Type I Interferons as Regulators of Human Antigen Presenting Cell Functions
Abstract
:1. Introduction
2. Type I IFNs in DC Development and Activation
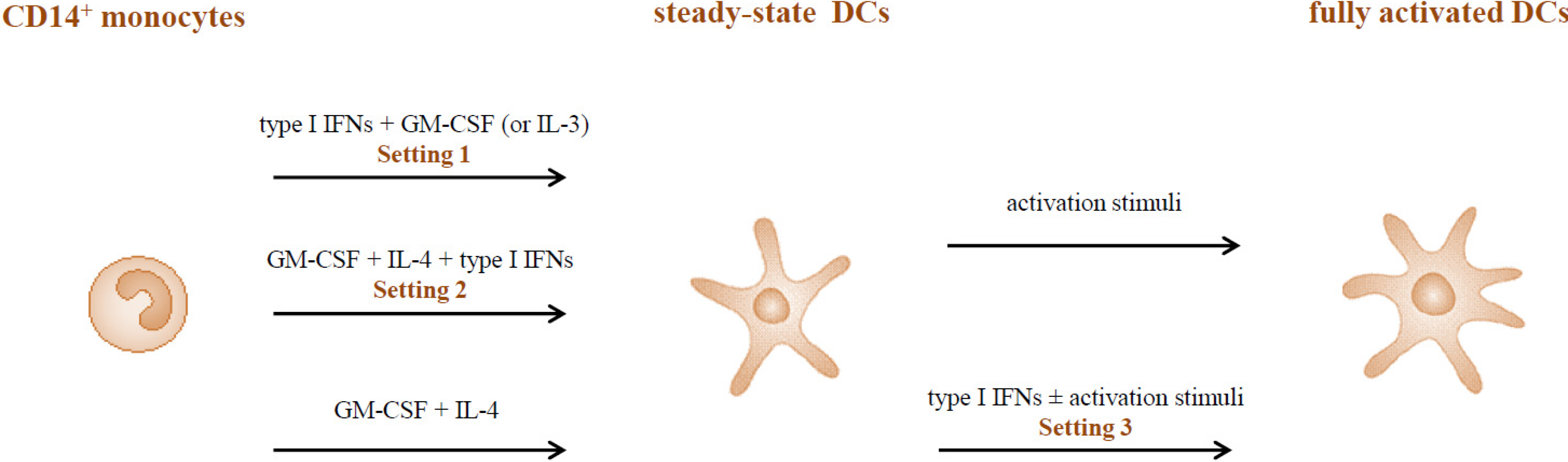
2.1. Type I IFNs Promote the in Vitro Generation of DCs (Setting 1)
| Properties | Partially activated | Fully activated | Ref. |
|---|---|---|---|
| Co-stimulatory molecules | CD25, CD40, CD80, CD86 | ↑ | [21,22,25,26,27,28,29] |
| MHC | Class I (A,B,C), class II (DR) | ↑ | |
| Adhesion molecules | CD44, CD54, CD58, LFA-1 | ↑ | |
| Macrophage markers | CD14 | N.D. | |
| pDC markers | CD123, BDCA1, BDCA4 | N.D. | [21,22,25,26,27,29,30,33,34,47] |
| NK cell markers | TRAIL CD56, granzyme B and M, defensin-α1 | ↑ | [21,26,34] |
| Activation markers | CD83 | ↑ | [21,25,28,35,36] |
| TLR | 1,2,3,4,5,6 | ↓ | [27,36] |
| 7,8 | ↑↓ | ||
| Chemokine receptors | CXCR4, CCR2, CCR5, CCR7 | N.D. | [28,31,37,38,39] |
| Cytokines | TNF-α, IL-1β, IL-6, IL-8, IL-10, IL-12 p40, IL-15, IL-18, intracellular IFN-γ | ↑ IL-12 p70, IL-1β, TNF-α, IL-6, IL-10, IL-15, IL-18, IL-23, IL-27, IL-1RA, type I IFNs | [21,25,27,28,31,33,34,35,36,43] |
| Chemokines | CXCL10, CCL2, CCL8, CCL19 | ↑ CCL2, CCL3, CCL4 | [35,37] |
| Migratory activity | CCL3, CCL4, CCL5, CCL19, CCL21 | N.D. | [37,38,39] |
| Antigen uptake | LOX-1 | ↓ LOX-1 | [27,28,40,41] |
| DC-SIGN, MR, CLEC7A/Dectin1 | |||
| Antigen processing/presentation | CD208/DC-LAMP, TAP-1/2, tapasin, PA28α, PA28β, LMP-2, LMP-7, MECL1 MHC I | ↑ MHC I | [21,27,40,42,43,44] |
| T cell polarization | Th0, Th1, Th2, Tr | ↑ Th0, Th1, Th17 | [21,25,30,33,42,45,46,47] |
2.2. Adjuvant versus Inhibitory Effects of Type I IFNs on the Differentiation of IL-4-DCs (Setting 2)
2.3. Type I IFNs as Activation Stimuli for Immature DCs (Setting 3)
| Observation | Culture conditions | Stimulus | Ref. |
|---|---|---|---|
| ↑ phenotypic activation (CD80, CD86, MHC I and II) | CD34+ progenitors derived DCs | IFN-α2, α8, β | [24] |
| ↑ phenotypic activation (CD86, MHC II) | IL-4-DCs | IFN-α | [22] |
| ↑ phenotypic (CD40, CD86, CD83, MHC II) and functional activation | IL-4-DCs + TNF-α | IFN-α2b | [23] |
| ↑ phenotypic (CD80, CD86, MHC I and II) and functional (CD8 priming) activation = CD83 and CCR7 expression | IL-4-DCs | IFN-α2a | [48,59] |
| ↑ CD38, CD83low | IL-4-DCs | IFN-α | [58] |
| ↑ CD38, CD83, CCR7 | IL-4-DCs | IFN-α + TLR ligands | [60] |
| ↑ apoptosis | IL-4-DCs | IFN-α + microbial stimulation | [51] |
| ↓ phagocytosis | IL-4-DCs | IFN-α2a + CD40L or IFN-α2a + PGE2 + TNF-α | [57] |
| ↑ MHC II expression | GM-CSF cultured | IFN-α2a, IFN-β | [61] |
| ↑ antigen uptake and cross-presentation | IL-4-DCs | IFN-α | [22] |
| ↑ cross-presentation | IL-4-DCs + TNF-α/PGE2/antigen | IFN-α, IFN-αβ | [62] |
| ↓ cross-presentation | IL-4-DCs | IFN-α + TNF-α + PGE2 + Ag | [62] |
3. Type I IFNs in Antigen Uptake/Processing and T Cell Response Generation
3.1. Antigen Recognition and Internalization
3.2. Antigen Processing for Cross-Presentation and CD8+ T Cell Priming
3.3. Antigen Processing for MHC II-Restricted Presentation
3.4. IFN-DC-Mediated T Cell Responses
4. Type I IFN Signature
4.1. Gene Expression Profiles
4.2. microRNA Expression Profiles
| Biological sample | Stimulus | Differentially Expressed gene | Ref. | Biological sample | Stimulus | Differentially Expressed microRNA | Ref. |
|---|---|---|---|---|---|---|---|
| IL4/IFN-DCs (Setting 2) | IFN-α | CXCL-9, CXCL-10, CXCL-11, MxA, MxB, ISG-15, ISG-56K, STAT-1, IRF7, PKR, 2-5OAS, IFP35, BST2 | [102] | IFN-DCs (Setting 1) | IFN-α | ↓ miR-23a; miR-27b; miR-30c; miR-32; miR-100; miR-146a; miR-1 25b; miR-let7e. | [111] |
| IL4-DCs (Setting 3) | IFN-α/ β TNF α/ PGE2/ IFN-α/β | STAT1 STAT4 | [62] | pDCs | IFN-α | ↑ miR-155 ↓ miR-155 * | [114] |
| IFN-DCs (Setting 1) | IFN-α/ω IFN-β | CXCL11 FCGR, MARCO, CLEC5A, DEFB1, IDO1 | [32] | MDMs | IFN-α/β | ↑ miR-28; miR-125b; miR-150; miR-382 | [116] |
| IFN-DCs (Setting 1) | IFN-α | LOX-1 | [40] | IL4-DCs (Setting 3) | RSV/ IFN-β * | ↑ miR Let7b | [117] |
| IFN-DCs (Setting 1) | IFN-α | TLR7 | [27] | PBMCs | Type I IFNs | ↑ miR146a | [118] |
| IFN-DCs (Setting 1) | IFN-α | TRAIL, granzymes, KLRs and other NK cell receptors, DCLAMP, CCR7 and CD49d | [26] | PBMCs | MS/ IFN-β ** | ↓ mir-29 family | [119] |
| IFN-DCs (Setting 1) | IFN-β | IL-6, IL-1β, IL-10, CCL20, CCL3, CCL5, CXCR4, CCR5, CCR2,CD44, TLR2, TLR4, CLECSF12, PRG1, TAP1, β2 microglobulin, CD74, CD1a, CD68 LAMP-3, NFkB2, SOD2, Cdc42, IFIT1 | [31] | PBMCs (healthy donors) | IFN-α | ↑ miR-1; miR-30; miR-128; miR-196; miR-296; | [120] |
5. Conclusions

Acknowledgments
Authors Contributions
Conflicts of Interest
References
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef]
