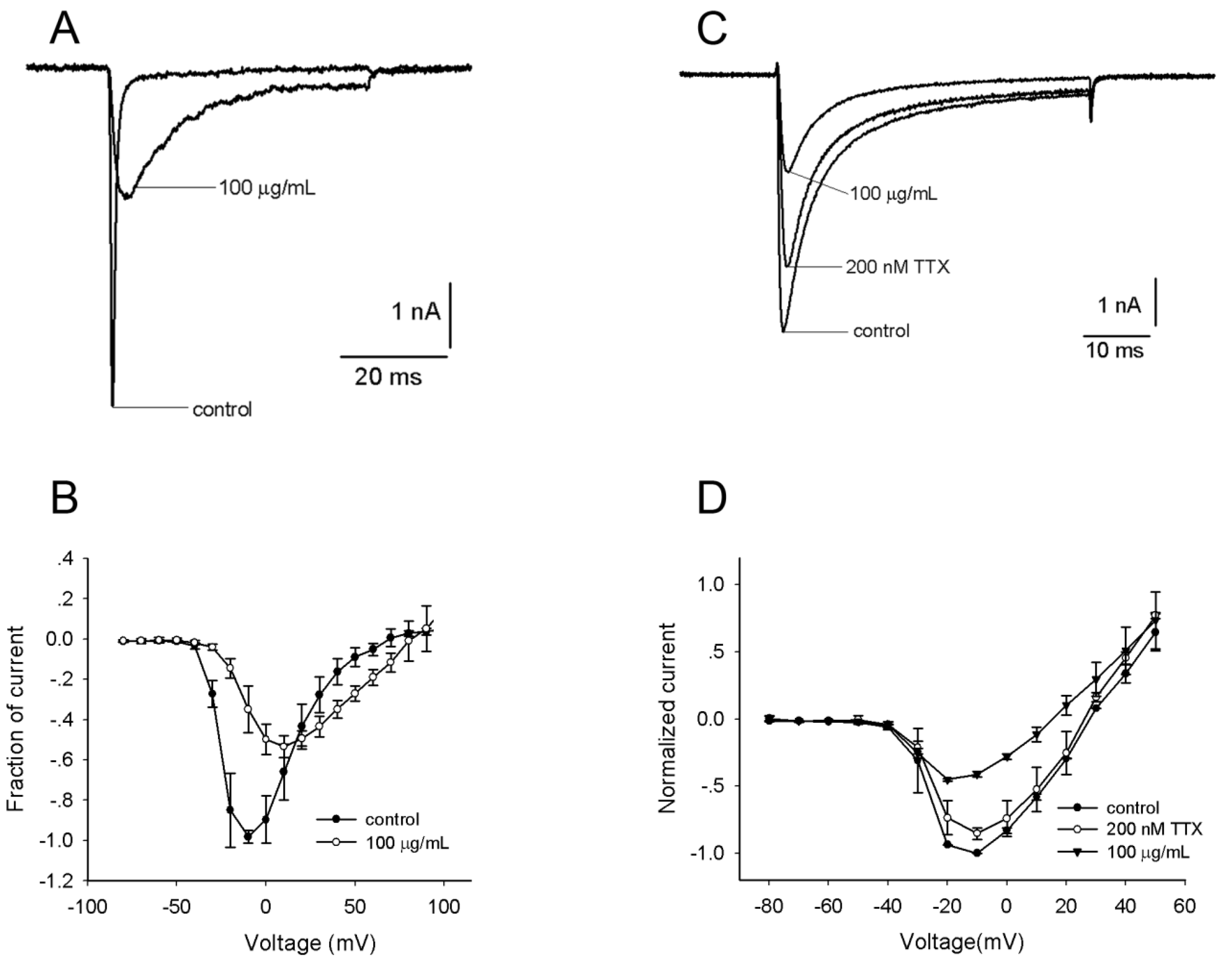
2.3. Effects of the Venom on Voltage-Gated Potassium Channels

2.4. Effects of the Venom on Voltage-Gated Calcium Channels

2.5. Bioactivity Assays
3. Experimental Section
3.1. Venom Collection
3.2. RP-HPLC Analysis
3.3. MALDI-TOF-MS Analysis
3.4. SDS-PAGE Analysis
3.5. Electrophysiological Test
3.6. Bioactivity Assays
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Coddington, J.A.; Levi, H.W. Systematics and evolution of spiders (Araneae). Annu. Rev. Ecol. Syst. 1991, 22, 565–592. [Google Scholar]
- Saez, N.J.; Senff, S.; Jensen, J.E.; Er, S.Y.; Herzig, V.; Rash, L.D.; King, G.F. Spider-venom peptides as therapeutics. Toxins 2010, 12, 2851–2871. [Google Scholar]
- Platnick, N.I. The World Spider Catalog, Version 13.0; American Museum of Natural History. Available online: http://www.research.amnh.org/iz/spiders/catalog (accessed on 18 December 2012).