- De Weerd, N.A.; Nguyen, T. The interferons and their receptors—Distribution and regulation. Immunol. Cell Biol. 2012, 90, 483–491. [Google Scholar] [CrossRef]
- Hertzog, P.J.; Williams, B.R. Fine tuning type I interferon responses. Cytokine Growth Factor Rev. 2013, 24, 217–225. [Google Scholar] [CrossRef]
- Vilcek, J. Fifty years of interferon research: Aiming at a moving target. Immunity 2006, 25, 343–348. [Google Scholar] [CrossRef]
- Gresser, I. The antitumor effects of interferon: A personal history. Biochimie 2007, 89, 723–728. [Google Scholar] [CrossRef]
- Wang, B.X.; Rahbar, R.; Fish, E.N. Interferon: Current status and future prospects in cancer therapy. J. Interferon Cytokine Res. 2011, 31, 545–552. [Google Scholar] [CrossRef]
- Belardelli, F.; Gresser, I. The neglected role of type I interferon in the t-cell response: Implications for its clinical use. Immunol. Today 1996, 17, 369–372. [Google Scholar] [CrossRef]
- Tough, D.F. Modulation of t-cell function by type I interferon. Immunol. Cell Biol. 2012, 90, 492–497. [Google Scholar] [CrossRef]
- Hervas-Stubbs, S.; Perez-Gracia, J.L.; Rouzaut, A.; Sanmamed, M.F.; Le Bon, A.; Melero, I. Direct effects of type I interferons on cells of the immune system. Clin. Cancer Res. 2011, 17, 2619–2627. [Google Scholar] [CrossRef]
- Le Bon, A.; Thompson, C.; Kamphuis, E.; Durand, V.; Rossmann, C.; Kalinke, U.; Tough, D.F. Cutting edge: Enhancement of antibody responses through direct stimulation of b and T cells by type I IFN. J. Immunol. 2006, 176, 2074–2078. [Google Scholar] [CrossRef]
- Stackaruk, M.L.; Lee, A.J.; Ashkar, A.A. Type I interferon regulation of natural killer cell function in primary and secondary infections. Expert Rev. Vaccines 2013, 12, 875–884. [Google Scholar] [CrossRef]
- Belardelli, F.; Ferrantini, M. Cytokines as a link between innate and adaptive antitumor immunity. Trends Immunol. 2002, 23, 201–208. [Google Scholar] [CrossRef]
- Bracci, L.; La Sorsa, V.; Belardelli, F.; Proietti, E. Type I interferons as vaccine adjuvants against infectious diseases and cancer. Expert Rev. Vaccines 2008, 7, 373–381. [Google Scholar] [CrossRef]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef]
- Steinman, R.M.; Cohn, Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973, 137, 1142–1162. [Google Scholar] [CrossRef]
- Shortman, K.; Naik, S.H. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 2007, 7, 19–30. [Google Scholar] [CrossRef]
- Schlitzer, A.; Ginhoux, F. Organization of the mouse and human dc network. Curr. Opin. Immunol. 2014, 26, 90–99. [Google Scholar] [CrossRef]
- Vieira, P.L.; de Jong, E.C.; Wierenga, E.A.; Kapsenberg, M.L.; Kalinski, P. Development of th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J. Immunol. 2000, 164, 4507–4512. [Google Scholar] [CrossRef]
- Zou, G.M.; Tam, Y.K. Cytokines in the generation and maturation of dendritic cells: Recent advances. Eur. Cytokine Netw. 2002, 13, 186–199. [Google Scholar]
- Conti, L.; Gessani, S. Gm-csf in the generation of dendritic cells from human blood monocyte precursors: Recent advances. Immunobiology 2008, 213, 859–870. [Google Scholar] [CrossRef]
- Santini, S.M.; Lapenta, C.; Logozzi, M.; Parlato, S.; Spada, M.; di Pucchio, T.; Belardelli, F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in hu-pbl-scid mice. J. Exp. Med. 2000, 191, 1777–1788. [Google Scholar] [CrossRef]
- Paquette, R.L.; Hsu, N.C.; Kiertscher, S.M.; Park, A.N.; Tran, L.; Roth, M.D.; Glaspy, J.A. Interferon-alpha and granulocyte-macrophage colony-stimulating factor differentiate peripheral blood monocytes into potent antigen-presenting cells. J. Leukoc. Biol. 1998, 64, 358–367. [Google Scholar]
- Radvanyi, L.G.; Banerjee, A.; Weir, M.; Messner, H. Low levels of interferon-alpha induce CD86 (B7.2) expression and accelerates dendritic cell maturation from human peripheral blood mononuclear cells. Scand. J. Immunol. 1999, 50, 499–509. [Google Scholar] [CrossRef]
- Luft, T.; Pang, K.C.; Thomas, E.; Hertzog, P.; Hart, D.N.; Trapani, J.; Cebon, J. Type I IFNs enhance the terminal differentiation of dendritic cells. J. Immunol. 1998, 161, 1947–1953. [Google Scholar]
- Della Bella, S.; Nicola, S.; Riva, A.; Biasin, M.; Clerici, M.; Villa, M.L. Functional repertoire of dendritic cells generated in granulocyte macrophage-colony stimulating factor and interferon-alpha. J. Leukoc. Biol. 2004, 75, 106–116. [Google Scholar]
- Korthals, M.; Safaian, N.; Kronenwett, R.; Maihofer, D.; Schott, M.; Papewalis, C.; Diaz Blanco, E.; Winter, M.; Czibere, A.; Haas, R.; et al. Monocyte derived dendritic cells generated by IFN-alpha acquire mature dendritic and natural killer cell properties as shown by gene expression analysis. J. Transl. Med. 2007, 5, 46. [Google Scholar] [CrossRef]
- Mohty, M.; Vialle-Castellano, A.; Nunes, J.A.; Isnardon, D.; Olive, D.; Gaugler, B. Ifn-alpha skews monocyte differentiation into toll-like receptor 7-expressing dendritic cells with potent functional activities. J. Immunol. 2003, 171, 3385–3393. [Google Scholar]
- Carbonneil, C.; Aouba, A.; Burgard, M.; Cardinaud, S.; Rouzioux, C.; Langlade-Demoyen, P.; Weiss, L. Dendritic cells generated in the presence of granulocyte-macrophage colony-stimulatingfactor and IFN-alpha are potent inducers of HIV-specific CD8 T cells. Aids 2003, 17, 1731–1740. [Google Scholar] [CrossRef]
- Renneson, J.; Salio, M.; Mazouz, N.; Goldman, M.; Marchant, A.; Cerundolo, V. Mature dendritic cells differentiated in the presence of interferon-beta and interleukin-3 prime functional antigen-specific CD8 T cells. Clin. Exp. Immunol. 2005, 139, 468–475. [Google Scholar] [CrossRef]
- Huang, Y.M.; Hussien, Y.; Yarilin, D.; Xiao, B.G.; Liu, Y.J.; Link, H. Interferon-beta induces the development of type 2 dendritic cells. Cytokine 2001, 13, 264–271. [Google Scholar] [CrossRef]
- Mazouz, N.; Detournay, O.; Buelens, C.; Renneson, J.; Trakatelli, M.; Lambermont, M.; Goldman, M.; Toungouz, M. Immunostimulatory properties of human dendritic cells generated using IFN-beta associated either with IL-3 or gm-csf. Cancer Immunol. Immunother. 2005, 54, 1010–1017. [Google Scholar]
- Garcin, G.; Bordat, Y.; Chuchana, P.; Monneron, D.; Law, H.K.; Piehler, J.; Uze, G. Differential activity of type I interferon subtypes for dendritic cell differentiation. PLoS ONE 2013, 8, e58465. [Google Scholar]
- Buelens, C.; Bartholome, E.J.; Amraoui, Z.; Boutriaux, M.; Salmon, I.; Thielemans, K.; Willems, F.; Goldman, M. Interleukin-3 and interferon beta cooperate to induce differentiation of monocytes into dendritic cells with potent helper t-cell stimulatory properties. Blood 2002, 99, 993–998. [Google Scholar]
- Papewalis, C.; Jacobs, B.; Wuttke, M.; Ullrich, E.; Baehring, T.; Fenk, R.; Willenberg, H.S.; Schinner, S.; Cohnen, M.; Seissler, J.; et al. Ifn-alpha skews monocytes into CD56+-expressing dendritic cells with potent functional activities in vitro and in vivo. J. Immunol. 2008, 180, 1462–1470. [Google Scholar] [CrossRef]
- Bracci, L.; Schumacher, R.; Provenzano, M.; Adamina, M.; Rosenthal, R.; Groeper, C.; Zajac, P.; Iezzi, G.; Proietti, E.; Belardelli, F.; et al. Efficient stimulation of T cell responses by human IFN-alpha-induced dendritic cells does not require toll-like receptor triggering. J. Immunother. 2008, 31, 466–474. [Google Scholar] [CrossRef]
- Farkas, A.; Tonel, G.; Nestle, F.O. Interferon-alpha and viral triggers promote functional maturation of human monocyte-derived dendritic cells. Br. J. Dermatol. 2008, 158, 921–929. [Google Scholar]
- Parlato, S.; Santini, S.M.; Lapenta, C.; di Pucchio, T.; Logozzi, M.; Spada, M.; Giammarioli, A.M.; Malorni, W.; Fais, S.; Belardelli, F. Expression of ccr-7, mip-3beta, and th-1 chemokines in type I IFN-induced monocyte-derived dendritic cells: Importance for the rapid acquisition of potent migratory and functional activities. Blood 2001, 98, 3022–3029. [Google Scholar] [CrossRef]
- Gauzzi, M.C.; Purificato, C.; Donato, K.; Jin, Y.; Wang, L.; Daniel, K.C.; Maghazachi, A.A.; Belardelli, F.; Adorini, L.; Gessani, S. Suppressive effect of 1alpha,25-dihydroxyvitamin d3 on type I IFN-mediated monocyte differentiation into dendritic cells: Impairment of functional activities and chemotaxis. J. Immunol. 2005, 174, 270–276. [Google Scholar] [CrossRef]
- Rouzaut, A.; Garasa, S.; Teijeira, A.; Gonzalez, I.; Martinez-Forero, I.; Suarez, N.; Larrea, E.; Alfaro, C.; Palazon, A.; Dubrot, J.; et al. Dendritic cells adhere to and transmigrate across lymphatic endothelium in response to IFN-alpha. Eur. J. Immunol. 2010, 40, 3054–3063. [Google Scholar] [CrossRef]
- Parlato, S.; Romagnoli, G.; Spadaro, F.; Canini, I.; Sirabella, P.; Borghi, P.; Ramoni, C.; Filesi, I.; Biocca, S.; Gabriele, L.; et al. Lox-1 as a natural IFN-alpha-mediated signal for apoptotic cell uptake and antigen presentation in dendritic cells. Blood 2010, 115, 1554–1563. [Google Scholar] [CrossRef]
- Cardone, M.; Ikeda, K.N.; Varano, B.; Belardelli, F.; Millefiorini, E.; Gessani, S.; Conti, L. Opposite regulatory effects of IFN-beta and IL-3 on c-type lectin receptors, antigen uptake, and phagocytosis in human macrophages. J. Leukoc. Biol. 2014, 95, 161–168. [Google Scholar]
- Lapenta, C.; Santini, S.M.; Spada, M.; Donati, S.; Urbani, F.; Accapezzato, D.; Franceschini, D.; Andreotti, M.; Barnaba, V.; Belardelli, F. Ifn-alpha-conditioned dendritic cells are highly efficient in inducing cross-priming CD8(+) T cells against exogenous viral antigens. Eur. J. Immunol. 2006, 36, 2046–2060. [Google Scholar] [CrossRef]