- Maretic, Z. Spider Venoms and Their Effects. In Ecophysiology of Spiders; Nentwig, W., Ed.; Springer-Verlag: New York, NY, USA, 1987; pp. 142–159. [Google Scholar]
- Zhu, M.S.; Zhang, R. Revision of the theraphosid spiders from China (Araneae: Mygalomorphae). J. Arachnol. 2008, 36, 425–447. [Google Scholar] [CrossRef]
- Klint, J.K.; Senff, S.; Rupasinghe, D.B.; Er, S.Y.; Herzig, V.; Nicholson, G.M.; King, G.F. Spider-venom peptides that target voltage-gated sodium channels: Pharmacological tools and potential therapeutic leads. Toxicon 2012, 60, 478–491. [Google Scholar] [CrossRef]
- Windley, M.J.; Herzig, V.; Dziemborowicz, S.A.; Hardy, M.C.; King, G.F.; Nicholson, G.M. Spider-venom peptides as bioinsecticides. Toxins 2012, 4, 191–227. [Google Scholar] [CrossRef]
- Kuhn-Nentwig, L.; Stöcklin, R.; Nentwig, W. Venom Composition and Strategies in Spiders: Is Everything Possible? In Advances in Insect Physiology; Academic Press: Burlington, MA, USA, 2011; Volume 40. [Google Scholar]
- Escoubas, P.; Sollod, B.; King, G.F. Venom landscapes: Mining the complexity of spider venoms via a combined cDNA and mass spectrometric approach. Toxicon 2006, 47, 650–663. [Google Scholar] [CrossRef]
- Yuan, C.; Jin, Q.; Tang, X.; Hu, W.; Cao, R.; Yang, S.; Xiong, J.; Xie, C.; Xie, J.; Liang, S. Proteomic and peptidomic characterization of the venom from the Chinese bird spider, Ornithoctonus huwena Wang. J. Proteome Res. 2007, 6, 2792–2801. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Y.; Hu, W.; Xu, D.; Yang, X.; Li, Y.; Jiang, L.; Liang, S. Molecular diversification of peptide toxins from the tarantula Haplopelma hainanum (Ornithoctonus hainana) venom based on transcriptomic, peptidomic, and genomic analyses. J. Proteome Res. 2010, 9, 2550–2564. [Google Scholar] [CrossRef]
- Liao, Z.; Cao, J.; Li, S.; Yan, X.; Hu, W.; He, Q.; Chen, J.; Tang, J.; Xie, J.; Liang, S. Proteomic and peptidomic analysis of the venom from Chinese tarantula Chilobrachys jingzhao. Proteomics 2007, 7, 1892–1907. [Google Scholar] [CrossRef]
- Mantegazza, M.; Curia, G.; Biagini, G.; Ragsdale, D.S.; Avoli, M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010, 9, 413–424. [Google Scholar] [CrossRef]
- Ogata, N.; Tatebayashi, H. Kinetic analysis of two types of Na+ channels in rat dorsal root ganglia. J. Physiol. 1993, 466, 9–37. [Google Scholar]
- Ho, C.; O’Leary, M.E. Single-cell analysis of sodium channel expression in dorsal root ganglion neurons. Mol. Cell. Neurosci. 2011, 46, 159–166. [Google Scholar] [CrossRef]
- Harriott, A.M.; Gold, M.S. Contribution of primary afferent channels to neuropathic pain. Curr. Pain Headache Rep. 2009, 13, 197–207. [Google Scholar] [CrossRef]
- Qiao, G.F.; Li, B.Y.; Zhou, Y.H.; Lu, Y.J.; Schild, J.H. Characterization of persistent TTX-R Na+ currents in physiological concentration of sodium in rat visceral afferents. Int. J. Biol. Sci. 2009, 5, 293–297. [Google Scholar]
- Rasband, M.N.; Park, E.W.; Vanderah, T.W.; Lai, J.; Porreca, F.; Trimmer, J.S. Distinct potassium channels on pain-sensing neurons. Proc. Natl. Acad. Sci. USA 2001, 98, 13373–13378. [Google Scholar] [CrossRef]