- Lattanzi, L.; Rozera, C.; Marescotti, D.; D’Agostino, G.; Santodonato, L.; Cellini, S.; Belardelli, F.; Gavioli, R.; Ferrantini, M. Ifn-alpha boosts epitope cross-presentation by dendritic cells via modulation of proteasome activity. Immunobiology 2011, 216, 537–547. [Google Scholar] [CrossRef]
- Tosello, V.; Zamarchi, R.; Merlo, A.; Gorza, M.; Piovan, E.; Mandruzzato, S.; Bronte, V.; Wang, X.; Ferrone, S.; Amadori, A.; et al. Differential expression of constitutive and inducible proteasome subunits in human monocyte-derived dc differentiated in the presence of IFN-alpha or IL-4. Eur. J. Immunol. 2009, 39, 56–66. [Google Scholar] [CrossRef]
- Carbonneil, C.; Saidi, H.; Donkova-Petrini, V.; Weiss, L. Dendritic cells generated in the presence of interferon-alpha stimulate allogeneic CD4+ T-cell proliferation: Modulation by autocrine IL-10, enhanced t-cell apoptosis and T regulatory type 1 cells. Int. Immunol. 2004, 16, 1037–1052. [Google Scholar] [CrossRef]
- Lapenta, C.; Santini, S.M.; Logozzi, M.; Spada, M.; Andreotti, M.; di Pucchio, T.; Parlato, S.; Belardelli, F. Potent immune response against hiv-1 and protection from virus challenge in hu-pbl-scid mice immunized with inactivated virus-pulsed dendritic cells generated in the presence of IFN-alpha. J. Exp. Med. 2003, 198, 361–367. [Google Scholar] [CrossRef]
- Santodonato, L.; D’Agostino, G.; Nisini, R.; Mariotti, S.; Monque, D.M.; Spada, M.; Lattanzi, L.; Perrone, M.P.; Andreotti, M.; Belardelli, F.; et al. Monocyte-derived dendritic cells generated after a short-term culture with IFN-alpha and granulocyte-macrophage colony-stimulating factor stimulate a potent epstein-barr virus-specific CD8+ T cell response. J. Immunol. 2003, 170, 5195–5202. [Google Scholar] [CrossRef]
- Padovan, E.; Spagnoli, G.C.; Ferrantini, M.; Heberer, M. Ifn-alpha2a induces ip-10/cxcl10 and mig/cxcl9 production in monocyte-derived dendritic cells and enhances their capacity to attract and stimulate CD8+ effector T cells. J. Leukoc. Biol. 2002, 71, 669–676. [Google Scholar]
- Cabanas, C.; Hogg, N. Ligand intercellular adhesion molecule 1 has a necessary role in activation of integrin lymphocyte function-associated molecule 1. Proc. Natl. Acad. Sci. USA 1993, 90, 5838–5842. [Google Scholar] [CrossRef]
- Gauzzi, M.C.; Canini, I.; Eid, P.; Belardelli, F.; Gessani, S. Loss of type I IFN receptors and impaired IFN responsiveness during terminal maturation of monocyte-derived human dendritic cells. J. Immunol. 2002, 169, 3038–3045. [Google Scholar] [CrossRef]
- Lehner, M.; Felzmann, T.; Clodi, K.; Holter, W. Type I interferons in combination with bacterial stimuli induce apoptosis of monocyte-derived dendritic cells. Blood 2001, 98, 736–742. [Google Scholar] [CrossRef]
- McRae, B.L.; Nagai, T.; Semnani, R.T.; van Seventer, J.M.; van Seventer, G.A. Interferon-alpha and -beta inhibit the in vitro differentiation of immunocompetent human dendritic cells from CD14(+) precursors. Blood 2000, 96, 210–217. [Google Scholar]
- Bartholome, E.J.; Willems, F.; Crusiaux, A.; Thielemans, K.; Schandene, L.; Goldman, M. IFN-beta interferes with the differentiation of dendritic cells from peripheral blood mononuclear cells: Selective inhibition of CD40-dependent interleukin-12 secretion. J. Interferon Cytokine Res. 1999, 19, 471–478. [Google Scholar] [CrossRef]
- Hussien, Y.; Sanna, A.; Soderstrom, M.; Link, H.; Huang, Y.M. Glatiramer acetate and IFN-beta act on dendritic cells in multiple sclerosis. J. Neuroimmunol. 2001, 121, 102–110. [Google Scholar] [CrossRef]
- Dauer, M.; Pohl, K.; Obermaier, B.; Meskendahl, T.; Robe, J.; Schnurr, M.; Endres, S.; Eigler, A. Interferon-alpha disables dendritic cell precursors: Dendritic cells derived from interferon-alpha-treated monocytes are defective in maturation and t-cell stimulation. Immunology 2003, 110, 38–47. [Google Scholar] [CrossRef]
- Ito, T.; Amakawa, R.; Inaba, M.; Ikehara, S.; Inaba, K.; Fukuhara, S. Differential regulation of human blood dendritic cell subsets by IFNs. J. Immunol. 2001, 166, 2961–2969. [Google Scholar] [CrossRef]
- Luft, T.; Luetjens, P.; Hochrein, H.; Toy, T.; Masterman, K.A.; Rizkalla, M.; Maliszewski, C.; Shortman, K.; Cebon, J.; Maraskovsky, E. Ifn-alpha enhances CD40 ligand-mediated activation of immature monocyte-derived dendritic cells. Int. Immunol. 2002, 14, 367–380. [Google Scholar] [CrossRef]
- Trepiakas, R.; Pedersen, A.E.; Met, O.; Svane, I.M. Addition of interferon-alpha to a standard maturation cocktail induces CD38 up-regulation and increases dendritic cell function. Vaccine 2009, 27, 2213–2219. [Google Scholar] [CrossRef]
- Svane, I.M.; Nikolajsen, K.; Walter, M.R.; Buus, S.; Gad, M.; Claesson, M.H.; Pedersen, A.E. Characterization of monocyte-derived dendritic cells maturated with IFN-alpha. Scand. J. Immunol. 2006, 63, 217–222. [Google Scholar] [CrossRef]
- Nguyen-Pham, T.N.; Lim, M.S.; Nguyen, T.A.; Lee, Y.K.; Jin, C.J.; Lee, H.J.; Hong, C.Y.; Ahn, J.S.; Yang, D.H.; Kim, Y.K.; et al. Type I and II interferons enhance dendritic cell maturation and migration capacity by regulating CD38 and CD74 that have synergistic effects with TLR agonists. Cell. Mol. Immunol. 2011, 8, 341–347. [Google Scholar] [CrossRef]
- Simmons, D.P.; Wearsch, P.A.; Canaday, D.H.; Meyerson, H.J.; Liu, Y.C.; Wang, Y.; Boom, W.H.; Harding, C.V. Type I IFN drives a distinctive dendritic cell maturation phenotype that allows continued class ii mhc synthesis and antigen processing. J. Immunol. 2012, 188, 3116–3126. [Google Scholar] [CrossRef]
- Longman, R.S.; Braun, D.; Pellegrini, S.; Rice, C.M.; Darnell, R.B.; Albert, M.L. Dendritic-cell maturation alters intracellular signaling networks, enabling differential effects of IFN-alpha/beta on antigen cross-presentation. Blood 2007, 109, 1113–1122. [Google Scholar]
- Nagai, T.; Devergne, O.; Mueller, T.F.; Perkins, D.L.; van Seventer, J.M.; van Seventer, G.A. Timing of IFN-beta exposure during human dendritic cell maturation and naive th cell stimulation has contrasting effects on th1 subset generation: A role for IFN-beta-mediated regulation of IL-12 family cytokines and IL-18 in naive th cell differentiation. J. Immunol. 2003, 171, 5233–5243. [Google Scholar]
- Wiesemann, E.; Sonmez, D.; Heidenreich, F.; Windhagen, A. Interferon-beta increases the stimulatory capacity of monocyte-derived dendritic cells to induce IL-13, IL-5 and IL-10 in autologous t-cells. J. Neuroimmunol. 2002, 123, 160–169. [Google Scholar] [CrossRef]
- Jensen, P.E. Recent advances in antigen processing and presentation. Nat. Immunol. 2007, 8, 1041–1048. [Google Scholar] [CrossRef]
- Wang, J.; Nikrad, M.P.; Travanty, E.A.; Zhou, B.; Phang, T.; Gao, B.; Alford, T.; Ito, Y.; Nahreini, P.; Hartshorn, K.; et al. Innate immune response of human alveolar macrophages during influenza a infection. PLoS One 2012, 7, e29879. [Google Scholar] [CrossRef]
- Song, Q.; Meng, Y.; Wang, Y.; Li, M.; Zhang, J.; Xin, S.; Wang, L.; Shan, F. Maturation inside and outside bone marrow dendritic cells (bmdcs) modulated by interferon-alpha (IFN-alpha). Int. Immunopharmacol. 2013, 17, 843–849. [Google Scholar] [CrossRef]
- Le Bon, A.; Etchart, N.; Rossmann, C.; Ashton, M.; Hou, S.; Gewert, D.; Borrow, P.; Tough, D.F. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 2003, 4, 1009–1015. [Google Scholar] [CrossRef]
- Schiavoni, G.; Mattei, F.; Gabriele, L. Type I interferons as stimulators of dc-mediated cross-priming: Impact on anti-tumor response. Front. Immunol. 2013, 4, 483. [Google Scholar]
- Kuchtey, J.; Chefalo, P.J.; Gray, R.C.; Ramachandra, L.; Harding, C.V. Enhancement of dendritic cell antigen cross-presentation by cpg DNA involves type I IFN and stabilization of class I mhc mrna. J. Immunol. 2005, 175, 2244–2251. [Google Scholar] [CrossRef]
- Gray, R.C.; Kuchtey, J.; Harding, C.V. Cpg-b odns potently induce low levels of IFN-alphabeta and induce IFN-alphabeta-dependent mhc-i cross-presentation in dcs as effectively as cpg-a and cpg-c odns. J. Leukoc. Biol. 2007, 81, 1075–1085. [Google Scholar]
- Hoebe, K.; Beutler, B. Lps, dsrna and the interferon bridge to adaptive immune responses: Trif, tram, and other tir adaptor proteins. J. Endotoxin Res. 2004, 10, 130–136. [Google Scholar] [CrossRef]
- Zietara, N.; Lyszkiewicz, M.; Gekara, N.; Puchalka, J.; Dos Santos, V.A.; Hunt, C.R.; Pandita, T.K.; Lienenklaus, S.; Weiss, S. Absence of IFN-beta impairs antigen presentation capacity of splenic dendritic cells via down-regulation of heat shock protein 70. J. Immunol. 2009, 183, 1099–1109. [Google Scholar]
- Lorenzi, S.; Mattei, F.; Sistigu, A.; Bracci, L.; Spadaro, F.; Sanchez, M.; Spada, M.; Belardelli, F.; Gabriele, L.; Schiavoni, G. Type I IFNs control antigen retention and survival of CD8alpha(+) dendritic cells after uptake of tumor apoptotic cells leading to cross-priming. J. Immunol. 2011, 186, 5142–5150. [Google Scholar] [CrossRef]
- Spadaro, F.; Lapenta, C.; Donati, S.; Abalsamo, L.; Barnaba, V.; Belardelli, F.; Santini, S.M.; Ferrantini, M. IFN-alpha enhances cross-presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood 2012, 119, 1407–1417. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Ng, C.; Lee, A.M.; Sullivan, B.M.; Sheehan, K.C.; Welch, M.; Schreiber, R.D.; de la Torre, J.C.; Oldstone, M.B. Persistent lcmv infection is controlled by blockade of type I interferon signaling. Science 2013, 340, 207–211. [Google Scholar] [CrossRef]