- Takeda, M.; Tsuboi, Y.; Kitagawa, J.; Nakagawa, K.; Iwata, K.; Matsumoto, S. Potassium channels as a potential therapeutic target for trigeminal neuropathic and inflammatory pain. Mol. Pain 2011, 7. [Google Scholar] [CrossRef]
- Roeper, J.; Pongs, O. Presynaptic potassium channels. Curr. Opin. Neurobiol. 1996, 6, 338–341. [Google Scholar] [CrossRef]
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef]
- Hofmann, F.; Biel, M.; Flockerzi, V. Molecular basis for Ca2+ channel diversity. Annu. Rev. Neurosci. 1994, 17, 399–418. [Google Scholar] [CrossRef]
- Olivera, B.M.; Miljanich, G.P.; Ramachandran, J.; Adams, M.E. Calcium channel diversity and neurotransmitter release: The omega-conotoxins and omega-agatoxins. Annu. Rev. Biochem. 1994, 63, 823–867. [Google Scholar] [CrossRef]
- Nelson, M.T.; Todorovic, S.M.; Perez-Reyes, E. The role of T-type calcium channels in epilepsy and pain. Curr. Pharm. Des. 2006, 12, 2189–2197. [Google Scholar] [CrossRef]
- Yaksh, T.L. Calcium channels as therapeutic targets in neuropathic pain. J. Pain 2006, 7, 13–30. [Google Scholar] [CrossRef]
- Cao, Y.Q. Voltage-gated calcium channels and pain. Pain 2006, 126, 5–9. [Google Scholar] [CrossRef]
- Liang, S.P.; Qin, Y.B.; Zhang, D.Y.; Pan, X.; Chen, X.D.; Xie, J.Y. Biological characterization of spider (Selenocosmia huwena) crude venom. Zool. Res. 1993, 14, 60–65. [Google Scholar]
- Liang, S.P. An overview of peptide toxins from the venom of the Chinese bird spider Selenocosmia huwena Wang [=Ornithoctonus huwena (Wang)]. Toxicon 2004, 43, 575–585. [Google Scholar] [CrossRef]
- Liu, Z.H.; Dai, J.; Dai, L.J.; Deng, M.C.; Hu, Z.; Hu, W.J.; Liang, S.P. Function and solution structure of Huwentoxin-X, a specific blocker of N-type calcium channels, from the Chinese bird spider Ornithoctonus huwena. J. Biol. Chem. 2006, 281, 8628–8635. [Google Scholar]
- Xiao, Y.C.; Liang, S.P. Inhibition of sodium channels in rat dorsal root ganglion neurons by hainantoxin-IV, a novel spider toxin. Acta Biochim. Biophys. Sin. 2003, 35, 82–85. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hu, Z.; Zhou, X.; Chen, J.; Tang, C.; Xiao, Z.; Ying, D.; Liu, Z.; Liang, S. The Venom of the Spider Selenocosmia Jiafu Contains Various Neurotoxins Acting on Voltage-Gated Ion Channels in Rat Dorsal Root Ganglion Neurons. Toxins 2014, 6, 988-1001. https://doi.org/10.3390/toxins6030988
Hu Z, Zhou X, Chen J, Tang C, Xiao Z, Ying D, Liu Z, Liang S. The Venom of the Spider Selenocosmia Jiafu Contains Various Neurotoxins Acting on Voltage-Gated Ion Channels in Rat Dorsal Root Ganglion Neurons. Toxins. 2014; 6(3):988-1001. https://doi.org/10.3390/toxins6030988
Chicago/Turabian StyleHu, Zhaotun, Xi Zhou, Jia Chen, Cheng Tang, Zhen Xiao, Dazhong Ying, Zhonghua Liu, and Songping Liang. 2014. "The Venom of the Spider Selenocosmia Jiafu Contains Various Neurotoxins Acting on Voltage-Gated Ion Channels in Rat Dorsal Root Ganglion Neurons" Toxins 6, no. 3: 988-1001. https://doi.org/10.3390/toxins6030988
APA StyleHu, Z., Zhou, X., Chen, J., Tang, C., Xiao, Z., Ying, D., Liu, Z., & Liang, S. (2014). The Venom of the Spider Selenocosmia Jiafu Contains Various Neurotoxins Acting on Voltage-Gated Ion Channels in Rat Dorsal Root Ganglion Neurons. Toxins, 6(3), 988-1001. https://doi.org/10.3390/toxins6030988