- Wilson, E.B.; Yamada, D.H.; Elsaesser, H.; Herskovitz, J.; Deng, J.; Cheng, G.; Aronow, B.J.; Karp, C.L.; Brooks, D.G. Blockade of chronic type I interferon signaling to control persistent lcmv infection. Science 2013, 340, 202–207. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Fu, M.L.; Weichselbaum, R.R.; Gajewski, T.F.; Guo, Y.; Fu, Y.X. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer Cell 2014, 25, 37–48. [Google Scholar] [CrossRef]
- Huber, J.P.; Farrar, J.D. Regulation of effector and memory t-cell functions by type I interferon. Immunology 2011, 132, 466–474. [Google Scholar] [CrossRef]
- Starbeck-Miller, G.R.; Xue, H.H.; Harty, J.T. Il-12 and type I interferon prolong the division of activated CD8 T cells by maintaining high-affinity IL-2 signaling in vivo. J. Exp. Med. 2014, 211, 105–120. [Google Scholar] [CrossRef]
- Santini, S.M.; Lapenta, C.; Donati, S.; Spadaro, F.; Belardelli, F.; Ferrantini, M. Interferon-alpha-conditioned human monocytes combine a th1-orienting attitude with the induction of autologous th17 responses: Role of IL-23 and IL-12. PLoS One 2011, 6, e17364. [Google Scholar]
- Trinchieri, G.; Pflanz, S.; Kastelein, R.A. The IL-12 family of heterodimeric cytokines: New players in the regulation of T cell responses. Immunity 2003, 19, 641–644. [Google Scholar] [CrossRef]
- Matsui, M.; Moriya, O.; Belladonna, M.L.; Kamiya, S.; Lemonnier, F.A.; Yoshimoto, T.; Akatsuka, T. Adjuvant activities of novel cytokines, interleukin-23 (IL-23) and IL-27, for induction of hepatitis c virus-specific cytotoxic T lymphocytes in hla-a*0201 transgenic mice. J. Virol. 2004, 78, 9093–9104. [Google Scholar] [CrossRef]
- Ha, S.J.; Kim, D.J.; Baek, K.H.; Yun, Y.D.; Sung, Y.C. Il-23 induces stronger sustained ctl and th1 immune responses than IL-12 in hepatitis c virus envelope protein 2 DNA immunization. J. Immunol. 2004, 172, 525–531. [Google Scholar] [CrossRef]
- Detournay, O.; Mazouz, N.; Goldman, M.; Toungouz, M. Il-6 produced by type I IFN dc controls IFN-gamma production by regulating the suppressive effect of CD4+ CD25+ regulatory T cells. Hum. Immunol. 2005, 66, 460–468. [Google Scholar] [CrossRef]
- Rodriguez, A.; Vigorito, E.; Clare, S.; Warren, M.V.; Couttet, P.; Soond, D.R.; van Dongen, S.; Grocock, R.J.; Das, P.P.; Miska, E.A.; et al. Requirement of bic/microrna-155 for normal immune function. Science 2007, 316, 608–611. [Google Scholar]
- Steiner, D.F.; Thomas, M.F.; Hu, J.K.; Yang, Z.; Babiarz, J.E.; Allen, C.D.; Matloubian, M.; Blelloch, R.; Ansel, K.M. Microrna-29 regulates t-box transcription factors and interferon-gamma production in helper T cells. Immunity 2011, 35, 169–181. [Google Scholar] [CrossRef]
- Thai, T.H.; Calado, D.P.; Casola, S.; Ansel, K.M.; Xiao, C.; Xue, Y.; Murphy, A.; Frendewey, D.; Valenzuela, D.; Kutok, J.L.; et al. Regulation of the germinal center response by microrna-155. Science 2007, 316, 604–608. [Google Scholar] [CrossRef]
- Bai, Y.; Qian, C.; Qian, L.; Ma, F.; Hou, J.; Chen, Y.; Wang, Q.; Cao, X. Integrin CD11b negatively regulates tlr9-triggered dendritic cell cross-priming by upregulating microrna-146a. J. Immunol. 2012, 188, 5293–5302. [Google Scholar] [CrossRef]
- Wang, P.; Gu, Y.; Zhang, Q.; Han, Y.; Hou, J.; Lin, L.; Wu, C.; Bao, Y.; Su, X.; Jiang, M.; et al. Identification of resting and type I IFN-activated human nk cell mirnomes reveals microrna-378 and microrna-30e as negative regulators of nk cell cytotoxicity. J. Immunol. 2012, 189, 211–221. [Google Scholar] [CrossRef]
- Ebert, M.S.; Sharp, P.A. Roles for micrornas in conferring robustness to biological processes. Cell 2012, 149, 515–524. [Google Scholar]
- Turner, M.L.; Schnorfeil, F.M.; Brocker, T. Micrornas regulate dendritic cell differentiation and function. J. Immunol. 2011, 187, 3911–3917. [Google Scholar] [CrossRef]
- El Gazzar, M.; McCall, C.E. Micrornas regulatory networks in myeloid lineage development and differentiation: Regulators of the regulators. Immunol. Cell Biol. 2012, 90, 587–593. [Google Scholar] [CrossRef]
- Zhan, Y.; Wu, L. Functional regulation of monocyte-derived dendritic cells by micrornas. Protein Cell 2012, 3, 497–507. [Google Scholar] [CrossRef]
- Moller, I.; Michel, K.; Frech, N.; Burger, M.; Pfeifer, D.; Frommolt, P.; Veelken, H.; Thomas-Kaskel, A.K. Dendritic cell maturation with poly(i:C)-based versus pge2-based cytokine combinations results in differential functional characteristics relevant to clinical application. J. Immunother. 2008, 31, 506–519. [Google Scholar] [CrossRef]
- Messmer, D.; Messmer, B.; Chiorazzi, N. The global transcriptional maturation program and stimuli-specific gene expression profiles of human myeloid dendritic cells. Int. Immunol. 2003, 15, 491–503. [Google Scholar] [CrossRef]
- De Veer, M.J.; Holko, M.; Frevel, M.; Walker, E.; Der, S.; Paranjape, J.M.; Silverman, R.H.; Williams, B.R. Functional classification of interferon-stimulated genes identified using microarrays. J. Leukoc. Biol. 2001, 69, 912–920. [Google Scholar]
- Samarajiwa, S.A.; Forster, S.; Auchettl, K.; Hertzog, P.J. Interferome: The database of interferon regulated genes. Nucleic Acids Res. 2009, 37, D852–D857. [Google Scholar] [CrossRef]
- Lynn, D.J.; Chan, C.; Naseer, M.; Yau, M.; Lo, R.; Sribnaia, A.; Ring, G.; Que, J.; Wee, K.; Winsor, G.L.; et al. Curating the innate immunity interactome. BMC Syst. Biol. 2010, 4, 117. [Google Scholar] [CrossRef]
- Korb, M.; Rust, A.G.; Thorsson, V.; Battail, C.; Li, B.; Hwang, D.; Kennedy, K.A.; Roach, J.C.; Rosenberger, C.M.; Gilchrist, M.; et al. The innate immune database (IIDB). BMC Immunol. 2008, 9. [Google Scholar] [CrossRef]
- Stroncek, D.F.; Basil, C.; Nagorsen, D.; Deola, S.; Arico, E.; Smith, K.; Wang, E.; Marincola, F.M.; Panelli, M.C. Delayed polarization of mononuclear phagocyte transcriptional program by type I interferon isoforms. J. Transl. Med. 2005, 3. [Google Scholar] [CrossRef]
- Schlaak, J.F.; Hilkens, C.M.; Costa-Pereira, A.P.; Strobl, B.; Aberger, F.; Frischauf, A.M.; Kerr, I.M. Cell-type and donor-specific transcriptional responses to interferon-alpha. Use of customized gene arrays. J. Biol. Chem. 2002, 277, 49428–49437. [Google Scholar] [CrossRef]
- Barnes, E.; Salio, M.; Cerundolo, V.; Medlin, J.; Murphy, S.; Dusheiko, G.; Klenerman, P. Impact of alpha interferon and ribavirin on the function of maturing dendritic cells. Antimicrob. Agents Chemother. 2004, 48, 3382–3389. [Google Scholar] [CrossRef]
- Izaguirre, A.; Barnes, B.J.; Amrute, S.; Yeow, W.S.; Megjugorac, N.; Dai, J.; Feng, D.; Chung, E.; Pitha, P.M.; Fitzgerald-Bocarsly, P. Comparative analysis of irf and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J. Leukoc. Biol. 2003, 74, 1125–1138. [Google Scholar]
- Takauji, R.; Iho, S.; Takatsuka, H.; Yamamoto, S.; Takahashi, T.; Kitagawa, H.; Iwasaki, H.; Iida, R.; Yokochi, T.; Matsuki, T. Cpg-DNA-induced IFN-alpha production involves p38 mapk-dependent stat1 phosphorylation in human plasmacytoid dendritic cell precursors. J. Leukoc. Biol. 2002, 72, 1011–1019. [Google Scholar]
- Forster, S. Interferon signatures in immune disorders and disease. Immunol. Cell Biol. 2012, 90, 520–527. [Google Scholar] [CrossRef]
- Arico, E.; Belardelli, F. Interferon-alpha as antiviral and antitumor vaccine adjuvants: Mechanisms of action and response signature. J. Interferon Cytokine Res. 2012, 32, 235–247. [Google Scholar] [CrossRef]
- Arico, E.; Castiello, L.; Urbani, F.; Rizza, P.; Panelli, M.C.; Wang, E.; Marincola, F.M.; Belardelli, F. Concomitant detection of ifnalpha signature and activated monocyte/dendritic cell precursors in the peripheral blood of ifnalpha-treated subjects at early times after repeated local cytokine treatments. J. Transl. Med. 2011, 9. [Google Scholar] [CrossRef]
- Rubins, K.H.; Hensley, L.E.; Jahrling, P.B.; Whitney, A.R.; Geisbert, T.W.; Huggins, J.W.; Owen, A.; Leduc, J.W.; Brown, P.O.; Relman, D.A. The host response to smallpox: Analysis of the gene expression program in peripheral blood cells in a nonhuman primate model. Proc. Natl. Acad. Sci. USA 2004, 101, 15190–15195. [Google Scholar] [CrossRef]
- David, M. Interferons and micrornas. J. Interferon Cytokine Res. 2010, 30, 825–828. [Google Scholar] [CrossRef]
- Parlato, S.; Bruni, R.; Fragapane, P.; Salerno, D.; Marcantonio, C.; Borghi, P.; Tataseo, P.; Ciccaglione, A.R.; Presutti, C.; Romagnoli, G.; et al. Ifn-alpha regulates blimp-1 expression via mir-23a and mir-125b in both monocytes-derived dc and pdc. PLoS ONE 2013, 8, e72833. [Google Scholar] [CrossRef]
- Tili, E.; Croce, C.M.; Michaille, J.J. Mir-155: On the crosstalk between inflammation and cancer. Int. Rev. Immunol. 2009, 28, 264–284. [Google Scholar] [CrossRef]
- Chen, T.; Li, Z.; Jing, T.; Zhu, W.; Ge, J.; Zheng, X.; Pan, X.; Yan, H.; Zhu, J. Microrna-146a regulates the maturation process and pro-inflammatory cytokine secretion by targeting CD40l in oxldl-stimulated dendritic cells. FEBS Lett. 2011, 585, 567–573. [Google Scholar]
- Zhou, H.; Huang, X.; Cui, H.; Luo, X.; Tang, Y.; Chen, S.; Wu, L.; Shen, N. Mir-155 and its star-form partner mir-155* cooperatively regulate type I interferon production by human plasmacytoid dendritic cells. Blood 2010, 116, 5885–5894. [Google Scholar]
- Karrich, J.J.; Jachimowski, L.C.; Libouban, M.; Iyer, A.; Brandwijk, K.; Taanman-Kueter, E.W.; Nagasawa, M.; de Jong, E.C.; Uittenbogaart, C.H.; Blom, B. Microrna-146a regulates survival and maturation of human plasmacytoid dendritic cells. Blood 2013, 122, 3001–3009. [Google Scholar] [CrossRef]
- Cobos Jimenez, V.; Booiman, T.; de Taeye, S.W.; van Dort, K.A.; Rits, M.A.; Hamann, J.; Kootstra, N.A. Differential expression of hiv-1 interfering factors in monocyte-derived macrophages stimulated with polarizing cytokines or interferons. Sci. Rep. 2012, 2. [Google Scholar] [CrossRef]
- Thornburg, N.J.; Hayward, S.L.; Crowe, J.E., Jr. Respiratory syncytial virus regulates human micrornas by using mechanisms involving beta interferon and nf-kappab. mBio 2012, 3. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, X.; Cui, H.; Ni, X.; Yuan, M.; Guo, Y.; Huang, X.; Zhou, H.; de Vries, N.; Tak, P.P.; et al. Microrna-146a contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009, 60, 1065–1075. [Google Scholar] [CrossRef]
- Hecker, M.; Goertsches, R.H.; Fatum, C.; Koczan, D.; Thiesen, H.J.; Guthke, R.; Zettl, U.K. Network analysis of transcriptional regulation in response to intramuscular interferon-beta-1a multiple sclerosis treatment. Pharmacogenomics J. 2012, 12, 134–146. [Google Scholar] [CrossRef]
- Scagnolari, C.; Zingariello, P.; Vecchiet, J.; Selvaggi, C.; Racciatti, D.; Taliani, G.; Riva, E.; Pizzigallo, E.; Antonelli, G. Differential expression of interferon-induced micrornas in patients with chronic hepatitis c virus infection treated with pegylated interferon alpha. Virol. J. 2010, 7. [Google Scholar] [CrossRef]
- Pedersen, I.M.; Cheng, G.; Wieland, S.; Volinia, S.; Croce, C.M.; Chisari, F.V.; David, M. Interferon modulation of cellular micrornas as an antiviral mechanism. Nature 2007, 449, 919–922. [Google Scholar] [CrossRef]
- Nathans, R.; Chu, C.Y.; Serquina, A.K.; Lu, C.C.; Cao, H.; Rana, T.M. Cellular microrna and p bodies modulate host-hiv-1 interactions. Mol. Cell 2009, 34, 696–709. [Google Scholar] [CrossRef]
- Wiesen, J.L.; Tomasi, T.B. Dicer is regulated by cellular stresses and interferons. Mol. Immunol. 2009, 46, 1222–1228. [Google Scholar] [CrossRef]
- Ahmed, S.; Maratha, A.; Butt, A.Q.; Shevlin, E.; Miggin, S.M. Trif-mediated TLR3 and TLR4 signaling is negatively regulated by adam15. J. Immunol. 2013, 190, 2217–2228. [Google Scholar] [CrossRef]
- Fang, T.C.; Schaefer, U.; Mecklenbrauker, I.; Stienen, A.; Dewell, S.; Chen, M.S.; Rioja, I.; Parravicini, V.; Prinjha, R.K.; Chandwani, R.; et al. Histone h3 lysine 9 di-methylation as an epigenetic signature of the interferon response. J. Exp. Med. 2012, 209, 661–669. [Google Scholar] [CrossRef]
- Lin, L.; Hou, J.; Ma, F.; Wang, P.; Liu, X.; Li, N.; Wang, J.; Wang, Q.; Cao, X. Type I IFN inhibits innate IL-10 production in macrophages through histone deacetylase 11 by downregulating microrna-145. J. Immunol. 2013, 191, 3896–3904. [Google Scholar] [CrossRef]
- Rizza, P.; Capone, I.; Moretti, F.; Proietti, E.; Belardelli, F. Ifn-alpha as a vaccine adjuvant: Recent insights into the mechanisms and perspectives for its clinical use. Expert Rev. Vaccines 2011, 10, 487–498. [Google Scholar] [CrossRef]
- Diamond, M.S.; Kinder, M.; Matsushita, H.; Mashayekhi, M.; Dunn, G.P.; Archambault, J.M.; Lee, H.; Arthur, C.D.; White, J.M.; Kalinke, U.; et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 2011, 208, 1989–2003. [Google Scholar] [CrossRef]
- Fuertes, M.B.; Woo, S.R.; Burnett, B.; Fu, Y.X.; Gajewski, T.F. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 2013, 34, 67–73. [Google Scholar] [CrossRef]
- Santini, S.M.; di Pucchio, T.; Lapenta, C.; Parlato, S.; Logozzi, M.; Belardelli, F. The natural alliance between type I interferon and dendritic cells and its role in linking innate and adaptive immunity. J. Interferon Cytokine Res. 2002, 22, 1071–1080. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gessani, S.; Conti, L.; Del Cornò, M.; Belardelli, F. Type I Interferons as Regulators of Human Antigen Presenting Cell Functions. Toxins 2014, 6, 1696-1723. https://doi.org/10.3390/toxins6061696
Gessani S, Conti L, Del Cornò M, Belardelli F. Type I Interferons as Regulators of Human Antigen Presenting Cell Functions. Toxins. 2014; 6(6):1696-1723. https://doi.org/10.3390/toxins6061696
Chicago/Turabian StyleGessani, Sandra, Lucia Conti, Manuela Del Cornò, and Filippo Belardelli. 2014. "Type I Interferons as Regulators of Human Antigen Presenting Cell Functions" Toxins 6, no. 6: 1696-1723. https://doi.org/10.3390/toxins6061696
APA StyleGessani, S., Conti, L., Del Cornò, M., & Belardelli, F. (2014). Type I Interferons as Regulators of Human Antigen Presenting Cell Functions. Toxins, 6(6), 1696-1723. https://doi.org/10.3390/toxins6061696





